Abstract
Climatic changes are commonly recognized to alter freshwater ecosystems. This chapter provides a unique perspective on the implications of climate change for reservoir inflows, water quality assessment, and management of aquatic contaminants influenced by site-specific pH. The various physical, biological, and chemical dynamics of reservoir zones are reviewed and a case study of four reservoirs in Texas demonstrates that reduced inflows and daily pH variability have direct implications for the collection, analysis and interpretation of water quality data. The chapter concludes with recommendations for reservoir water quality assessment and management, particularly given the prospect of increased frequency and duration of drought conditions in the southwestern and south-central U.S.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Climatic changes are commonly recognized to alter the structure and functions of freshwater ecosystems [18]. In fact, a recent special issue of Limnology and Oceanography focused on lakes and reservoirs as sentinels [1], integrators [68], and regulators of climatic change [127]. From impacts on shortening ice cover [117] to increased risks from harmful algal blooms [91, 100, 136] and anthropogenic contaminants [7, 63, 75, 87], understanding the implications of climate change on environmental assessment and management is critical [104, 141].
Climate change can result in strong shifts in precipitation patterns that alter instream flows [4, 8]. Cayan et al. [16] recently projected sustained periods of drought for the twenty-first century in the southwestern U.S., which will challenge water resource management efforts [47, 74]. In fact, drought conditions occurred during late 2005 and 2006 in Texas, resulting in elevated temperatures, reduced precipitation and decreased freshwater inflows to reservoirs. In Sect. 26.5 of this chapter we present a case study of the influence of drought on instream flows, reservoir pool levels, and implications for site-specific environmental assessments. Similar increases in temperature and decreases in precipitation, inflows, and reservoir pool levels have been observed [22, 78] and predicted elsewhere [92] during summer and autumn months. Our previous efforts in Texas reservoirs focused on developing an approach to support water quality assessments in different reservoir locations during drought conditions [11]. We further highlighted the importance of impoundment and sampling location depth during studies of reservoir carbon and nitrogen dynamics [40, 41, 108]. Valenti et al. [133] identified the importance of such drought conditions on reduced instream flows, diel pH variability, and resulting site-specific uncertainty associated with aquatic risk assessments of ionizable contaminants.
In this chapter we provide a unique perspective on the implications of climate change for reservoir inflows, reservoir water quality assessment and management of aquatic contaminants influenced by site-specific pH. Understanding these relationships is critical given the recent linkage among El Niño Southern Oscillation to decreased inflows and decreased dissolved oxygen levels in a reservoir near Barcelona, Spain [78]. Thus, an objective of this study is to explore how reduced inflows to reservoirs can influence site-specific hazards of aquatic contaminants. Before we do so, however, it is important to first recognize that the unique hydrology of reservoirs classifies them as intermediates between river and lake ecosystems [64]. Therefore, we begin with a review of the effects of reservoir inflows on the physical, chemical, and biological factors that influence reservoir water quality. We then provide a case study that emphasizes the impacts of drought and reduced reservoir inflows on pH-related contaminant hazards to aquatic life.
2 River and Lake Hybrids: The Importance of Considering Reservoir Zones
Early investigators knew that reservoirs differed from lakes and detailed the measurable physical, chemical, and biological gradients that developed along the axis of impoundments [5, 48, 60, 102]. Similarly, stream ecologists have documented impoundment effects on the hydrology, chemistry, and biology of downstream and upstream systems [138, 139]. Focusing on this early descriptive work, Thornton et al. [122] proposed a heuristic model of reservoir zonation based on morphology (width and depth), hydrology (flow velocity and turbulence), and sedimentation rates. The model described three reservoir zones—riverine, transition, and lacustrine—that respectively exhibit decreasing downgradient advective energy associated with turbulence from river inputs. The zonation model proposed by Thornton et al. [122] remains the most widely used descriptor of reservoir spatial patterns, although locating reliable boundaries between riverine-transition and lacustrine zones has remained a challenge [40]. Not surprisingly, this reservoir zonation model has been expanded in numerous reviews to include a variety of other limnological characteristics (Table 26.1).
Numerous authors have used Thornton’s heuristic model as a basis for interpreting observed gradients in water quality parameters. Allochthonous inputs from watersheds are often major sources of nutrients to a reservoir; thus, riverine zones are generally high in nutrient concentrations, particularly during higher inflows [61]. The influence of the watershed on up-reservoir water quality is also evident from increased turbidity due to suspended sediment loadings of allochthonous organic matter [124] (Table 26.1). These patterns are exemplified in a study by Pickett and Harvey [94] that demonstrated decreasing total nitrogen and phosphorus concentrations and increasing Secchi transparency along the longitudinal axis of a South Carolina reservoir. Similarly, Doyle et al. [24] observed decreased turbidity along the riverine-lacustrine gradient of Lake Waco, an impoundment in Texas.
The deepening and widening of reservoir basins often causes a dissipation of advective energy in the water column and increases in the sedimentation of fine particulates [125]. These trends result in the development of transition zones with increasing water transparency. The reservoir basin eventually deepens and widens to a point of maximum volume, where advective energy due to river inflow is minimized and water transparency is maximized (Table 26.1). Within the lacustrine zone, advective nutrient input is minimal and nutrient cycling is dominated by internal processes [61]. Mean chlorophyll a concentrations respond to such transparency gradients along the reservoir gradient. Specifically, water column chlorophyll a values have been shown to be relatively low in the riverine zone and then increase significantly in the transition zone before again falling in the lacustrine region [65]. A decreasing trend in particle-associated parameters (total Kjeldahl nitrogen, total phosphorus, ammonium, and total iron concentrations) along the riverine-lacustrine gradient has been reported for seven Kentucky reservoirs [20]. Filstrup and Lind [35] recently defined similar sedimentation patterns and quantified sediment resuspension along the riverine-lacustrine continuum in Lake Waco, Texas.
The dynamics of biological communities have also been explored in the context of Thornton’s reservoir zonation model. Although relatively high levels of available nutrients are often present in the riverine zone, primary production is predicted to be low due to light limitation in turbid waters [65]. However, increasing water transparency results in increased phytoplankton production in the transition zone before a subsequent decrease in the lacustrine zone as nutrients become the growth-limiting factor [65]. Buckavekas and Crain [13] demonstrated increasing phytoplankton nutrient limitation and occasional spatial shifts in the nutrient limitation of algal production along a Kentucky reservoir gradient. Some evidence has supported complex seasonal shifts in phytoplankton nutrient limitation status [26]; however, few studies have demonstrated similar spatial complexity [49]. Increased nutrient limitation of near-dam stations relative to up-reservoir locations is commonly [49] but not universally [115] reported. Havel and Pattinson [54] reported strong longitudinal patterns in the densities of algae and zooplankton with cyanobacteria populations being most abundant in up-reservoir and tributary sites in Bull Shoals Lake, Arkansas. More recently, Scott et al. [108] and Doyle et al. [24] identified reservoir transition zones as potential hot spots for nitrogen-fixing cyanobacterial blooms.
Because primary production in riverine zones is relatively low, the ratio of primary production to respiration (P:R) in this zone has historically been believed to be less than 1 (Table 26.1). The trend of P < R is usually considered a formula for anoxia and stress to aquatic life; however, the riverine zone of reservoirs is expected to maintain some degree of oxygen stability through mixing from turbulent energy provided by river inflows. In the transition zone, respiration should remain high but as previously mentioned, primary production is expected to increase, increasing P:R to approximately 1 (Table 26.1). The lack of turbulent energy in transition zones decreases reaeration, which may cause high oxygen demand and large diel swings in dissolved oxygen concentrations [17, 101]. Historically, community respiration is expected to be minimized in lacustrine zones and P:R is therefore predicted to increase to a value greater than 1 (Table 26.1).
Predicted spatial trends in reservoir ecosystem metabolism may not hold true because reservoir age, land use, and reduced inflows impact hydrodynamic and thus biological processes. For example, we recently demonstrated net heterotrophic conditions (P < R) for lacustrine zones of seven Texas reservoirs during low-flow conditions [41]. Such considerations are important because P:R dynamics influence diel dissolved oxygen patterns and the prevalence of anoxic conditions, which are critically important for the protection of habitat for aquatic life. The relationships among P:R, dissolved oxygen, and pH are further explored in Sect. 26.4.
Changes in bacterial communities along reservoir gradients have been less frequently investigated. However, Simek et al. [109] studied the changes in the epilimnetic bacterial community of a Spanish reservoir. They found strong longitudinal zonation of bacterial community composition and food web structure that were driven primarily by hydrology and high localized inputs of river-borne organic matter [109]. Lind [69] suggested that bacterioplankton production may be high in riverine zones due to allochthonous dissolved carbon inputs. Production in the transition zone should also be high, supported by increased production of autochthonous dissolved carbon (Table 26.1). Finally, production in lacustrine zones may be lower because phytoplankton productivity is lower and this zone exhibits higher rates of algal cell loss due to sedimentation. Further, Lind et al. [71] examined data for several reservoirs and demonstrated a consistent increase in trophic states along the riverine-lacustrine gradient. Specifically, riverine zones are expected to be more eutrophic than other reservoir locations, and lacustrine zones should be the least eutrophic [71]. Lind and Barcena [70] showed that storm events can temporarily shift the trophic relationships proposed by Lind [69] as floodwater inflows pass through the impoundment. Again, this perspective may need to be modified as reservoirs age and watershed development occurs, as evidenced by the recent work of McCallister and del Giorgio [80] and Forbes et al [41].
The longitudinal gradients of morphometry, hydrology, biology, and ultimately water quality reviewed above and their responses to inflows and droughts are fundamentally recognized by researchers working in reservoir systems. Because reservoir zones represent uniquely different habitats for aquatic life, Brooks et al. [11] questioned whether water quality criteria and standards should be developed for specific reservoir zones. However, water resource managers, water board and commission members, and the general public are much less aware of the spatiotemporal uncertainties related to quantifying the water quality of a reservoir [71]. Therefore, Lind et al. [71] recommended that reservoir zonation should be considered in sampling, assessment, and reservoir management. In fact, Lind et al. [71] further recommended that the normal changes along the longitudinal gradients should form the basis of a water resource management plan for multiple uses of the reservoir (e.g., aquatic life use, contact recreation, drinking water withdrawals). Additionally, Hobson et al. [56] recently demonstrated the effects of stormwater inflows on longitudinal gradients of natural organic matter and its influence on source water withdrawals for drinking water treatment.
Unfortunately, consideration of reservoir zones and their responsiveness to inflows are seldom integrated in water quality regulatory frameworks in the U.S. For example, in Texas, water quality criteria and standards only exist for water bodies generically classified as either streams or “lakes,” even though there is only one naturally occurring lake in the State (Caddo Lake) that is large enough to qualify for water quality protection. Although delineation of reservoir zonation has largely been based on qualitative changes, numerous studies have interpreted their research findings within the framework of Thornton’s zonation paradigm. For example, Brooks et al. [11] proposed coupling hydrodynamic modeling with multivariate statistical analyses of physical, chemical, and biological factors to differentiate various reservoir zones or habitats. This approach would allow for site-specific determination of reservoir zone locations as they may be influenced by inflow events and water withdrawals.
3 Influence of Inflows on Reservoirs
As previously noted, influences of climate change on river hydrology may have profound effects on reservoir zonation and water quality. Altered inflows can dramatically influence physical, chemical, and biological processes, which may in turn complicate reservoir water quality assessment and management. Reservoir limnologists routinely consider the dynamic nature of reservoir gradients and how inflow variability may cause them to shift in time and space. For example, Wetzel [142] noted that lakes routinely receive inflows from lower-order streams and more diffuse sources because lake watersheds are comparatively smaller than those of impoundments. Thus, reservoirs generally receive a majority of inflows from higher-order streams and rivers associated with larger drainage areas [142]. A number of researchers have commented on the dynamic nature of the riverine and transition zones in particular, noting that these locations have variable and somewhat unpredictable physical, chemical, and biological dynamics. Kimmel and Groeger [64] proposed a view of reservoirs as semi-fluvial environments and pointed out the likely influence of flushing rate or water retention time on the spatial and temporal heterogeneity of these systems. As we examine below, others have likewise recognized the importance of hydrologic flushing and stressed that the reservoir zonation model, while valuable, is likely to vary dramatically in space and time, largely depending on inflow patterns.
The transition zone is clearly recognized as the most dynamic of the reservoir zones [59]. Furthermore, it is widely acknowledged that the position of this zone is subject to substantial fluctuations related to hydrodynamic forces [27]. Under high-flow events, the exchange between a cove and the main reservoir may be dominated by advective momentum of the entering stream. However, for most reservoir tributaries located at lower latitudes in semiarid and arid regions, inflows may be low enough that the advective momentum dissipates rapidly in the lotic system. In Lake Lewisville, Texas, for example, fluctuations of riverine and transition zone locations were related to drought and water withdrawal patterns [120]. Kennedy et al. [59] suggested that the upstream and downstream boundaries of the transition zone may correspond to the location of the plunge point under low-flow and high-flow conditions, respectively. When density of inflowing waters is greater than that of the reservoir surface layer, then water from the inflowing stream will “plunge beneath” the surface layer of the reservoir, resulting in an underflow [42]. However, the position of the plunge point is dynamic and responsive to inflows, reservoir volumes and water column densities. Accordingly, in reservoirs where a plunge point is generally detectable, the boundary between the riverine and the transition zones would be the plunge point under low-flow conditions.
Kimmel et al. [65] discussed the Thornton zonation model and commented on numerous exceptions resulting from the dynamic nature of reservoir zones. For example, they cited evidence of numerous rapidly-flushed, run-of-the-river impoundments where riverine-like conditions persist throughout most or all of the reservoir. Additionally, reservoirs with lower inflows and long residence times had highly compressed riverine and transition zones and likely function as “lakes” over most of their extent, despite changes in morphology in the upstream direction. Changes in reservoir zonation were also observed by An and Jones [3] for a Korean reservoir based on annual monsoon pattern. In years with strong monsoon, there was sharp longitudinal zonation with a turbid, light-limited riverine zone followed by productive transition and lacustrine zones. However, when the monsoon was weak and inflows were low, there was little to no apparent riverine zone and the lacustrine zone dominated the reservoir [3]. Thus, reservoir retention time (R) was strongly influenced by inflows. Straskraba et al. [114] discussed the likely role of R on the extent of the three reservoir zones. They speculated that under short retention times (R < 10 days) the whole reservoir may be considered to be within the riverine zone. Conversely, when there is little advective inflow, and retention times are very long (R > 200 days), virtually all of the reservoir will be within the lacustrine zone [114].
Zooplankton communities are often found to exhibit spatial trends that are related to reservoir zonation and responses to inflows. Marzolf [140] pointed out that zooplankton quickly respond to resource gradients. In addition, reservoir gradients resulted in a pattern of zooplankton distribution with elevated population densities in the transition zone of a reservoir, though the location of this zone clearly was dependent on inflows [140]. Havel and Pattinson [54] reported pronounced spatial structure of zooplankton with the highest densities occurring in the transition zone; this observation is consistent with patterns seen in Kansas [140] and Texas [43]. Soballe and Threlkeld [112] found that short residence times nullified the stimulation of inflowing nutrients on algal biomass in smaller reservoirs of Oklahoma. Dirnberger and Threlkeld [21] and Threlkeld [126] further showed that flushing markedly influenced the structure of reservoir zooplankton communities.
Phytoplankton communities are also strongly influenced by inflow events, primarily through hydraulic flushing and increased nutrient pulses [100]. As reviewed above, phytoplankton production is driven by hydrology, nutrient availability, light limitation, and other physical factors in the various zones of reservoir systems [65, 137] (Table 26.1). Previous authors have also examined how climate change may increase the likelihood, expansion and severity of harmful algal blooms [7, 90, 91, 98, 99]. For reservoirs, freshwater inflows are increasingly reported to influence harmful algal blooms. Vilhena et al. [136] predicted development of an observed major cyanobacterial bloom when an Australian reservoir with drought-induced low pool levels received a large inflow event. Specifically, river inflows high in nutrients apparently stimulated development of a bloom dominated by Microcystis sp [136]. Conversely, several studies identified how inflows may terminate harmful blooms of cyanobacteria and other harmful algae. Mitrovic et al. [144] demonstrated how increased flows terminated a cyanobacterial bloom located upstream of a low-water dam on the Lower Darling River, Australia. Similarly, Brooks et al. [9] identified that increased inflows ameliorated a harmful bloom of the haptophyte Prymnesium parvum in Dunkard Creek, Pennsylvania. This bloom decimated fish and shellfish in the river, which is impounded by several low-water dams [9].
Such observations are consistent with those of Roelke et al. [98], who documented how inflows to Lake Granbury, an impoundment of the Brazos River in central Texas abruptly terminated a large-scale fish kill resulting from bloom formation of P. parvum. Prior to those inflows, severe drought conditions had promoted increased salinity and development of a severe P. parvum bloom [98]. With 30% hydraulic dilution, the harmful bloom was terminated, ambient toxicity was ameliorated, and zooplankton densities quickly increased by over 200-fold [106]. Roelke et al. [99] further demonstrated the decade-long impacts of decreased inflows and drought-induced salinization on harmful blooms of P. parvum among a chain of three impoundments, which included Lake Granbury. Thus, Roelke et al. [99] and Mitrovic et al. [144] suggested that managing instream flows represents a potentially useful approach to minimize harmful blooms. Under drought conditions, Grover et al. [50] predicted that harmful blooms may initiate in coves that are hydraulically isolated from the main channel of a reservoir. Thus, such coves, which may not receive inflows under non-storm conditions, represent useful management units for preemptive mitigation of harmful bloom formation using various manipulations [50]. As explored further in our case study presented below, decreased inflows commonly occur in southwestern and south-central U.S. reservoirs during or following drought conditions, which are predicted to increase with climate change [98, 99].
In semi-arid and arid regions, inflows to reservoirs may receive, be dominated by, or even be dependent on return flow effluents from wastewater treatment plants. This trend is likely to increase, as the number of permitted discharges increases in response to urbanization [10]. Effluent-dominated streams present unique challenges to water resource managers and introduce a variety of chemical stressors to surface waters [10, 52, 57]. In addition to containing complex mixtures of various anthropogenic contaminants (e.g., metals, pesticides, pharmaceuticals, other industrial chemicals), effluent-dominated streams are also known to often have elevated nutrient levels [134]. The quality of discharged effluent varies temporally although the volume of annual effluent loadings to reservoirs remains relatively stable [10]. What is less understood is how loadings of nutrients and other contaminants from effluent-dominated streams influence limnological processes and water quality management in reservoirs, particularly under low-flow conditions. However, with climate change predictions of increased drought [16], the influence of effluent-dominated streams on base flows of rivers and streams deserves additional study [10]. This is particularly true for streams that were formerly intermittent tributaries, and during droughts because waste load allocations and ambient water quality monitoring efforts (e.g., for chemical constituents, ambient toxicity, and bioassessment) are generally performed during low-flow periods (e.g., defined by 7Q2 or 7Q10) [129]. How climate change may introduce uncertainty in environmental assessments that rely on currently defined low-flow conditions for regulatory compliance also deserves attention in the future [133].
4 Reservoir P:R, Diel Ph and Dissolved Oxygen, and Site-Specific Contaminant Hazards
As noted above, climatological conditions may affect ecosystem processes that influence the transportation and redistribution of nutrients in lakes and reservoirs and therefore determine the nature and intensity of biological and biogeochemical processes [15]. For example, the ionic composition of surface waters may be affected by seasonal or annual variability in precipitation that influence hydrology and transportation of allochthonous nutrients from surrounding watersheds [14, 36, 96, 113]. Climate also affects successional shifts of phytoplankton and macrophyte communities that could influence the carbon dioxide demand of aquatic systems. Maberly [73] described scenarios in which inorganic carbon in surface waters may become uncoupled from concentrations of carbon dioxide in the atmosphere due to high rates of biological transformation related to primary production.
Several researchers have noted substantial diel oscillation in pH at both riverine [12, 44, 51, 85, 123] and lacustrine sites [15, 58, 73, 119]. These diel pH patterns are attributed to assimilation of inorganic carbon for photosynthesis during light hours, followed by continued community respiration at night. Several studies have noted that eutrophication of surface waters can potentiate extreme diel pH oscillations due to high rates of primary production and greater ecosystem biomass [51, 73, 76, 79, 82,145]. Similarly, eutrophication may also spur higher rates of respiration that may eventually lead to increased oxygen demand due to cessation and decomposition of species. Figure 26.1 illustrates this perspective by depicting 24-h data for a representative riverine site in Lake Conroe, Texas, during July 2005, where diel pH values respond directly to dissolved oxygen dynamics.
The interplay of community primary production and respiration and their effects on oxygen dynamics in surface water have been long recognized [88], and the importance of diel patterns of dissolved oxygen are emphasized by protocols for current water quality monitoring efforts [121]. Monitoring efforts for dissolved oxygen recommend that 24-h data profiles are collected, or at minimum, measurements are recorded at multiple intervals throughout the day with at least one targeting the early morning hours [121]. The additional effort for these monitoring approaches is to ensure collection of data representing worst-case scenarios of anoxia. For example, surface water impacted by eutrophication may have elevated levels of dissolved oxygen during the day due to high rates of primary production; however, biological oxygen demand due to community respiration or decomposition may deplete the available oxygen throughout the night when photosynthesis is no longer occurring. These same processes can influence surface water pH and therefore daily discrete measurements may not provide sufficient resolutions for the accurate definition of site-specific pH conditions (Fig. 26.1). Consequently, the failure to monitor and account for diel pH oscillations may introduce uncertainty in environmental risk assessment and management [131, 133].
The potential for contaminants to cause toxicity in aquatic ecosystems is often influenced by site-specific factors [33, 86, 116, 135]. Surface water pH is particularly important because it can change the physicochemical properties of compounds or affect how they interact with other constituents in the water column. Consequently, predictions of environmental fate, bioavailability, and potency for some contaminants can vary appreciably over environmentally relevant surface water pH gradients. Scientists are cognizant of the biological relevance that such variability in pH has on ecological risk assessment and substantial effort has been made to accommodate for site-specific distinctions to reduce uncertainty. Examples of these efforts include the integration of site-specific pH adjustment factors for the derivation of National Ambient Water Quality Criteria (NAWQC) for contaminants such as ammonia [130] and pentachlorophenol (PCP) [128]. The importance of site-specific factors (including pH) for regulatory efforts is also emphasized by the development of biotic ligand models (BLMs) to predict metal speciation and acute toxicity [86].
The completion of bioassays in the laboratory under controlled conditions has allowed researchers to identify pH-dependent toxicity relationships for various traditional contaminants such as ammonia [132], heavy metals [29, 72, 81, 93, 105], contaminants of emerging concern such as pharmaceuticals [28, 63, 84, 131], and harmful algal toxins [46, 134]. However, the effective utilization and application of pH-dependent toxicity data for regulatory efforts is contingent on appropriate characterization of site-specific conditions.
Defining site-specific conditions is challenging for surface water pH because it culminates from various interactions among the atmosphere, hydrology, climate, geomineralogy, and physical morphology that fluctuate both in time and space on various scales [2, 38, 55, 97, 103]. Consequently, understanding site-specific pH and putting it in the context of contaminant hazards for surface waters in general and reservoirs in particular is thus challenged by the spatiotemporal variability observed within and between watersheds [25, 38, 55, 97]. As noted above, within a specific impoundment, site-specific conditions vary both spatially and temporally in different reservoir zones. Whereas differences in surface water pH are partially explained by environmental heterogeneity (e.g., distinctions in geominerology, physical morphology, hydrology, and land use between watersheds), less understood is how the temporal factors causing pH variability introduce uncertainty in environmental risk assessments. Below we present a case studying examining the influence of inflows on diel pH swings in reservoirs and subsequent implications for how sites that are driven by P:R dynamics may influence hazards of select aquatic contaminants.
5 Climate Influences on Reservoir Dissolved Oxygen and Ph: A Case Study
5.1 Study Locations and Approach
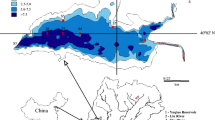
For this case study we selected four impoundments in Texas: Cedar Creek Reservoir, Lake Lewisville, Lake Conroe and Aquilla Lake (Fig. 26.2). Cedar Creek Reservoir is eutrophic, located in the Trinity River Basin, and has a surface area of 138.8 km2 and a watershed area of 2,589 km2. Lake Lewisville is also located in the Trinity River Basin and is eutrophic. Lake Lewisville’s watershed is highly urbanized, and has a surface area of 94.2 km2 and a watershed area of 4,299 km2. Lake Conroe is also a eutrophic reservoir located in the San Jacinto River Basin, and has a surface area of 84.9 km2 and a watershed area of 1,153 km2. Aquilla Lake is mesotrophic and located in the Brazos River Basin. Aquilla Lake has a surface area of 13.3 km2 and a watershed area of 660 km2.
Land use characteristics associated with drainage basins of each study reservoir are provided elsewhere [40]. The designated uses under the U.S. Clean Water Act for each study reservoir include contact recreation, high aquatic life use, and public water supply. As expected for any reservoir, pool elevations are known to fluctuate based largely on climatic factors and water use patterns. For example, during the 5-year period of 1999–2003, pool elevations in these reservoirs fluctuated from 6 to 20 ft with 74% and 24% of the total pool observations from the reservoirs reported below and above the conservation pool elevation, respectively.
We selected monitoring stations along two riverine to lacustrine gradients in each reservoir to identify the extent of longitudinal changes or gradients of water quality. In addition, a main lake station was chosen for each study reservoir to provide water quality information from the lacustrine zone. Continuous measurements of temperature, pH, dissolved oxygen, and conductivity were continuously monitored at surface depth (∼0.3 m) using a YSI 600 XLM® multiparameter datasonde configured with a YSI 650 MDS® multiparameter display system [121]. All water quality data used for statistical analyses passed pre- and post-calibration checks [121]. Methodology used to calculate community P:R followed common approaches [62]. Station locations within either riverine, transition or lacustine zones were further identified using field collected parameters and hydrodynamic modeling [11]. More information on various methodologies, which were employed under a U.S. Environmental Protection Agency-approved Quality Assurance Project Plan, is provided elsewhere [11, 40, 41, 108].
Reservoirs were sampled during summer conditions of 2005 and 2006. During this period, temperatures were warmer than normal and precipitation was lower than normal for most of Texas, though coastal areas received an excess of rain. For example, between April and August 2006 in the Dallas/Ft. Worth region of north-central Texas, mean temperature was 4.5°F warmer and total rainfall was ∼60% lower than normal. Such precipitation decreases resulted in lower inflows to reservoirs and decreased reservoir volumes, which is depicted as mean daily pool levels of four reservoirs during 2005 and 2006 (Fig. 26.3).
5.2 Community P:R and Diel Dissolved Oxygen and Ph Dynamics
As noted above, Forbes et al. [41] previously reported that when both 2005 and 2006 datasets were considered, Lake Conroe, Cedar Creek Reservoir, and Lake Lewisville were net heterotrophic. In the present case study, we identified that community P:R (Fig. 26.4) did not conform to historic expectations of P:R ratios along the riverine to lacustrine gradient (Table 26.1). In fact, lacustrine stations were net heterotrophic (P < R). During the drought conditions of 2006, net heterotrophic conditions were more pronounced with both transition and lacustrine zones, which were characterized by P:R less than 1. As noted above, diel dissolved oxygen and pH are coupled (Fig. 26.1). Thus, we examined the magnitude of dissolved oxygen and pH change over 24 h from each sampling station (Fig. 26.5). In 2005, relatively limited dissolved oxygen and pH change was observed in riverine sites, whereas statistically significant relationships were observed between dissolved oxygen and pH change at transition and lacustrine sites (Fig. 26.6). However, such observations were not observed in transition or lacustrine zones during 2006 (Fig. 26.5). Further, pH and dissolved oxygen variability in riverine zones during the drought conditions of 2006 resembled transition zone locations during 2005 (Fig. 26.6). Thus, it appears that reduced inflows during 2006 reduced community primary production relative to heterotropic respiration (Fig. 26.4), which reduced the magnitude of diel dissolved oxygen and pH variability, particularly in the transition and lacustrine zones of these reservoirs (Fig. 26.6). In fact, such observations in these Texas reservoirs are generally consistent with those of Marce et al. [78], who identified decreasing stream flows over a 44-year period to cause an approximate 20% decrease in oxygen levels in Sau Reservoir near Barcelona, Spain.
Below we examine the role of pH on bioavailability and toxicity of metals and ionizable contaminants. We further examine how the pH variability between more traditional (2005) and drought conditions (2006) influenced site-specific predictions of NAWQC values for several model contaminants in riverine, transition and lacustrine zones of the reservoirs examined in this case study. We specifically estimated acute NAWQC concentrations for an ionizable weak acid (PCP), a weak base (ammonia) and copper, using the BLM.
5.3 Ph Influences on Metal Speciation, Ionization, and Site-Specific Water Quality Criteria
5.3.1 Ph Influences on Metal Speciation and Aquatic Toxicity
Trace element speciation analysis is an approach in which various forms or phases of an element (e.g., simple inorganic species, organic complexes, and absorbed to colloidal particles) are quantified in an environmental sample on an individual basis rather than cumulatively as the total measured concentration [39, 118]. Of course, multiple elemental species may coexist in an environmental matrix simultaneously and their prevalence is determined by various environmental factors. Therefore, defining site-specific conditions is often imperative for grasping either biological or geochemical cycling of elements in the environment [39]. While various factors influence elemental speciation in surface waters (e.g., cations and anions, dissolved organic carbon), pH is often critical because it controls the magnitude and intensity of interactions with other variables.
Elemental speciation is important for environmental risk assessment because the various physiochemical forms of elements generally have unique potencies to aquatic life [39]. Furthermore, differences in site-specific conditions may also dramatically affect environmental fate, transport, partitioning behavior, bioavailability, bioconcentration, and ultimately toxicity [23, 95]. The total elemental concentration in an environmental sample seldom provides sufficient information to accurately infer hazard [31]. For example, many fish and other aquatic species can effectively cope with high concentrations of heavy metals in the food chain or sediments due to their natural defenses against ingested heavy metals, which can be eliminated from the gut and detoxified by metallothioneins [67]. However, it appears that relatively seldom have aquatic species evolved mechanisms to defend them against elemental species that may be rapidly absorbed across the water-gill interface (e.g., free metal ions or toxic lipid-soluble complexes). Therefore, the site-specific risk that a contaminant poses to aquatic organisms is often more accurately predicted by the proportion of that contaminant in a free form [31].
The BLM was developed for heavy metals to support determinations of how site-specific conditions affect metal speciation, an understanding of ambient aquatic toxicity, and risk characterization and management [23]. To illustrate the influence of pH on copper speciation, predictions of the prevalence of free ionic copper (Cu2+) in synthetic waters with varying hardness are illustrated in Fig. 26.7 at 25° C and for a total concentration of 25 μg Cu/L. This observation is critical because ionic copper concentrations are most highly correlated with acute toxicity to aquatic life [23, 95]. In Fig. 26.7, the effects of pH are clear regardless of hardness concentrations. The greatest magnitude of differences in free Cu2+ concentration is expected in soft water with a predicted 2000-fold difference between pH 6 and 9.5, whereas the predicted differences for moderately hard and hard water were 1400- and 780-fold, respectively (Fig. 26.7). In the present case study with various reservoir sites, we modeled acute copper NAWQC values using default BLM assumptions, hard water conditions, and maintained temperature at 25°C (the highest possible temperature allowed by the model).
5.3.2 Ph Influences on Ionization State and Aquatic Toxicity
Scientists have long recognized that the biological activity of some xenobiotics is markedly influenced by their ionization state [6, 107, 110, 111]. The physiochemical properties of ionizable compounds may vary in the environment depending on their dissociation, which is controlled by structural components intrinsic to the compound and the pH of the medium in which it resides. Researchers will often estimate acid dissociation constant (pK a) for compounds based on their structural attributes, and this value can be related to pH to infer the degree of ionization (or dissociation). Weak acids are generally ionized in solutions when pH > pK a, whereas weak bases are more ionized when pKa > pH. The unionized forms of either weak acids or bases are often considered more bioavailable because they have greater propensity to cross cellular membranes due to their lower polarity and greater lipophilicity.
Various chemicals that may be transported or discharged into aquatic systems, such as pesticides, various metabolites, pharmaceuticals, and personal care products, may be ionizable. In fact, some contaminants are specifically designed to be ionizable to maximize their efficacy for intended use. For example, approximately 75% of the essential medicinal drugs described by the World Health Organization and approximately one-third of modern pesticides have ionizable groups [77]. In the case of pharmaceuticals, many are often administered orally and therefore must first pass through the digestive tract and then be transported via the blood to the target area. Because various regions of the body have different pH (stomach: pH 4; blood: pH 7; CNS: pH 8), the bioavailability of drugs will often change within the body [32]. Thus, the influences of pH on bioavailability, efficacy, and toxicity are critical considerations for weak acids and bases found in various environmental or biological matrices.
The importance of ionization state to environmental risk assessment is emphasized by the integration of site-specific pH adjustment factors in NAWQC for compounds such as ammonia (NH4) [132] and PCP [128]. As expected, both the weak base NH4 (pK a = 9.3) and the weak acid PCP (pK a = 4.7) are more toxic to aquatic life when they occur in the environment predominately as the unionized forms. To account for pH-dependent effects on potency, pH adjustment factors are derived by relating site-specific pH to laboratory-derived toxicological data from experiments completed with the compound of interest at various ambient pH levels (e.g., ranging from pH 6 to 9). Site-specific pH consideration can result in markedly different acceptable loads in receiving systems; varying by 13-fold for NH4 and 60-fold for PCP between sites with contrasting pH values of 6 and 9.
However, analysis of effects during environmental risk assessment is often traditionally completed by collecting data for laboratory toxicity tests in which individuals of a single species are exposed to a known concentration of contaminants [63, 131]. These exposures are typically completed under specific conditions that are stable to allow for repeatability; however, this approach may not accurately capture the potential for all contaminants to cause biological effect. For a select number of ionizable organic contaminants in which their physicochemical properties are known to change appreciably depending on the pH of the solution where they reside, including ammonia, PCP, and more recently for some pharmaceuticals [63, 84, 131] and antimicrobials [89], experiments have quantified the magnitude of difference in biological responses over environmentally relevant pH gradients. Kim et al. [63] recently noted greater toxicity at lower pH for several acidic pharmaceuticals, which was attributed to the ionization of the compounds and ultimately their bioavailability being pH-driven.
5.3.3 Site-Specific Ph Influences on Reservoir Water Quality Criteria
In the present case study, variation in surface water pH profiles led to statistically significant differences (p < 0.05) in acceptable criterion maximum concentration (CMC) NAWQC for copper, ammonia and the weak acid pesticide PCP among the reservoir zones between study years (Fig. 26.8; Table 26.2). Using the BLM, the predicted NAWQC for copper was substantially higher in Cedar Creek Reservoir and Lake Conroe due to the higher ambient pH observed at these reservoirs compared to Aquilla Lake and Lake Lewisville (Fig. 26.8). Interestingly, predicted NAWQC varied substantially at similar sites within the same reservoir between 2005 and 2006. For example, there was nearly a 10-fold difference in the NAWQC for copper between the lacustrine zone stations of Cedar Creek as mean concentrations for 2005 and 2006 were modeled to be 238 and 31 μg/L, respectively. Temporal effects were less pronounced in some of the other studied reservoirs as mean copper CMC NAWQC were nearly identical between 2005 and 2006 for transition and lacustrine zones and only varied by 28% in the riverine zone of Aquilla Lake.
In addition to variability between reservoirs, notable differences in NAWQC for copper were also observed among the riverine, transition, and lacustrine zones within the same reservoir, especially during drought conditions (Fig. 26.8). For example, the magnitude of effect related to longitudinal gradation was evident for copper in Cedar Creek during drought (2006) as mean NAWQC CMC for riverine and transition zones were 7–10 times higher compared to the lacustrine zone. Alternatively, NAWQC for copper in Lewisville Reservoir showed an opposite pattern as the lacustrine zone had values 1.7 times greater than the riverine zone during 2005.
The predicted NAWQC CMC for ammonia and PCP varied appreciably among the study reservoirs (Fig. 26.8). For ammonia, a weak ionizable base, NAWQC were markedly lower in Conroe and Cedar Creek due to higher surface water pH. The opposite was observed for the weak acid PCP and both Aquilla and Lewisville had appreciably lower acceptable water column concentrations than the other reservoirs (Fig. 26.8). The effects of flow on the potential hazard of these ionizable contaminants were emphasized by substantial differences in predicted NAWQC between 2005 and 2006. For example, mean ammonia NAQWC values for transition and lacustrine zones of Cedar Creek were 11 and 2 times higher in 2006 than in 2005 (Fig. 26.8). The effect of year was also apparent in the mean NAQWC for PCP in Cedar Creek and again the magnitude of effect was most pronounced at the lacustrine sites as the respective means varied by 4- and 8-fold between the 2 years (Fig. 26.8). However, unlike for ammonia, the NAWQC for PCP in Cedar Creek was markedly lower in 2006 compared to 2005.
There were also clear distinctions among mean NAWQC values for ionizable contaminants over longitudinal gradients in several of the reservoirs and magnitude of these effects appeared related to hydrology. For example, in 2006 the mean ammonia NAWQC was approximately 14 and 7 times lower the riverine and transition zones, respectively, compared to the lacustrine zone of Cedar Creek (Fig. 26.8). Longitudinal effects were also apparent for Conroe and Lewisville in 2005 as allowable ammonia concentrations were 3–4 times higher and far more variable in riverine zones compared to the transition or lacustrine zones.
5.4 Implications for Site-Specific Reservoir Water Quality Assessment and Management
Several researchers have demonstrated how diel cycles in pH and dissolved oxygen at riverine sites may affect surface water concentrations of trace metals if they are released from sediments. For example, Nimick et al. [85] recorded minimum metal concentrations for several metals in the mid-afternoon, but these levels increased by 100–500% during the evening and peaked just before sunrise. Gammons et al. [45] noted that dissolved concentrations of Fe(II) and Cu in streams decreased during the day by 10- and 2.4-fold, respectively. The substantial variability in observed metal concentrations over the course of the day emphasizes that biogeochemical processes affecting their environmental behavior are dynamic. Perhaps more importantly, short-term variability in metal concentrations and speciation associated with hourly or daily time scales can in fact be of similar or greater magnitude than those previously attributed to seasonal or yearly variability [83, 123]. For example, Nagorski et al. [83] describe scenarios in which short-term (daily, bi-hourly) variation of several geochemical parameters actually accounted for a majority of the variability once thought to represent fluctuations due to much coarser seasonal time scales. In other words, the timing of collection of discrete samples over the course of the day may introduce more uncertainty to the analysis of exposure phase of environmental risk assessment than collecting samples at much broader seasonal or yearly intervals.
Studies have also shown that changes in ionization state due to differences in site-specific pH can affect bioconcentration factors (BCFs) for some organic compounds Endo and Onozawa [28], Fisher et al. [37], and others have demonstrated that increased BCFs correspond to heightened toxicological responses [34, 66, 84]. When the site-specific differences in pH observed in reservoirs presented in the present case study are considered, it appears highly probable that the environmental risk of some ionizable contaminants that may be found in these reservoirs vary depending on P:R dynamics. For a gradient of wadeable streams in central Texas, Valenti et al. [133] demonstrated the importance of climate change on instream flows, diel pH variability, and aquatic hazards during the drought conditions of 2006 and extremely wet conditions of 2007. Specifically, Valenti et al. [133] described scenarios in which NAWQC for ammonia varied 20-fold at a site over the course of the day due to diel pH variability. Furthermore, the magnitude of difference among site-specific potencies for ammonia and the weak base pharmaceutical sertraline were actually considerably greater than the relative difference in mean NAWQC or predicted toxicological responses between years (2006, 2007) with strikingly different instream flows [133].
Other studies have demonstrated that patterns of diel pH oscillation have potential water quality implications because internal contaminant exposure scenarios for individuals may vary over the course of the day. In a study not focused on steady state concentrations, Hargreaves and Kucuk [53] demonstrated rather that total ammonia-nitrogen concentrations in the plasma of juvenile hybrid striped bass, channel catfish, and blue tilapia varied as a result of environmentally relevant daily oscillations in exposure pH. Similar trends in changing internal doses could occur for other ionizable contaminants, but less understood is how the magnitude, frequency, and duration of sporadic fluctuations in concentrations at specific target sites within the body will affect pharmacological or toxicological responses in aquatic organisms. Thus, how long and how often must site-specific pH be above or below a specific threshold before it affects estimates of environmental hazard for ionizable contaminants?
This question could be answered by examining site-specific pH in relation to pH-dependent toxicological relationships integrated over the course of a day. For example, a probabilistic analysis could support determining the duration and magnitude of contaminant hazard exceeding an acceptable threshold of toxicity for specific proportions of the day. Thus, instead of determining toxicological thresholds (e.g., LC50) as a function of pH, it would also be useful to define time to adverse responses (e.g., LT50) with diel pH variability. Such an approach deserves additional consideration.
The question could also be addressed by developing aquatic toxicokinetic models that take into account rates of uptake and deprivation under stochastic environmental conditions. These models allow for extension beyond the traditional approaches of simply measuring contaminant concentrations in a water sample and thus further support considerations of how site-specific conditions may affect internal body concentrations or doses of xenobiotics [19]. Recently, it has become apparent that uptake of ionizable organic contaminants cannot solely be explained by the availability of the unionized moiety [30]. While this may be partially attributed to uptake by means other than passive transport at the gill (e.g., by transporters) or other dermal surfaces, it may also be caused by an organism’s ability to maintain xenobiotics once they are absorbed. Wheeler and Hellebust [143] described how differences in the internal pH of some organelles in plant cells may serve as reservoirs for alkylamines. For examples, pumping H+ into vacuoles could reduce pH and allow some cells to accumulate amines at concentrations 103- to 104-fold higher than external media. Similar mechanisms may be at play for aquatic organisms based on the pharmacokinetic principle that body compartments may vary in their specific pH. Therefore, by melding toxicokinetic with pharmacokinetic principles and relating modes-of-action to specific target locations within the body, aquatic scientists could more appropriately determine the relative and site-specific risks of different ionizable contaminants, including contaminants of emerging concern [19].
6 Conclusions and Recommendations
Here we reviewed the various physical, biological, and chemical dynamics of reservoir zones, which distinguish them as uniquely different aquatic habitats, and we examined the role of inflows on reservoir water quality. Further, using a case study of four reservoirs in Texas, we demonstrated that daily variability has direct implications for how we should collect, analyze, and interpret site-specific water quality data, particularly among reservoir zones when inflows are influenced by climatic variability. Based on our experiences, we provide the following recommendations for reservoir water quality assessment and management, particularly given the prospects of increased frequency and duration of drought conditions in the southwestern and south-central U.S [16, 74].
Regulatory, management, and assessment efforts should account for the reservoir-specific characteristics that constitute the major drivers for water quality. These include reservoir morphology, hydraulic retention times, watershed land uses, timing, quantities and sources of inflows, and resident biological communities that constitute threats (e.g., harmful algal blooms) or represent sensitive resources (e.g., threatened and endangered species). Protecting such resources essentially involves managing worst-case scenarios that, for example, could occur in summer months during periods of drought. Thus, the timing and location of assessment sampling should be chosen so as to best evaluate the greatest threats to the site-specific resources.
We specifically recommend the integration of an alternative approach for defining site-specific pH at riverine, transition, and lacustrine sites using 24-h continuous monitoring data. This approach would allow researchers to determine the probability of encountering a specific pH at a site. Alternatively, we recommend that samples should be taken both in the early morning before sunrise and in the late afternoon near sunset, when net community respiration and net community production, respectively, are expected to be greatest (Fig. 26.1), to identify diel pH variability at sampling sites. At a minimum, diel pH variability should be considered in the design of study plan and long-term monitoring efforts. Current approaches for defining site-specific pH for NAWQC often include using the 15th centile of a minimum of 30 discrete samples [121]; however, as demonstrated here and elsewhere [131, 133], this approach may introduce substantial uncertainty in environmental risk assessment due to diel variability of pH.
Previously, there has been substantial effort towards defining pH-dependent toxicological relationships for traditional contaminants such as ammonia, PCP, and heavy metals. However, we advise that similar strides must be taken to better understand the potential hazard of contaminants of emerging concern, such as parent compounds, metabolites, and degradates of pharmaceuticals and other industrial chemicals. Often, the effects of pH on the physicochemical properties of pharmaceuticals are extensively studied such that pharmacokinetics and environmental fates can be predicted; however, a similar emphasis should also be made to identify how pH may affect their potency to aquatic life [131].
As demonstrated in this study, water quality parameters may shift appreciably over the longitudinal gradients of various reservoir habitats in response to inflows. This presents an environmental management challenge as changes in site-specific conditions can alter the aquatic hazard of some contaminants. Monitoring reservoir water quality only at dam (e.g., lacustrine) sites, for example, fails to provide representative information for the assessment and management of water quality in riverine and transition zones of reservoirs, particularly when inflow patterns are modified by climatic changes.
References
Adrian R, O’Reilly CM, Zagarese H, Baines SB, Hessen DO, Keller W, Livingstone DM, Sommaruga R, Straile D, Van Donk E, Weyhenmeyer CA, Winder M (2009) Lakes as sentinels of current climate change. Limnol Oceanogr 54:2283–2297
Allan DJ (1995) Stream ecology: structure and function of running waters. Chapman and Hall, London
An K, Jones J (2002) Reservoir response to the Asian Monsoon with an emphasis on longitudinal gradients. J Freshw Ecol 17:151–160
Barnett TP, Pierce DW, Hidalgo HG, Bonfils C, Santer BD, Das T, Bala G, Wood AW, Nozawa T, Mirin AA, Cayan DR, Dettinger MD (2008) Human-induced changes in the hydrology of the western United States. Science 319:1080–1083
Baxter RM (1977) Environmental effects of dams and impoundments. Annu Rev Ecol Syst 8:255–283
Blackman GE, Robertson-Cuningham RC (1953) The influence of pH on the phytotoxicity of 2:4-dichlorophenxyacetic acid to Lemna minor. New Phytol 52:71–75
Boxall AB, Hardy A, Beulke S, Boucard T, Burgin L, Falloon PD, Haygarth PM, Hutchinson T, Kovats RS, Leonardi G, Levy LS, Nichols G, Parsons SA, Potts L, Stone D, Topp E, Turley DB, Walsh K, Wellington EM, Williams RJ (2009) Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ Health Perspect 117:508–514
Brooks BW, Chambliss CK, Stanley JK, Ramirez AJ, Banks KE, Johnson RD, Lewis RJ (2005) Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem 24:464–469
Brooks BW, Grover JP, Roelke DL (2011) Accepted. Prymnesium parvum, an invasive harmful algal species in inland waters: challenges and recommendations for environmental risk assessment and management. Environ Toxicol Chem
Brooks BW, Riley TM, Taylor RD (2006) Water quality of effluent-dominated stream ecosystems: ecotoxicological, hydrological, and management considerations. Hydrobiologia 556:365–379
Brooks BW, Scott JT, Forbes MG, Valenti TW, Doyle RD, Dean KE, Patek J, Palachek RM, Taylor RD, Koenig L (2008) Reservoir zonation and water quality: science, management and regulations. LakeLine 28(4):39–43
Brooks BW, Stanley JK, White JC, Turner PK, Wu KB, La Point TW (2004) Laboratory and field responses to cadmium in effluent-dominated stream mesocosms. Environ Toxicol Chem 23:464–469
Buckavekas PA, Crain AS (2002) Inter-annual, seasonal and spatial variability in nutrient limitation of phytoplankton production in a river impoundment. Hydrobiologia 481:19–31
Carpenter SR, Fisher SG, Grimm NB, Kitchell JF (1992) Global change and freshwater ecosystems. Annu Rev Ecol Syst 23:119–139
Cavalcante PRS, MdS R, Barroso MFS, Barbieri R, Serra CLM, Oliveira RCA (2007) Diel variation of limnological parameters in a reservoir in northeastern Brazil (Boa Esperaca, Maranhao/Piaui): rainy period. Lakes Reservoirs Res Manage 12:35–42
Cayan DR, Das T, Pierce DW, Barnett TP, Tyree M, Gershunov A (2010) Future dryness in the southwest US and the hydrology of the early 21st century drought. Proc Natl Acad Sci USA 107:21271–21276
Cole T, Hannan H (1990) Dissolved oxygen dynamics. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 71–108
Cole JJ, Pace ML, Carpenter SR, Kitchell JF (2000) Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnol Oceanogr 45:1718–1730
Daughton CG, Brooks BW (2011) Active pharmaceuticals ingredients and aquatic organisms. In: Meador J, Beyer N (eds), Environmental contaminants in wildlife: interpreting tissue concentrations, 2nd edn. Taylor and Francis, pp 281–341
Davis SE III, Reeder BC (2001) Spatial characterization of water quality in seven eastern Kentucky reservoirs using multivariate analyses. Aquat Ecosyst Health Manag Soc 4:463–477
Dirnberger JM, Threlkeld ST (1986) Advective effects of a reservoir flood on zooplankton abundance and dispersion. Freshw Biol 16:387–396
Dirnberger JM, Weinberger J (2005) Influences of lake level changes on reservoir water clarity in Allatoona Lake, Georgia. Lake Reservoir Manage 21:24–29
DiToro DM, Allen HE, Bergman HL, Meyer JS, Santore RC (2000) The biotic ligand model: a computational approach for assessing the ecological effect of metals in aquatic systems. International Copper Association, New York
Doyle RD, Scott JT, Forbes MG (2010) Hot spots and hot moments of planktonic nitrogen fixation in a eutrophic southern reservoir. Lake Reservoir Manage 26:95–103
Driscoll CT, Yatsko CP, Unangst FJ (1987) Longitudinal and temporal trends in the water chemistry of the north branch of the Moose River. Biogeochemistry 3:37–61
Effler SW, Bader AP (1988) A limnological analysis of Cannonsville Reservoir, NY. Lake Reservoir Manage 14:125–139
Effler S, O’Donnell D (2001) Resolution of spatial patterns in three reservoirs with rapid profiling instrumentation. Hydrobiologia 450:197–208
Endo T, Onozawa M (1987) Effects of pH and temperature on the uptake of oxolinic acid in goldfish. Nippon Suisan Gakkaishi 53:551–555
Erickson RJ, Brooke LT, Kahl MD, Benter FV, Harting SL, Markee TP, Spehar RL (1998) Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. Environ Toxicol Chem 17:572–578
Erickson RJ, McKim JM, Lien GJ, Hoffman AD, Batterman SL (2006) Uptake and elimination of ionizable organic chemicals at fish gills: I. Model formulation, parameterization, and behavior. Environ Toxicol Chem 25:1512–1521
Escher BI, Hermens JLM (2004) Internal exposure: linking bioavailability to effects. Environ Sci Technol 38:455–462
Escher BI, Schwarzenbach RP (2000) Evaluation of liposome-water partitioning of organic acids and bases. 1. Development of a sorption model. Environ Sci Technol 32:3954–3961
Farrington JW (1991) Biogeochemical processes governing exposure and uptake of organic pollutant compounds in aquatic organisms. Environ Health Perspect 90:75–84
Fent K, Looser PW (1995) Bioaccumulation and bioavailability of tributyltin chloride: Influence of pH and humic acid. Water Res 29:1631–1637
Filstrup CT, Lind OT (2010) Sediment transport mechanisms influencing spatiotemporal resuspension patterns in a shallow, polymictic reservoir. Lake Reservoir Manage 26:85–94
Findlay S (1995) Importance of surface-subsurface exchange in stream ecosystems: The hyporheic zone. Limnol Oceanogr 40:159–164
Fisher SW, Hwang H, Atanasoff M, Landru PF (1999) Lethal body residues for pentachlorophenol in zebra mussels (Dreissena polymorpha) under varying conditions of temperature and pH. Ecotoxicol Environ Saf 43:274–283
Fitzhugh RD, Furman T, Webb JR, Cosby BJ, Driscoll CT (1999) Longitudinal and seasonal patterns of stream acidity in a headwater catchment on the Appalachian Plateau, West Virginia, USA. Biogeochemistry 47:39–62
Florence TM, Morrison GM, Stauber JL (1992) Determination of trace element speciation and role of speciation in aquatic toxicity. Sci Total Environ 125:1–13
Forbes MG, Doyle RD, Scott JT, Stanley JK, Huang H, Brooks BW (2008) Physical factors control carbon and nitrogen fixation in eight Texas reservoirs. Ecosystems 11:1181–1197
Forbes MG, Doyle RD, Scott JT, Stanley JK, Huang H, Fulton BA, Brooks BW (2011) Carbon sink to source: longitudinal gradients of planktonic P:R ratios in subtropical reservoirs. Biogeochemistry. doi: 10.1007/s10533-010-9533-3
Ford D (1990) Reservoir transport processes. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 15–42
Franks JL, Clyde GA, Dickson KL (2001) Zooplankton community structure and seasonal dynamics in Lake Texoma (Oklahoma-Texas). Tex J Sci 53:203–220
Gammons CH, Milodragovich L, Belanger-Woods J (2007) Influence of diurnal cycles on metal concentrations and loads in stream draining abandoned mine lands: an example from High Ore Creek, Montana. Environ Geol 53:611–622
Gammons CH, Nimick DA, Parker SR, Cleasby TE, McCleskey RB (2005) Diel behavior of iron and other heavy metals in a mountain stream with acidic to neutral pH: Fisher Creek, Montana, USA. Geochim Cosmochim Acta 69:2505–2516
Gert-Jan de Maagd P, Hendriks AJ, Seinen W, Sijm DTHM (1999) pH-dependent hydrophobicity of the cyanobacteria toxin microcystin-LR. Water Res 33:677–680
Gleick P (2010) Roadmap for sustainable water resources in southwestern North America. Proc Natl Acad Sci USA 107:21300–21305
Gloss SP, Mayer LM, Kidd DE (1980) Advection control of nutrient dynamics in the epilimnion of a large reservoir. Limnol Oceanogr 25:219–228
Groeger AW, Kimmel BL (1988) Photosynthesis carbon metabolism by phytoplankton in a nitrogen limited reservoir. Can J Fish Aquat Sci 45:720–730
Grover JP, Crane KW, Baker JW, Brooks BW, Roelke DL (2011) Spatial variation of harmful algae and their toxins in flowing-water habitats: a theoretical exploration. J Plankton Res 33:211–228
Guasch H, Armengol J, Marti E, Sabater S (1998) Diurnal variation in dissolved oxygen and carbon dioxide in two low-order streams. Water Res 32:1067–1074
Halstead BG, Tash JC (1982) Unusual diel pH in water as related to aquatic vegetation. Hydrobiologia 96:217–224
Hargreaves JA, Kucuk S (2001) Effects of diel un-ionized ammonia fluctuation on juvenile hybrid striped bass, channel catfish, and blue tilapia. Aquaculture 195:163–181
Havel J, Pattinson K (2004) Spatial distribution and seasonal dynamics of plankton in a terminal multiple-series reservoir. Lake Reservoir Manage 20:14–26
Hill T, Neal C (1997) Spatial and temporal variation in pH, alkalinity and conductivity in surface runoff and groundwater from the Upper River Severn catchment. Hydrol Earth Syst Sci 1:697–715
Hobson P, Fabris R, Develter E, Linden LG, Burch MD, Brookes JD (2010) Reservoir inflow monitoring for improved management of treated water quality—A South Australian experience. Water Resour Manage 24:4161–4174
Johnson LT, Tank JL (2009) Diurnal variations in dissolved oxygen matter and ammonium uptake in six open-canopy streams. J N Am Benthol Soc 28:694–708
Kanna V, Job SV (1980) Diurnal, seasonal, and vertical study of primary production in Sathiar reservoir. Hydrobiologia 70:171–178
Kennedy RH, Thornton KW, Ford DE (1985) Characterization of the reservoir ecosystem. In: Gunnison G (ed) Microbial processes in reservoirs. Junk Publishing, Dordrecht, pp 27–38
Kennedy R, Thornton K, Gunkel R Jr (1982) The establishment of water quality gradients in reservoirs. Can Water Resour J 7:71–87
Kennedy R, Walker W (1990) Reservoir nutrient dynamics. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 109–132
Kent R, Belitz K, Burton CA (2005) Algal productivity and nitrate assimilation in an effluent dominated concrete lined stream. J Am Water Resour Assoc 41:1109–1128
Kim J, Park J, Kin PG, Lee C, Choi K, Choi K (2010) Implications of global environmental changes on chemical toxicity-effect of water temperature, pH, and ultraviolet B irradiation on acute toxicity of several pharmaceuticals in Daphnia magna. Ecotoxicology 19:662–669
Kimmel BL, Groeger AW (1984) Factors controlling primary production in lakes and reservoirs; a perspective. Lake Reservoir Manage 2:277–281
Kimmel BL, Lind OT, Paulson L (1990) Reservoir primary production. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 133–194
Kishino T, Kobayashi K (1995) Relation between toxicity and accumulation of chlorophenols at various pH, and their absorption mechanism in fish. Water Res 29:431–442
Langston WJ, Bebianno MJ (eds) (1998) Metal metabolism in aquatic environments. Chapman and Hall, New York
Leavitt PR, Fritz SC, Anderson NJ, Baker PA, Blenckner T, Bunting L, Catalan J, Conley DJ, Hobbs WO, Jeppesen E, Korhola A, McGowan S, Rühland K, Rusak JA, Simpson GL, Solovieva N, Werne J (2009) Paleolimnological evidence of the effects on lakes of energy and mass transfer from climate and humans. Limnol Oceanogr 54:2330–2348
Lind O (2002) Reservoir zones: microbial production and trophic state. Lake Reservoir Manage 18:129–137
Lind O, Barcena E (2003) Response of riverine and transition zone bacterioplankton communities to a pulsed river inflow. Hydrobiologia 504:79–85
Lind OT, Terrell TT, Kimmel BL (1993) Problems in reservoir trophic-state classification and implications for reservoir management. In: Straskraba M, Tundisi JG, Duncan A (eds) Comparitive reservoir limnology and water quality management. Kluwer Academic Press, Dordrecht, pp 57–68
Lithner G (1989) Some fundamental relationships between metal toxicity in freshwater, physicochemical properties and background levels. Sci Total Environ 84:365–380
Maberly SC (1996) Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshw Biol 35:579–598
Mace RE, Wade SC (2008) In hot water? How climate change may (or may not) affect the groundwater resources of Texas. Gulf Coast Assoc Geol Soc Trans 58:655–668
Macneale KH, Kiffney PM, Scholtz NL (2010) Pesticides, aquatic food webs, and the conservation of Pacific salmon. Front Ecol Environ 8:475–482
Magnuson JJ, Robertson DM, Benson BJ, Wynne RH, Livingstone DM, Arai T, Assel RA, Barry RG, Card V, Kuusisto E, Granin NG, Prowse TD, Stewart KM, Vuglinski VS (2000) Historical trends in lake and river ice cover in the Northern Hemisphere. Science 289:1743–1746
Manallack DT (2007) The pKa distribution of drugs: application to drug discovery. Perspect Med Chem 1:25–38
Marce R, Rodriguez-Arias MA, Garcia JC, Armengol J (2010) El Nino Southern Oscillation and climate trends impact reservoir water quality. Glob Change Biol 16:2857–2865
Marzolf GR (1990) Reservoirs as environments for zooplankton. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 195–208
McCallister SL, del Giorgio PA (2008) Direct measurement of the δ13C signature of carbon respired by bacteria in lakes: linkages to potential carbon sources, ecosystem baseline metabolism, and CO2 fluxes. Limnol Oceanogr 53:1204–1216
Meador JP (1991) The interaction of pH, dissolved organic carbon, and total copper in the determination of ionic copper and toxicity. Aquat Toxicol 19:13–31
Mitrovic SM, Hardwick L, Dorani F (2011) Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J Plankton Res 33:229–241
Nagorski SA, Moore JN, McKinnon TE, Smith DB (2003) Scale-dependent variation in stream water geochemistry. Environ Sci Technol 37:859–864
Nakamura Y, Yamamoto H, Sekizawa J, Kondo T, Hirai N, Tatarazako N (2008) The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 70(5):865–873; Nimick DA, CH Gammons, TE Cleasby, JP Madison, D Skaar, CM Brick (2003) Diel cycles in dissolved metal concentrations in streams: occurrence and possible causes. Water Resour Res 39:2.1–2.17
Niyogi S, Wood CM (2004) Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ Sci Technol 38:6177–6192
Noyes PD, McElwee MK, Miller HD, Clark BW, Tiem LAV, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Int 35:971–986
Odum HT (1956) Primary production in flowing waters. Limnol Oceanogr 1:102–117
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21:1338–1349
Paerl HW, Huisman J (2009) Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep 1:27–37
Paerl HW, Scott JT (2010) Throwing fuel on the fire: synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ Sci Technol 44:7756–7758
Park GA, Ahn SR, Lee YJ, Shin HJ, Park MJ, Kim SJ (2009) Assessment of climate change on the inflow and outflow of two agricultural reservoirs in Korea. Trans ASABE 52:1869–1883
Peterson HG, Healey FP, Wagmann R (1984) Metal toxicity to algae: a highly pH dependent phenomenon. Can J Fish Aquat Sci 41:974–979
Pickett JR, Harvey RM (1988) Water quality gradients in the Santee-Cooper lakes, South Carolina. Lake Reservoir Manage 4:11–20
Playle RC, Dixon DG, Burnison K (1993) Copper and cadmium binding to fish gills: modification by dissolved organic carbon and synthetic ligands. Can J Fish Aquat Sci 50:2667–2677
Rascher CM, Driscoll CT, Peters NE (1987) Concentration and flux of solutes from snow and forest floor during snowmelt in the West-Central Adirondack region of New York. Biogeochemistry 3:209–224
Rebsdorf A, Thyssen N, Erlandsen M (1991) Regional and temporal variation in pH, alkalinity and carbon dioxide in Danish streams, related to soil type and land use. Freshw Biol 25:419–435
Roelke DL, Gable GM, Valenti TW, Grover JP, Brooks BW, Pinckney JL (2010) Hydraulic flushing as a Prymnesium parvum bloom-terminating mechanism in a subtropical lake. Harmful Algae 9:323–332
Roelke DL, Grover JP, Brooks BW, Glass J, Buzan D, Southard GM, Fries L, Gable GM, Schwierzke-Wade L, Byrd M, Nelson J (2011) A decade of fish-killing Prymnesium parvum blooms in Texas: roles of inflow and salinity. J Plankton Res 33:243–254
Roelke DL, Pierce RH (2011) Effects of inflow on harmful algal blooms: some considerations. J Plankton Res 33:205–209
Romero J, Imberger J (1999) Seasonal horizontal gradients of dissolved oxygen in a temperate Austral reservoir. In: Tundisi JG, Straškraba M (eds) Theoretical reservoir ecology and its applications. Backhuys Publishers, London, pp 211–226
Ryder RA (1978) Ecological heterogeneity between north-temperate reservoirs and glacial lake systems due to differing succession rates and cultural uses. Verh Internationalen Ver Limnol 20:1568–1574
Santschi PH (1988) Factors controlling the biogeochemical cycles of trace elements in fresh and coastal marine waters as revealed by artificial radioisotopes. Limnol Oceanogr 33:848–866
Schiedek D, Sundelin B, Readman JW, Macdonald RW (2007) Interactions between climate change and contaminants. Mar Pollut Bull 54:1845–1856
Schubauer-Berigan MK, Dierkes JR, Monson PD, Ankley GT (1993) pH-dependent toxicity of Cd, Cu, Ni, Pb, and Zn to Ceriodaphnia dubia, Pimephales promelas, Hyalella azteca, and Lumbriculus variegates. Environ Toxicol Chem 12:1261–1266
Scott JT, Stanley JK, Doyle RD, Forbes MG, Brooks BW (2009) River–reservoir transition zones are nitrogen fixation hot spots regardless of ecosystem trophic state. Hydrobiologia 625:61–68
Simek K, Armengol J, Comerma M, Garcia JC, Kojecka P, Nedoma J, Hejzlar J (2001) Changes in the epilimnetic bacterial community composition, production, and protest-induced morality along the longitudinal axis of a highly eutrophic reservoir. Microb Ecol 42:359–371
Simon EW, Beever H (1952) The effect of pH on the biological activities of weak acids and base: II. Other relationships between pH and activity. New Phytol 51:191–197
Simon EW, Beevers H (1951) The quantitative relationship between pH and the activity of weak acids and bases in biological experiments. Science 114:124–126
Simon EW, Beevers H (1952) The effect of pH on the biological activities of weak acids and bases: I. The most usual relationship between pH and activity. New Phytol 51:163–190
Smakhtin VU (2001) Low flow hydrology: a review. J Hydrol 240:147–186
Soballe DM, Threlkeld ST (1985) Advection, phytoplankton biomass, and nutrient transformations in a rapidly flushed impoundment. Arch Hydrobiol 105:187–203
Sterner RW (1994) Seasonal and spatial patterns in macro and micronutrient limitation in Joe Pool Lake, Texas. Limnol Oceanogr 39:535–550
Straškraba M, Tundisi J, Duncan A (1993) State-of-the-art reservoir limnology and water quality management. In: Straškraba M, Tundisi J, Duncan A (eds) Comparative reservoir limnology and water quality management. Kluwer Academic Publishers, Boston, pp 213–288
Suter GW (2006) Ecological risk assessment, 2nd edn. CRC Press, Boca Raton
Tack FMG, Verloo MG (1995) Chemical speciation and fractionation in soil and sediment heavy metal analysis: a review. Int J Environ Anal Chem 59:225–238
Talling JF (1976) The depletion of carbon dioxide from lake water by phytoplankton. J Ecol 64:79–121
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J N Am Benthol Soc 29:118–146
Taylor RD (2002) Water quality aspects of an intermittent stream and backwaters in an Urban North Texas Watershed: Pecan Creek, Denton County, Texas. University of North Texas, Denton. PhD Dissertation, 134 pp
Tercier-Waeber LM, Hezard T, Masson M, Schafer J (2009) In situ monitoring of the diurnal cycling of dynamic metal species in a stream under contrasting photobenthic biofilm activity and hydrological conditions. Environ Sci Technol 49:7237–7244
Texas Commission of Environmental Quality (2003) Surface water quality monitoring procedures, vol 1: Physical and chemical monitoring methods for water, sediment and tissue, Publication NO. RG-415, Dec 2003, Austin
Thornton KW (1990) Perspectives on reservoir limnology. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 1–14
Thornton KW (1990) Sedimentary processes. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 43–70
Thornton KW, Kennedy RH, Carroll JH, Walker WW, Gunkel RC, Ashby S (1981) Reservoir sedimentation and water quality- a heuristic model. In: Stefan H (ed) Proceedings of the symposium on surface water impoundments, vol I, pp 654–661
Threlkeld ST (1986) Life table responses and population dynamics of 4 cladoceran zooplankton during a reservoir flood. J Plankton Res 8:639–647
Tranvik LJ, Downing JA, Cotner JB, Loiselle SA, Striegl RG, Ballatore TJ, Dillon P, Finlay K, Fortino K, Knoll LB, Kortelainen PL, Kutser T, Larsen S, Laurion I, Leech DM, McCallister SL, McKnight DM, Melack JM, Overholt E, Porter JA, Prairie Y, Renwick WH, Roland F, Sherman BS, Schindler DW, Sobek S, Tremblay A, Vanni MJ, Verschoor AM, von Wachenfeldt E, Weyhenmeyer GA (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54:2298–2314
U.S. Environmental Protection Agency (1986) Ambient water quality criteria for pentachlorophenol- 1986. EPA-440-5-86-009. Office of Regulations and Standards, Criteria and Standards Division, Washington, DC
U.S. Environmental Protection Agency (1991) Guidance for water quality-based decisions: the TMDL process. EPA 440/4-91-001, United States Environmental Protection Agency, Washington DC
U.S. Environmental Protection Agency (1999) 1999 update of ambient water quality criteria for ammonia. EPA-822-R-99-014. Office of Regulations and Standards, Criteria and Standards Division, Washington, DC
U.S. Environmental Protection Agency (2009) Draft 2009 update aquatic life ambient water quality criteria for ammonia-Freshwater. EPA-822-D-09-001. Office of Water, Washington, DC
Valenti TW, Taylor JM, JA Back, RS King, BW Brooks (In review) Hydrological and nutrient influences on diel pH in wadeable streams: implications for ecological risk assessment of ionizable contaminants
Valenti TW, James SV, Lahousse MJ, Schug KA, Roelke DL, Grover JP, Brooks BW (2010) A mechanistic explanation for pH-dependent ambient aquatic toxicity of Prymnesium parvum carter. Toxicon 55:990–998
Valenti TW, Perez-Hurtado P, Chambliss CK, Brooks BW (2009) Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ Toxicol Chem 28:2685–2694
Van Wezel AP (1998) Chemical and biological aspects of ecotoxicological risk assessment of ionizable and neutral organic compounds in fresh and marine waters: a review. Environ Rev 6:123–137
Vanni MJ, Andrews JS, Renwick RH, Gonzalez MJ, Noble SJ (2006) Nutrient and light limitation of reservoir phytoplankton in relation to storm-mediated pulses in stream discharge. Arch Hydrobiol 167:421–445
Vilhena LC, Hillmer I, Imberger J (2010) The role of climate change in the occurrence of algal blooms: Lake Burragorang, Australia. Limnol Oceanogr 55:1188–1200
Ward JV, Stanford JA (eds) (1979) The ecology of regulated streams. Plenum Press, New York, 398 pp
Ward JV, Stanford JA (1983) The serial discontinuity concept of lotic ecosystems. In: Fontaine TD, Bartell SM (eds) Dynamics of lotic ecosystems. Ann Arbor Science Publishers, Ann Arbor, pp 29–42
Wenning RJ, Finger SE, Guilhermino L, Helm RC, Hooper MJ, Landis WG, Menzie CA, Munns WR, Rombke J, Stahl RG (2010) Global climate change and environmental contaminants: a SETAC call for research. Integr Environ Assess Manage 6:197–198
Wetzel RG (1990) Reservoir ecosystems: conclusions and speculations. In: Thornton K, Kimmel B, Payne F (eds) Reservoir limnology: ecological perspectives. Wiley-Interscience, New York, pp 227–238
Weyhenmeyer GA, Livingstone DM, Meili M, Jensen O, Benson B, Magnuson JJ (2011) Large geographical differences in the sensitivity of ice-covered lakes and rivers in the Northern Hemisphere to temperature changes. Glob Change Biol 17:268–275
Wheeler PA, Hellebust JA (1981) Uptake and concentration of alkylamines by marine diatoms. Plant Physiol 67:367–372
Wilcox BP (2010) Transformative ecosystem change and ecohydrology: ushering in a new era for watershed management. Ecohydrology 3:126–130
Williamson CE, Saros JE, Vincent WF, Smol JP (2009) Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol Oceanogr 54:2273–2282
Zeis B, Horn W, Gigengack U, Koch M, Paul RJ (2010) A major shift in Daphnia genetic structure after the first ice-free winter in a German reservoir. Freshw Biol 55:2296–2304
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this paper
Cite this paper
Brooks, B.W. et al. (2011). Influence of Climate Change on Reservoir Water Quality Assessment and Management. In: Linkov, I., Bridges, T. (eds) Climate. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1770-1_26
Download citation
DOI: https://doi.org/10.1007/978-94-007-1770-1_26
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-1769-5
Online ISBN: 978-94-007-1770-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)