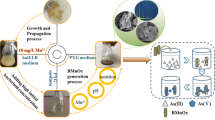
Abstract
The secondary effluent from biological treatment process in chemical industrial plant often contains refractory organic matter, which deserves to be further treated in order to meet the increasingly stringent environmental regulations. In this study, the key role of biogenic manganese oxides (BioMnOx) in enhanced removal of highly recalcitrant 1,2,4-triazole from bio-treated chemical industrial wastewater was investigated. BioMnOx production by acclimated manganese-oxidizing bacterium (MOB) consortium was confirmed through scanning electronic microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) analysis. Pseudomonas and Bacillus were found to be the most predominant species in acclimated MOB consortium. Mn2+ could be oxidized optimally at neutral pH and initial Mn2+ concentration below 33 mg L−1. However, 1,2,4-triazole removal by BioMnOx produced occurred optimally at slightly acidic pH. High dosage of both Mn2+ and 1,2,4-triazole resulted in decreased 1,2,4-triazole removal. In a biological aerated filter (BAF) coupled with manganese oxidation, 1,2,4-triazole and total organic carbon removal could be significantly enhanced compared to the control system without the participation of manganese oxidation, confirming the key role of BioMnOx in the removal of highly recalcitrant 1,2,4-triazole. This study demonstrated that the biosystem coupled with manganese oxidation had a potential for the removal of various recalcitrant contaminants from bio-treated chemical industrial wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
1,2,4-Triazole is a frequently prescribed N-heterocyclic compound (NHC) widely used in pesticide products (e.g., insecticides, herbicides, and plant growth regulators) and medicine products (e.g., fungicides, antitumor, antivirus, and antibacterial agents) (Wang et al. 2011; Fan et al. 2010; Tang et al. 2013). The widespread application of 1,2,4-triazole has raised serious concerns because 1,2,4-triazole and its derivatives may pose threats to human health by inducing carcinogenesis and chromatic aberration and do harm to ecosystem by causing bacterial resistance development due to their recalcitrant, persistent, and toxic nature (Kinnberg et al. 2007; Wu et al. 2016; Liu et al. 2015). Conventional biological wastewater treatment processes based on activated sludge often aim at removing biodegradable contaminants and nutrients; however, various recalcitrant contaminants such as 1,2,4-triazole are often resistant to mineralize in conventional biological systems (Terners et al. 2004; Wu et al. 2016). As a result, 1,2,4-triazole can penetrate through biological wastewater treatment systems, with abundant 1,2,4-triazole remaining in the effluent.
Over the past decades, various technologies including adsorption (Jia et al. 2007), chemical oxidation (Schulze and Schubert 2014), electrochemical oxidation (Han et al. 2014), and membrane separation (Zimmermann et al. 2011) have been developed for the abatement of pollution caused by recalcitrant organic pollutants such as 1,2,4-triazole and its derivatives. However, these physicochemical methods have been proven to be costly and have the inherent drawbacks due to the tendency of the formation of secondary toxic materials (Benner and Ternes 2009; Watanabe et al. 2005). Thus, it is of great emergency to develop a cost-effective method to remove 1,2,4-triazole and its derivatives from wastewater.
Manganese oxide (MnOx) has been extensively studied as the strongest oxidants naturally found in a wide range of environment settings (Tebo et al. 2004; Spiro et al. 2009). Numerous studies demonstrated that MnOx is processed in biogeochemical cycles, and it has been found to be capable of oxidizing a wide range of recalcitrant compounds, including antibacterials and related compounds with phenolic and fluoroquinolonic moieties, aromatic N-oxides, triclosan, and estrogenic compounds such as the synthetic hormone 17α-ethinylestradiol (Zhang and Huang 2003; Forrez et al. 2010; Jiang et al. 2014). MnOx may act as an electron acceptor in the presence of various micropollutants, resulting in the reduction of MnOx to Mn2+ and the oxidation of various micropollutants (de Rudder et al. 2004). Manganese-oxidizing bacteria (MOB), which can oxidize Mn2+ to biogenic manganese oxides (BioMnOx), are widely distributed in nature (Cowen et al. 1986; Fuller and Harvey 2000; Kay et al. 2001). The mechanism for Mn2+ oxidation by microorganisms involves the oxidation of Mn2+ to Mn(IV) by multicopper oxidase-like enzymes through one-electron transfer reaction (Tebo et al. 2004; Webb et al. 2005). In addition, this process is often accompanied by the occurrence of soluble or enzyme-complexed Mn(III) intermediates (Webb et al. 2005; Van Wassbergen et al. 1996). Both Mn(III) and Mn(IV) oxides are often embedded in the biofilms or bacterial sheaths formed by extracellular polymeric substances (Tebo et al. 2004). Both Mn(III) and Mn(IV) oxides as nanoscale particles have excellent sorption and oxidation properties due to their high specific surface area and high number of Mn(IV) vacancies (Villalobos et al. 2003). A number of studies have been pursued to clarify the structure of BioMnOx in recent years. According to Bargar et al. (2005) and Villalobos et al. (2003), the structures of BioMnOx produced by Pseudomonas putida strain MnB1 and Bacillus sp. strain SG-1 were mixed-valent layered Mn(III/IV)Ox compounds. The mineral structure of BioMnOx is poorly crystalline, mostly similar to δ-MnO2 or acid birnessite in terms of the relatively high Mn oxidation state and the relatively low defect levels in the octahedral Mn layer (Tebo et al. 2004).
Compared to chemically synthetic MnOx, BioMnOx can potentially be utilized to remove recalcitrant organic micropollutants in water treatment processes, due to their high specific surface area and high catalytic reactivity (Villalobos et al. 2006; Zhang et al. 2008). Biofilter coupled with BioMnOx has been employed for the removal of pollutants such as 17α-ethinylestradiol (Forrez et al. 2009), pharmaceuticals (Zhang et al. 2015) and biocides (Forrez et al. 2011). It was reported by Forrez et al. (2009) that a flow-through reactor with MnOx showed its efficiency in removal of 17α-ethinylestradiol from the effluent of wastewater treatment plant. Zhang et al. (2015) reported that the addition of Mn2+ into the feed facilitated the growth of iron/MOB and the removal of diclofenac and sulfamethoxazole. The enhanced contaminant removal could be attributed to the participation of MOB, which was quite essential for the oxidative removal of various pollutants. Meerburg et al. (2012) further found that diclofenac removal efficiency as high as 96 ± 2% within 44 h could be achieved in a manganese-bio-oxidizing system inoculated by P. putida MnB6; however, inactivation of this manganese-bio-oxidizing system by heat resulted in the complete failure in terms of diclofenac removal. As reported by Forrez et al. (2010), the addition of either sodium azide or cell lysis agent to the manganese-bio-oxidizing system resulted in a significant inhibition of diclofenac oxidation. Therefore, other mechanisms might coexist to promote the pollutant removal, which occurred with the participation of active MOB. However, continuous addition or regeneration of MnOx or BioMnOx is quite essential in order to achieve practical application of chemical MnOx or BioMnOx. The ability of MOB to regenerate BioMnOx within the biosystem makes the application of BioMnOx sustainable and cost-effective, compared with the addition of MnOx produced abiotically (Forrez et al. 2010; De Rudder et al. 2004). Thus, the manganese-bio-oxidizing system based on BioMnOx is showing wide prospect in the field of wastewater treatment (Karolina et al. 2015).
However, many problems deserve to be further resolved in order to achieve field application, especially for the polishing of chemical industrial wastewater. For example, the adaptability of pure cultures for manganese bio-oxidization is questionable when they are applied in real wastewater treatment system. The use of a natural bacterial consortium containing MOB may be more practical in the remediation of wastewater or soil, compared with pure MOB strains (Zhou et al. 2016). However, by far, most studies were mainly focused on BioMnOx produced by pure bacteria. The available information is still very limited on BioMnOx generation by bacterial consortium in the manganese-bio-oxidizing system for the remediation of various recalcitrant contaminants such as 1,2,4-triazole. In addition, the improved contaminant removal in the manganese-bio-oxidizing system is closely related with the specific microbial community structure in it. However, limited information in terms of the microbial community structure in the manganese-bio-oxidizing system is now available.
Therefore, this study was performed to obtain an acclimated microbial consortium and investigate the potential of BioMnOx to remove 1,2,4-triazole. The BioMnOx produced through Mn2+ oxidation was characterized through XRD, X-ray photoelectron spectroscopy (XPS), and SEM. The bacterial community of MOB was characterized by high-throughput sequencing analysis. The effect of various parameters on BioMnOx production and 1,2,4-triazole removal was studied. The key role of BioMnOx in the polishing of bio-treated chemical industrial wastewater was investigated in biological aerated filters (BAFs).
Materials and methods
Soil sampling, growth conditions, and acclimation
Soil samples taken near the scenic spot of Sun Yat-sen Mausoleum in Nanjing, China (32° 03′ N, 118° 50′ E), were used for the cultivation of Mn2+ oxidation bacteria consortium. Thirty samples were randomly collected in sterile containers from 5 cm below the land surface. Soil samples were air-dried and then mixed and ground in an agate mortar to pass through a 2-mm nylon sieve. About 2-g ground soil sample described earlier was inoculated into 100-mL modified Leptothrix medium supplemented with MnCl2 at an initial Mn2+ concentration of 11 mg L−1. Per liter modified Leptothrix medium contained 0.125 g yeast extract, 0.25 g casamino acids, 0.125 g glucose, 2.38 g N-2-hydroxyethylpiperazine-N′-2-ethanesulfonicacid (HEPES), 0.05 g CaCl2, 0.20 g MgSO4, 0.6 mg FeCl3, and 1-mL trace element solution. The pH was adjusted to 7.0 through the addition of NaOH and HCl. Per liter trace element solution contained 10 mg CuSO4·5H2O, 44 mg ZnSO4·7H2O, 20 mg CoCl2·6H2O, and 13 mg Na2MoO4·2H2O. In order to enrich MOB consortium, the mixture was incubated on a rotary shaker at 180 rpm and 30 °C. A series of 250-mL Erlenmeyer flask were used as batch reactors, and each flask contained a 100-mL medium described earlier. About 2 months later, a color change from pale yellow to dark brown was observed in one of the flasks, indicating the formation of BioMnOx. For the acclimation and enrichment of MOB consortium, 10-mL dark brown media were transferred into a 90-mL fresh modified Leptothrix medium containing 22 mg L−1 Mn2+ and incubated under identical conditions. Thereafter, the cultures were transferred successively to fresh medium. Five months later, the bacteria consortium with stable Mn2+ oxidation ability was obtained and named after C-S1, which was used as inocula in further study.
Mn2+ oxidation and 1,2,4-triazole removal in batch tests
Batch tests were performed in a series of 250-mL Erlenmeyer flasks on a rotary shaker at 180 rpm and 30 °C in order to investigate the optimal Mn2+ oxidation conditions, i.e., pH and Mn2+ dosage. In order to investigate the effect of pH, the pH values of the media with 22.0 mg L−1 Mn2+ were adjusted to 5.0, 6.0, 6.5, 7.0, 7.5, 8.0, and 9.0 by adding NaOH or HCl into the medium. In order to investigate the effect of initial Mn2+ concentration, the initial Mn2+ concentration of the medium varied from 11.0 to 121.0 mg L−1, with initial pH maintained at 7.0. The inoculum, i.e., C-S1, was inoculated at an inoculum size of 10%.
In order to investigate the effect of various conditions on 1,2,4-triazole removal by BioMnOx in batch reactors, a 1,2,4-triazole stock solution was added into the batch reactors at desired concentrations after the formation of BioMnOx. The incubation was carried out on a rotary shaker at 180 rpm and 30 °C. The effect of pH on 1,2,4-triazole removal by BioMnOx was firstly investigated. The pH values of the media after the formation of BioMnOx were adjusted to 5.0, 6.0, 7.0, and 8.0 while 5.0 mg L−1 1,2,4-triazole was added into every batch reactor. BioMnOx was produced with an initial Mn2+ concentration of 33.0 mg L−1. The effect of initial Mn2+ concentration on 1,2,4-triazole removal was investigated within an initial Mn2+ concentration range of 11.0–121.0 mg L−1 and at an initial 1,2,4-triazole concentration of 5.0 mg L−1, with initial pH maintained at 5.0. In order to investigate the effect of 1,2,4-triazole concentration on 1,2,4-triazole removal, the initial 1,2,4-triazole concentration varied from 1.0 to 20.0 mg L−1 and initial Mn2+ concentration was controlled at 33.0 mg L−1, with initial pH maintained at 5.0.
A control experiment regarding 1,2,4-triazole removal by the same bacterial consortium suspension but without the precipitation of Mn2+ was used to evaluate the degradation or sorption of 1,2,4-triazole by bacterial consortium. In addition, 1,2,4-triazole removal by BioMnOx suspension autoclaved at 121 °C for 40 min was evaluated in order to investigate the chemical degradation or adsorption of 1,2,4-triazole by BioMnOx. In order to evaluate the degradation of 1,2,4-triazole by chemical MnO2, batch experiments with chemical MnO2 (δ-MnO2) were initiated by adding 50.0 mg L−1 chemical MnO2 and 5.0 mg L−1 1,2,4-triazole to 100 mL of the modified Leptothrix medium. To distinguish 1,2,4-triazole removal through adsorption or catalytic degradation by BioMnOx, BioMnOx was solubilized by 10 mg mL−1 ascorbic acid to release 1,2,4-triazole adsorbed by BioMnOx before the determination of 1,2,4-triazole concentration.
1,2,4-Triazole removal in BAFs
In this study, Mn2+ oxidation and 1,2,4-triazole removal was tested in continuous BAFs, which was often used for the polishing of bio-treated chemical industrial wastewater. As illustrated in Fig. S1, two BAFs, named after R1 (with Mn2+ in the influent) and R0 (without Mn2+ in the influent), respectively, consisted of polymethyl methacrylate column (inner diameter 8 cm, effective height 120 cm, packed height 80 cm). The porous ceramic particles were used to pack these two BAFs, reaching an effective volume of 1.5 L and a headspace of 1.5 L. The parameters of the ceramic particles can be described as follows: 2.0–4.0 mm in diameter, 1.6–1.8 g cm−3 in true density, 800–900 kg m−3 in bulk density, 4.11 m2 g−1 in specific surface area, <2% in solubility in HCl, >30% in inner porosity, and >42% in outer porosity.
The bio-treated chemical industrial wastewater was pumped into the base of BAFs through two peristaltic pumps at desired influent rate. Both BAFs were aerated through a fine-bubble membrane diffuser at the bottom. The aeration rate was regulated with a needle valve at approximately 150 L h−1. The bio-treated chemical industrial wastewater was sampled from the secondary sedimentation tank after activated sludge tank in a pesticide factory located in Jiangsu Province, China. The characteristic of the bio-treated chemical industrial wastewater could be described as follows: 90–110 mg L−1 1,2,4-triazole, 60–70 mg L−1 total organic carbon (TOC), and pH 6.5–7.0. In addition, desired Mn2+ was added into the feedings of R1 to promote the growth of MOB.
Both BAFs were initially inoculated with 250 mL C-S1 consortium. In addition, 500 mL activated sludge (MLSS at 15 mg L−1) was added as supplementary inocula. In order to increase the biomass attachment onto ceramic particles, 1.5 L bio-treated chemical industrial wastewater was added into these two BAFs on the first day, with aeration turned on but with influent and effluent stream turned off. After 2 days, the rectors were continuously fed with the bio-treated chemical industrial wastewater at a hydraulic retention time (HRT) of 1.5 days, with the treatment performance evaluated. Considering the low organic compound concentration in the influent, biomass increase in both R0 and R1 was insignificant. Therefore, frequency backwashing of the BAFs was unnecessary. Periodic backwashing through the loosening of ceramic packing by means of air was carried out every 3 months, with the backwashing sludge produced discharged directly.
Analysis and characterization methods
The determination of dissolved manganese was performed by centrifuging 5-mL samples of bacteria suspension (15 min at 8000 rpm) to remove the biomass and particulate manganese. The supernatants were collected, acidified with 1% HNO3 (7 M), and stored at 4 °C prior to measurement with atomic absorption spectrometry (AAS, PinAAcle 900T, PerkinElmer, USA). To determine the sorption of Mn2+ to BioMnOx in opposite to formation of particulate MnOx, the previously mentioned particulate manganese was washed twice in deionized water and resuspended in 50 mM CuSO4 for 12 h to extract the Cu(II)-exchangeable Mn2+ (Tani et al. 2003). The total unoxidized Mn2+ was determined as the sum of the adsorbed and dissolved Mn2+. BioMnOx yield was calculated as equivalent MnO2 concentration. Briefly, the concentration of BioMnOx was measured colorimetrically with the Leco Berbelin blue (LBB) assay, and 1-mL pre-blended BioMnOx media were added to 5 mL of 0.04% LBB in 45 mM acetic acid. The samples were then stored in the dark for 30 min, and the absorbance was determined at 620 nm using a UV-vis spectrophotometry (Lambda 25, PerkinElmer, USA). KMnO4 was used to plot the standard curve (Francis et al. 2001). Any cells present in the samples were removed by centrifugation prior to measurement of the absorbance. Adsorption of 1,2,4-triazole onto BioMnOx surface under different pH values was evaluated by comparing the measured 1,2,4-triazole concentrations from two different reaction quenching methods (i.e., ascorbic acid addition vs centrifugation). By adding ascorbic acid (0.1 mM), BioMnOx was reductively dissolved yielding Mn2+ ions; thus, the adsorbed or unreacted 1,2,4-triazole could be released and measured. Besides, control tests were also conducted to verify the stability of 1,2,4-triazole in the presence of ascorbic acid (Zhang and Huang 2003).
After incubation of the acclimated C-S1 for 2 weeks, the flasks were allowed to stand for 30 min to allow the settling of BioMnOx. About 80% of the liquid was decanted, and the mixture was centrifuged to precipitate the particles. The collected solid samples were washed three times with deionized water and stored at 4 °C before analysis. For X-ray diffraction (XRD, D8 Advance, Bruker, Germany) analysis, these samples were vacuum-dried at 25 °C, crushed using a mortar and pestle, and mounted on an Al sample holder. XRD recorded in the 2θ range from 20° to 80° was obtained with a Philips X-Pert diffractometer using Cu Kα radiation. The surface of the BioMnOx was observed by scanning electronic microscopy (SEM, JSM-6380, JEOL, Japan) equipped with an energy-dispersive spectrometer (EDS) system at an accelerating voltage of 30 kV and a spot of 3.5. XPS (PHI, USA) analysis was acquired using a Thermo ESCALAB 250 instrument.
For reactor operation, water samples were withdrawn at preset time points and filtered through a 0.22 μm membrane and stored at 4 °C before analysis. 1,2,4-Triazole concentration was quantified by high-performance liquid chromatography (HPLC, Waters 2996, Waters Incorporation, USA) with a Inertsil® ODS-SP column (5 μm, 4.6 × 250 mm) and a diode array detector through authentic standard. The mobile phase was a mixture of 10% methanol and 90% ultrapure water pumped at a flow rate of 1.00 mL min−1. The sample injection volume was 10 μL. The analysis was performed at 195 nm, with a column temperature of 40 °C. Total organic carbon (TOC) was quantified by a TOC tester (Vario TOC, Elementar, Germany). DNA extraction, PCR, high-throughput sequencing, and data analysis were carried out according to Ou et al. (2016) and Jiang et al. (2016).
Results and discussion
Acclimation of MOB
After the inoculation of sampled soil for about 2 months, a color change of the modified Leptothrix media from pale yellow to dark brown was firstly observed, indicating the possibility for BioMnOx formation. Thereafter, Mn2+ oxidation and BioMnOx formation performance of microbial consortium was firstly assayed, as illustrated in Fig. 1. During the first batch of acclimation period, Mn2+ concentration decreased from initial 21.8 to 4.9 mg L−1 after 20 days. Correspondingly, BioMnOx concentration, which was assessed based on MnO2, increased from initial 1.5 to 26.9 mg L−1 after 20 days. However, after acclimation for about 130 days, the rate for both Mn2+ oxidation and BioMnOx formation increased obviously. As indicated in Fig. 1, from batch 12 to batch 14, Mn2+ could be always removed completely after about 10 days, and the concentration of generated BioMnOx always reached peak values after 10 days. The stable Mn2+ removal and BioMnOx formation after 150-day acclimation suggested that stable MOB consortium was obtained, which was named after C-S1 and used as inocula in further study.
Characterization of BioMnOx produced
The generated BioMnOx and MOB consortium was characterized through SEM observations, as shown in Fig. S2. The MOB consortium cultivated in modified Leptothrix media without Mn2+ mainly appeared as rod shaped, with the surface turned out to be smooth (Fig. S2a, b). From Fig. S2c, d, it could be seen that MOB consortium still sustained their cell morphotypes, but with something like collapsed ropes adhering tightly onto the bacterial capsular. Furgal et al. (2015) observed that BioMnOx types formed accumulated on the surface of MOB cells. As reported by Tebo et al. (2004), extracellular polymeric substances could be produced by MOB to generate biofilms or bacterial sheaths, which coated on or trapped BioMnOx. Similar morphology was also observed in this study; BioMnOx produced by C-S1 appeared to be encased by the biofilm, which appeared as collapsed ropes owing to desiccation of the samples. From EDS analysis in the inset of Fig. S2c, it could be inferred that C, O, N, Mn, and Fe were present on the cell surface, indicating the possible formation of Mn oxides.
In order to characterize the BioMnOx types formed, XPS analysis of the sediment formed after incubation was carried out, as presented in Fig. S3. According to Zou et al. (2009), binding energies (BE) of Mn 2P3/2 for MnO2, Mn2O3, and MnO were 642.4, 641.8, and 640.9 eV, respectively. Peaks for both Mn(IV) and Mn(III) were clearly visible in Fig. S3a; however, the peaks for Mn(II) were not pronounced. The presence of Mn(III) in the BioMnOx samples indicated that two sequential one-step electron transfer processes occurred for the oxidation of Mn2+ to Mn(III) and Mn(III) to Mn(IV), which was also proved by a Mn(III) intermediate occurring in the oxidation of Mn2+ by Bacillus sp. strain SG-1 (Webb et al. 2005). The important role of Mn(III) as an intermediate during the oxidation of Mn2+ to Mn(IV) by microorganism was also proposed by Tebo et al. (2004). Both Mn(III) and Mn(IV) oxides are powerful oxidants, which can oxidize a wide range of organic compounds and are ubiquitous in a wide range of environmental settings (Remucal and Ginder-Vogel 2014). From Fig. S3b, the obtained Mn 3s multiplet spitting values (ΔE) were calculated to be 4.5. The average oxidation state (AOS) of Mn in the sediment was calculated to be 3.9 through the relationship AOS = 8.956–1.126 (ΔE) (Tu et al. 2014). From XPS analysis, it could be inferred that BioMnOx with high valance Mn could be successfully obtained through the acclimation of MOB consortium.
The crystalline structure of the sediment generated by C-S1 consortium was further characterized by XRD. As presented in Fig. 2, for the diffraction pattern of the sediment formed in incubation system without the addition of Mn2+, no additional peak other than the peak at 2θ = 19.3° was observed. However, another two peaks at 36.6° and 65.7° appeared in the sediment formed in the incubation system with the addition of Mn2+. Tu et al. (2014) indicated that the peak at around 2θ = 19° could be attributed to the organic and biological media, whereas the two peaks at around 2θ = 37° and 66° could be ascribed to the (100) crystal planes of δ-MnO2 or birnessite composite of discrete or poorly ordered stacking of adjacent layers, respectively. The basic unit of birnessite is MnO6 octahedra, which is normally organized into either layer or tunnel structures. The tunnel structures are generally composed of single, double, or triple chains of edge-sharing MnO6 octahedra, and the tunnels of square or rectangular cross section are formed by chains of MnO6 octahedra through sharing corners (Tebo et al. 2004). It was probably that δ-MnO2 has the similar structural unit (Miyata et al. 2007). The primary Mn oxides generated via enzyme catalyzing are most similar to δ-MnO2 or acid birnessite (Tebo et al. 2004). As reported by McKeown and Post (2001), natural Mn oxides often are poorly crystalline solids. For example, Acremonium sp. KR21-2 and other aquatic ascomycetes formed Mn(IV) oxides similar to δ-MnO2 in liquid cultures, and a birnessite-like phase was also reported in aquatic Paraconiothyrium sp. (Miyata et al. 2006; Sasaki et al. 2006). The XRD analysis suggested that poorly crystalline BioMnOx was formed through Mn2+ oxidation by C-S1 consortium.
Composition shift in the microbial community during acclimation
The microbial community structure of both soil sample and MOB consortium at phylum and genus levels was profiled using high-throughput sequencing technology. As shown in Fig. 3a, the phylum with the highest relative abundance in initial soil sample was Protebacteria (56.05%), followed by Firmicutes (20.69%), Bacteroidetes (14.76%), Verrucomicrobia (1.65%), Gemmatimonadetes (1.62%), Acidobacteria (1.45%), and Actinobacteria (0.12%). The MOB consortium named C-S1 with stable Mn2+ oxidation ability was obtained after 150-day acclimation. It could be clearly seen that the relative abundance of Protebacteria, Bacteroidetes, and Actinobacteria in C-S1 increased to 65.13, 19.74, and 3.86%, respectively. However, Acidobacteria, Gemmatimonadetes, and Verrucomicrobia disappeared completely, whereas the abundance of Firmicutes decreased to 6.57%. The dominance of Protebacteria in both initial soil sample and C-S1 was similar to a manganese remediation system aiming at coal mine drainage treatment (Chaput et al. 2015). In addition, it was reported by Zhou et al. (2016) that after acclimation of a marine microbial consortium named PJ-1-2mM with excellent Mn2+ oxidation capacity, Protebacteria and Bacteroidetes accounted for 76.12 and 21.64%, respectively. Tebo et al. (2004) indicated that MOB were primarily identified in the phylum of Firmicutes, Protebacteria, and Actinobacteria. The dominance of Protebacteria at the phylum level indicated the richness of MOB in the C-S1 after 150-day acclimation.
The initial soil sample and C-S1 also exhibited significant difference in the genus level, as shown in Fig. 3b. The dominant genera in initial soil sample were Cupriavidus (22.35%), Lysobacter (11.75%), Janthinobacterium (10.33%), Flavihumibacter (8.90%), and Pseudomonas (7.75%). For C-S1, Lysobacter, Janthinobacterium, Flavihumibacter, and Flavobacterium were found to disappear completely while the relative abundance of Cupriavidus decreased to 1.42%. However, after 150-day acclimation, the abundance of Pseudomonas, Bacillus, Bdellovibrio, and Pedobacter increased to 27.24, 15.96, 15.23, and 7.80%, respectively. It has been extensively reported that Bacillus such as Bacillus sp. strain SG-1 and Bacillus sp. PL-2 and Pseudomonas such as P. putida strains MnB1 and P. putida strains GB-1 have strong Mn2+-oxidizing capacity (Villalobos et al. 2003; de Vrind et al. 2003; Dick et al. 2008). The dominance of Pseudomonas and Bacillus in C-S1 indicated the probability of this MOB consortium to oxidize Mn2+. Besides, the increase of the relative abundance of Bdellovibrio, Pedobacter, and Sediminibacterium after the acclimation process indicated their potential role in Mn2+ oxidation or 1,2,4-triazole biodegradation.
Effect of various operation conditions on BioMnOx generation
As indicated by Boogerd and de Vrind (1986), pH values play an important role in Mn2+ oxidation because Mn2+ oxidation activity of MOB was strongly pH dependent. As shown in Fig. 4a, with the increase of pH from 5.0 to 7.0, Mn2+ oxidation efficiency and BioMnOx generation increased from 1.2% and 1.3 mg L−1 to 99.3% and 34.1 mg L−1, respectively. However, with the further increase of pH to 9.0, Mn2+ oxidation efficiency and BioMnOx generation decreased sharply to 1.4% and 0.4 mg L−1, respectively. From this result, it could be inferred that the optimal Mn2+ oxidation efficiency and BioMnOx generation occurred at neutral condition. However, poor Mn2+ oxidation was found at both acidic and alkaline conditions, probably due to the inhibitory effect on the activity of intracellular enzyme of the bacteria caused by acidity or alkalinity. This result was in good accordance with the previous studies, where pure MOB was used for Mn2+ oxidation (Villalobos et al. 2003).
Furthermore, Mn2+ concentration in influent was another significant factor affecting Mn2+ oxidation and BioMnOx generation. As shown in Fig. 4b, with the initial Mn2+ concentration increased from 11.0 to 33.0 mg L−1, BioMnOx production increased gradually from 17.2 to 51.3 mg L−1, but with residual Mn2+ always lower than 0.5 mg L−1. However, with the further increase of initial Mn2+ concentration from 33.0 to 77.0 mg L−1, BioMnOx production appeared to stop increasing; meanwhile, the incremental concentrations of residual Mn2+ from 1.1 to 46.5 mg L−1 were observed. The phenomenon observed earlier was probably due to the toxicity of high Mn2+ concentration, which significantly inhibited the overall growth of microbes. Poor Mn2+ oxidation rate by a marine microbial consortium at high Mn2+ concentration was also observed by Zhou et al. (2016). Subsequently, pH was controlled at 7.0 and the initial Mn2+ concentration was set as 33.0 mg L−1 for achieving optimal Mn2+ oxidation performance.
Figure 5 demonstrates the time course for Mn2+ oxidation, BioMnOx generation, and biomass growth under the optimal condition within 10 days. The OD600 of the C-S1 consortium quickly grew up to 1.1 within 2 days and then gradually decreased to 0.7 on day 10. However, Mn2+ oxidation and BioMnOx generation were unremarkable within 2 days, but with obvious Mn2+ oxidation observed after 2 days. Mn2+ oxidation efficiency and BioMnOx generation quickly increased from 9.9% and 5.1 mg L−1 on day 2 to 99.9% and 51.3 mg L−1 on day 10. Similar phenomenon regarding Mn2+ oxidation was also observed in a previous work (Villalobos et al. 2003), where the cell growth reached the stationary phase after 24 h, and then, the cells aggregated, accompanied by Mn2+ oxidation after approximately 36 h. In this study, the optimal Mn2+ oxidation capacity of the MOB consortium, namely C-S1, was found to be better than some pure cultured MOB strains. For example, Wang et al. (2009) found that Brachybacterium sp. strain Mn32 was failed to oxidize Mn2+ at a concentration higher than 7.2 mg L−1, even with incubation time more than 9 days. In addition, Francis et al. (2001) discovered that a marine proteobacterium namely Erythrobacter sp. strain SD-21 could only convert 4.4 mg L−1 Mn2+ into BioMnOx within 160 h in the dark.
1,2,4-Triazole removal in batch tests
The effect of pH
The pH values of the media after the formation of BioMnOx were adjusted to 5.0, 6.0, 7.0, and 8.0 while 5.00 mg L−1 1,2,4-triazole was added into every batch reactor. As shown in Fig. 6a, with the pH of the BioMnOx culture increased from 5.0 to 8.0, 1,2,4-triazole removal seemed to be decelerated, which was rather interesting. At initial pH of 5.0, 1,2,4-triazole concentration decreased sharply to 0.89 mg L−1 within the first 2 h and then decreased slowly to 0.12 mg L−1 within 160 h. However, at initial pH of 8.0, 1,2,4-triazole concentration decreased slowly during 160 h, with residual 1,2,4-triazole concentration as high as 4.77 mg L−1 after 160 h. Similarly, the degradation of 1,2,4-triazole mediated by chemical MnO2 was also pH depended. 1,2,4-Triazole removal by chemical MnO2 at pH 5.0 was obviously greater than that at pH 7.0. The pH dependence of the oxidation of selected organic compounds by MnO2 or BioMnOx has been comprehensively reported (Forrez et al. 2010; Jiang et al. 2009). According to Remucal and Ginder-Vogel (2014), the redox potentials of MnO2 decreased linearly from 0.99 V at pH 4 to 0.76 V at pH 8. Besides, electron transfer was facilitated at lower pH values because protons were required for the reduction of MnO2. In this study, 1,2,4-triazole removal by BioMnOx was also considered to be a surface reaction, which depended strongly on surface charge of BioMnOx. According to Xu et al. (2008), at pH of 4.0, the surface of MnO2 was presumed to bear a negative charge, which increases with increased pH. The increased charge would make the MnO2 surface more hydrophilic, which would result in the blockage of the surface sites and thus limitation of the surface reaction.
It was interesting to found that in the control system of Mn2+-free C-S1 consortium culture at pH 7.0 (without BioMnOx), only a slight removal of 1,2,4-triazole was observed after 160 h, indicating the key role of BioMnOx in 1,2,4-triazole removal. Therefore, the slight decrease of 1,2,4-triazole in the Mn2+-free C-S1 consortium culture might be related to the role of MOB consortium, which was likely to absorb 1,2,4-triazole slightly. The adsorption properties of the bacteria cells were illustrated by Tebo et al. (2004), and the study demonstrated that the sorption was even more extensive when it occurred during the Mn(II) oxidation by bacteria. Compared to the BioMnOx system at pH 5.0, a slight decrease of 1,2,4-triazole removal was observed in the control BioMnOx system lacking of C-S1 consortium due to autoclave, indicating that the participation of active MOB was beneficial for 1,2,4-triazole removal. The important role of MOB involved in the removal of various organic pollutants was also elucidated in previous works. For example, an obvious inhibition of diclofenac oxidation by BioMnOx was observed by Forrez et al. (2010), which could be attributed to the inhibition of MOB through the addition of either lysozyme or sodium azide. The key role of BioMnOx in the degradation of 1,2,4-triazole was also supported by the relatively slight removal of 1,2,4-triazole by chemical MnO2 at pH 5.0. As indicated in Fig. 6a, in the oxidation system by chemical MnO2, 1,2,4-triazole concentration decreased from 5.00 to 1.46 mg L−1 within 160 h, with residual 1,2,4-triazole concentration higher than that in oxidation system by BioMnOx. Relatively slight decrease of 1,2,4-triazole removal by chemical MnO2 compared with that by BioMnOx illustrated the advantage of BioMnOx over chemical MnO2.
The effect of initial Mn2+ concentration
The removal of 1,2,4-triazole by BioMnOx at initial Mn2+ concentration ranged from 11.0 to 121.0 mg L−1 was investigated, as shown in Fig. 6b. With the initial Mn2+ concentration increased from 11.0 to 33.0 mg L−1, 1,2,4-triazole removal seemed to be accelerated. However, with the further increase of initial Mn2+ concentration to 121.0 mg L−1, 1,2,4-triazole removal seemed to be decelerated and even stopped at 121.0 mg L−1. At initial Mn2+ concentration of 33.0 mg L−1, 1,2,4-triazole concentration decreased sharply to 1.22 mg L−1 within the first 2 h and then decreased slowly to 0.10 mg L−1 within 160 h. However, at initial Mn2+ concentration of 121.0 mg L−1, 1,2,4-triazole concentration decreased slowly during 160 h, with residual 1,2,4-triazole concentration as high as 4.60 mg L−1 after 160 h. As shown in Fig. 4b, BioMnOx production increased significantly from 17.2 to 51.3 mg L−1 with the Mn2+ concentration increased from 11.0 to 33.0 mg L−1, resulting in more surface sites of BioMnOx provided for 1,2,4-triazole removal. Although BioMnOx production in the media containing 55.0 or 77.0 mg L−1 Mn2+ was close to that in the media containing 33.0 mg L−1 Mn2+, 1,2,4-triazole removal efficiency in the media with initial Mn2+ concentration of 55.0 or 77.0 mg L−1 was much lower than that in the media with initial Mn2+ concentration of 33.0 mg L−1. As reported by Barrett and McBride (2005), metal ions could inhibit the decomposition of reactants by complexing with both dissolved and adsorbed reactants or by occupying reactive surface sites of the oxides. Compared to Zn2+ and Cu2+, Mn2+ could be adsorbed more strongly onto the surface of MnO2 (Xu et al. 2008). The suppressive role of Mn2+ at high initial concentration was especially interesting, suggesting that the residual Mn2+ blocked on the surface of BioMnOx might lead to the reduced reaction efficiency of 1,2,4-triazole. In addition, the decreased 1,2,4-triazole removal could be attributed to the toxicity of Mn2+ towards microorganisms involved in 1,2,4-triazole removal, especially at high initial Mn2+ concentration (Tu et al. 2014; Xu et al. 2008). Mn oxides were well known for their cation exchange behavior, metal sorption, and redox properties. However, metal sorptions by BioMnOx and synthetic Mn oxides are significantly different due to that the former are often actively growing in the presence of contaminant metal ions, which effectively removes or decreases the activation barrier required to incorporate metals into Mn oxide structures (Post 1999; Tebo et al. 2004). MOB can produce extracellular polymeric substances that form biofilms or bacterial sheaths. These materials coat or trap the BioMnOx, which likely affect the sorptive properties (Tebo et al. 2004). From the results of SEM in this study, BioMnOx produced by C-S1 was most likely to be trapped by the biofilm, the BioMnOx and biofilm had formed a whole system, and thus, it was most likely that the whole BioMnOx system took effect. The results of the control tests in 3.5.1 also illustrated the important role of the whole BioMnOx system.
The effect of initial 1,2,4-triazole concentration
1,2,4-Triazole removal at initial 1,2,4-triazole concentration ranging from 1.00 to 20.00 mg L−1 was also investigated, as illustrated in Fig. 6c. It could be observed clearly that when the initial 1,2,4-triazole concentration was lower than 5.00 mg L−1, 1,2,4-triazole could be completely removed after 160 h. However, with the further increase of initial 1,2,4-triazole concentration to 10.00 mg L−1 and finally to 20.00 mg L−1, the curve appeared to level off, resembling Langmuir-type surface saturation effect in the presence of higher concentration of 1,2,4-triazole. This phenomenon was similar to the observation in previous studies (Zhang and Huang 2003, 2005), where an increase in the removal of carbadox or triclosan at a fixed loading of MnO2 quickly tapered off with the increase of contaminant concentration. In this study, 1,2,4-triazole removal was obviously decreased when 1,2,4-triazole concentration was above 5.00 mg L−1 with BioMnOx produced at 33.0 mg L−1 Mn2+. As reported by Klausen et al. (1997), the organic compound removal by MnOx was a surface-controlled process, which was controlled by the accessibility of the surface to organic matter. The oxidation rates decreased as the reaction proceeds due to the accumulation of reaction products on the surface, and thus, less reactive sites could be available for organic compounds (Furgal et al. 2015). In this study, a surface reaction regarding 1,2,4-triazole removal by BioMnOx was proposed. The formation of surface complex, which was probably the first step of the 1,2,4-triazole removal by BioMnOx, was dependent on the number of active surface sites. However, the transformation products of 1,2,4-triazole and the newly formed Mn2+ might occupy the oxide surface, and thus, greater reaction inhibition effect could be observed as the reaction proceeds. In addition, the high toxicity nature of 1,2,4-triazole at higher concentrations might inhibit the activities of microorganisms involved in both manganese oxidation and 1,2,4-triazole removal, resulting in the poor 1,2,4-triazole removal at higher concentrations (Kinnberg et al. 2007).
The mechanisms involved in 1,2,4-triazole removal
At the initial incubation stage, the fast removal of 1,2,4-triazole could be due to the adsorption of 1,2,4-triazole by BioMnOx. In order to assess the adsorption of 1,2,4-triazole by BioMnOx, the adsorbed 1,2,4-triazole was quantified after desorption by ascorbic acid. As indicated in Fig. 6d, the adsorption of 1,2,4-triazole to BioMnOx surface after 1 h decreased with the increasing of pH. With the increase of incubation pH from 5.0 to 8.0, the adsorbed ratio of 1,2,4-triazole onto BioMnOx decreased from 42.3 ± 2.6 to 6.1 ± 1.5%, which was consistent with the results from Fig. 6a, where 1,2,4-triazole could be removed efficiently at low pH values during the initial incubation stage. However, it was worth noting that desorbed 1,2,4-triazole was negligible at the end of 160-h incubation, suggesting that 1,2,4-triazole adsorbed at the initial incubation stage was probably removed through the degradation by BioMnOx. In view of the results achieved in this study, it was proposed that the mechanisms involved in 1,2,4-triazole removal in manganese oxidation system might follow two steps. Firstly, 1,2,4-triazole was adsorbed rapidly by BioMnOx, which was produced by MOB at the early stage. Then, the adsorbed 1,2,4-triazole was directly oxidized by BioMnOx, with the easily biodegradable intermediates formed. During the batch experiment, the breakdown intermediates of 1,2,4-triazole were investigated through HPLC-MS and GC-MS; however, no breakdown intermediates of 1,2,4-triazole could be observed, probably due to the low concentration of 1,2,4-triazole in the influent.
Based on the results of 1,2,4-triazole removal in batch tests and the literature on the oxidation of other organic substances such as triclosan, hydroquinone, and substituted phenols by MnO2 (Stone 1987; Laha and Luthy 1990; Zhang and Huang 2005; Jiang et al. 2009), a surface reaction mechanism involving MnOx proposed by Stone (1987) seems likely suitable to elaborate the removal of 1,2,4-triazole by BioMnOx. In summary, the occurrence of redox reaction could be initiated by generating a surface complex between 1,2,4-triazole and BioMnOx. Electron transfer occurred at the closely associated surface complex followed by release of the organic oxidation products and Mn2+ from the oxide. The surface complex formation and electron transfer were believed to be rate limiting. The properties of organic reductant and MnOx might also affect the precursor complex formation and electron transfer reactions. Zhang and Huang (2003) indicated that the tendency of surface complex formation might be assessed by adsorption of the organic substrate to BioMnOx. Higher adsorption of the oxidizable organic substrate onto BioMnOx could lead to the formation of more surface complexes to undergo redox transformation, resulting into higher reactivity of the substrate. In this study, when the incubation pH was decreased, 1,2,4-triazole removal by BioMnOx increased as the adsorption of 1,2,4-triazole onto BioMnOx increased (Fig. 6d), which was well agreed with the previous expectation. The surface mechanism was also reported by Zhang and Huang (2005), where the rate of ciprofloxacin oxidation by MnO2 increased with the increase of ciprofloxacin adsorption by MnO2 when the pH was varied. However, the fact that lomefloxacin and pipemidic acid adsorbed by MnO2 were obviously weaker than ciprofloxacin might also contribute partially to the observed lower reactivity of these two compounds. Furthermore, the surface complex formation was also affected by the relative concentration of organic reductant and MnOx (Zhang and Huang 2003). As observed in this study, at a fixed amount of 1,2,4-triazole, the removal of 1,2,4-triazole increased as expected with increasing amount of BioMnOx due to the increased number of active surface sites and consequently increased precursor complex formation. Similarly, the removal of 1,2,4-triazole increased with increasing of 1,2,4-triazole concentration (1–5 mg L−1) at a fixed amount of BioMnOx. However, once the surface sites were saturated by organics, additional organic reductant would not increase the formation of surface complex. The actual number of active surface sites on the BioMnOx was unknown; however, the slow reaction rate of 1,2,4-triazole even with an excess amount of BioMnOx indicated that number of active surface sites was relatively rare, which was well agreed with the fact that high initial 1,2,4-triazole concentration often resulted in poor removal.
Fate of 1,2,4-triazole in BioMnOx biofilter
The variation of Mn2+, 1,2,4-triazole, and TOC in R1 and R0 fed with bio-treated chemical industrial wastewater is illustrated in Fig. 7. Mn2+ concentration of R1 effluent decreased from 32.6 mg L−1 on day 1 to 12.7 mg L−1 on day 18, probably due to the adsorption of Mn2+ by sludge and ceramic particles. Meanwhile, adsorption of 1,2,4-triazole and TOC also occurred in both R1 and R0 at the initial stage, as indicated by the significant decrease of 1,2,4-triazole and TOC concentration in the effluent from both reactors of R1 and R0 from day 1 to day 30. Thereafter, Mn2+ concentration of R1 effluent slowly increased from 12.8 mg L−1 on day 18 to 23.0 mg L−1 on day 46. 1,2,4-Triazole and TOC concentration of R1 effluent increased from 66.70 and 36.87 mg L−1 on day 30 to 94.20 and 57.50 mg L−1 on day 50, respectively, probably due to the saturation of adsorption by sludge and ceramic particles. The similar variations of 1,2,4-triazole and TOC concentration of R0 effluent were also observed at the same period.
After day 46, Mn2+ in the effluent of R1 decreased from 22.9 mg L−1 on day 46 to below 1.0 mg L−1 on day 60, probably due to the biological oxidation of manganese on the surface of the ceramsite. At this stage, black sediment could be clearly found on ceramsite in R1. It was found that R1 performed apparently better than R0 in terms of 1,2,4-triazole and TOC removal after day 50. 1,2,4-Triazole in R1 effluent decreased from 94.20 mg L−1 on day 50 to 52.90 mg L−1 on day 86 and kept stable at around 52.10 mg L−1 from day 86 to day 122. Similarly, TOC in R1 effluent decreased from 57.60 mg L−1 on day 50 to 31.20 mg L−1 on day 70 and kept stable at around 31.10 mg L−1 from day 70 to day 122. However, only slight removal of 1,2,4-triazole and TOC was observed in R0 after day 50, with residual 1,2,4-triazole and TOC well above 90.15 and 50.20 mg L−1, respectively. The poor performance of R0 in terms of 1,2,4-triazole and TOC removal indicated the slight role of biodegradation by microorganisms within the activated sludge or alternative C-S1 culture. It was proposed that the significant difference of R1 and R0 in the removal of 1,2,4-triazole and TOC at this phase could be attributed to the formation of BioMnOx in R1, which played a key role in the removal of both 1,2,4-triazole and TOC. The slight removal of 1,2,4-triazole and TOC in R0 indicated that the biological activity of some heterotrophic bacteria could not act efficiently in pollutant removal. The results in this study provided an alternative solution for acclimation of the MOB consortium within a simple biofilter. As reported by Zhang et al. (2015), after 30-day acclimation, MOB consortium could be formed in a biofilter fed with wastewater containing Mn2+, resulting in enhanced sulfamethoxazole removal in the biofilter, which could be attributed to the oxidation reaction by BioMnOx formed.
However, when Mn2+ concentration in the influent increased to 87.3 mg L−1, the residual Mn2+ in R1 increased gradually to 49.1 mg L−1 on day 160. Residual 1,2,4-triazole and TOC in the effluent of R1 increased gradually to 77.90 and 50.20 mg L−1 on day 160, respectively. The failure to remove Mn2+ completely in R1 indicated the inhibition of MOB activities at high Mn2+ dosage, which resulted in the failure of 1,2,4-triazole and TOC removal. It was hypothesized that 1,2,4-triazole was susceptible to be oxidized by BioMnOx, generating Mn2+ and oxidized intermediates. As shown in Fig. S4, a new peak with the retention time of 3.6 min appeared in the HPLC chromatogram of BAF effluent, which was identified as semicarbazide through authentic standard. As indicated by Wu et al. (2016), semicarbazide was identified as the main intermediate during 1,2,4-triazole biodegradation by Shinella sp. NJUST26. The detection of semicarbazide in BAF effluent provided the key evidence for the cleavage of 1,2,4-triazole ring and the involving of BioMnOx in 1,2,4-triazole biodegradation.
Implications of this work
As indicated by Hastings and Emerson (1986), MOB could promote the rate of manganese oxidization compared with abiotic process. BioMnOx exhibited much higher oxidation and sorption capacities for various pollutants than synthetic MnOx (Murray and Tebo 2007). In this study, as illustrated in Fig. 6a, at the presence of BioMnOx and chemical MnO2 at pH 5.0, 1,2,4-triazole concentration decreased from 5.00 to 0.12 mg L−1 and 1.46 mg L−1 within 160 h, respectively, indicating the advantage of BioMnOx over chemical MnO2. As reported by Forrez et al. (2010), the oxidation removal of diclofenac by BioMnOx was ten times faster than that by chemically synthetic MnO2 at neutral pH. The main advantage of BioMnOx over chemically synthetic MnO2 could be attributed to the ability of the bacteria to re-oxidize the formed Mn2+, which was found to be capable of inhibiting diclofenac oxidation (Forrez et al. 2010). In addition, the widely distributed MOB in nature, e.g., in soil, in sediment, and in freshwater, enhanced the implementation simplicity of the acclimation of MOB (Tebo et al. 2004). In this study, 33.0 mg L−1 Mn2+ could be completely oxidized within 9 days by the C-S1, which was superior to some pure cultured bacteria (Francis et al. 2001; Wang et al. 2009). In both natural and engineered environments, microbes often lived in multispecies communities, where interspecific interaction was comprehensive. Thus, the cooperative Mn2+ oxidation by multispecies communities was more likely to occur in natural aquatic ecosystems, which contributed directly to the advantage of multispecies communities over pure cultured species (Liang et al. 2016).
BAFs have been extensively applied in advanced treatment of wastewater, due to its lots of advantages, such as small footprint and excellent performance in terms of the removal of various contaminants (Wu et al. 2011; Hasan et al. 2015). Coupling of manganese oxidation into BAFs has turned out to be a favorable alternative for the removal of various contaminants, such as sulfamethoxazole (Zhang et al. 2015). Particularly, the effective removal of 1,2,4-triazole through a simple coupling of manganese oxidation with BAFs in this study highlighted the practical application of microbial consortium containing MOB in BAFs for wastewater treatment.
Conclusion
Enhanced removal of highly recalcitrant 1,2,4-triazole from bio-treated chemical industrial wastewater by BioMnOx was achieved in this study. Mn2+ could be effectively oxidized to BioMnOx by MOB consortium obtained through acclimation. Pseudomonas and Bacillus were found to be the most predominant species in acclimated MOB consortium. Mn2+ oxidation occurred optimally at neutral pH and initial Mn2+ concentration below 0.6 mM. However, 1,2,4-triazole removal by BioMnOx produced occurred optimally at slightly acidic pH. High dosage of both Mn2+ and 1,2,4-triazole resulted in decreased 1,2,4-triazole removal. In the BAF coupled with manganese oxidation, the system performance in terms of 1,2,4-triazole and TOC removal could be significantly enhanced, confirming the key role of BioMnOx in the removal of highly recalcitrant 1,2,4-triazole. The BAF coupled with manganese oxidation was proven to show a potential for the removal of various highly recalcitrant contaminants from bio-treated chemical industrial wastewater.
References
Bargar JR, Tebo BM, Bergmann U, Webb SM, Glatzel P, Chiu VQ, Villalobos M (2005) Biotic and abiotic products of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Amer Mineral 90(1):143–154
Barrett KA, McBride MB (2005) Oxidative degradation of glyphosate and aminomethylphosphonate by manganese oxide. Environ Sci Technol 39(23):9223–9228
Benner J, Ternes TA (2009) Ozonation of propranolol: formation of oxidation products. Environ Sci Technol 43(13):5086–5093
Boogerd FC, De Vrind JPM (1986) Manganese oxidation by Leptothrix discophora. J Bacteriol 169(2):489–494
Chaput DL, Hansel CM, Burgos WD, Santelli CM (2015) Profiling microbial communities in manganese remediation systems treating coal mine drainage. Appl Environ Microb 81(6):2189–2198
Cowen JP, Massoth GJ, Baker ET (1986) Bacterial scavenging of Mn and Fe in a mid-to-far-field hydrothermal particle plume. Nature 322:169–171
De Rudder J, Van de Wiele T, Dhooge W, Comhaire F, Verstraete W (2004) Advanced water treatment with manganese oxide for the removal of 17α-ethinylestradiol (EE2). Water Res 38(1):184–192
de Vrind J, de Groot A, Brouwers GJ, Tommassen J, de Vrind-de Jong E (2003) Identification of a novel Gsp-related pathway required for secretion of the manganese-oxidizing factor of Pseudomonas putida strain GB-1. Mol Microbiol 47(4):993–1006
Dick GJ, Torpey JW, Beveridge TJ, Tebo BM (2008) Direct identification of a bacterial manganese (II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl Environ Microb 74(5):1527–1534
Fan Z, Yang Z, Zhang H, Mi N, Wang H, Cai F, Song H (2010) Synthesis, crystal structure, and biological activity of 4-methyl-1,2,3-thiadiazole-containing 1, 2, 4-triazolo [3, 4-b][1, 3, 4] thiadiazoles. J Agr Food Chem 58(5):2630–2636
Forrez I, Carballa M, Noppe H, De Brabander H, Boon N, Verstraete W (2009) Influence of manganese and ammonium oxidation on the removal of 17α-ethinylestradiol (EE2). Water Res 43(1):77–86
Forrez I, Carballa M, Verbeken K, Vanhaecke L, Ternes T, Boon N, Verstraete W (2010) Diclofenac oxidation by biogenic manganese oxides. Environ Sci Technol 44(9):3449–3454
Forrez I, Carballa M, Fink G, Wick A, Hennebel T, Vanhaecke L, Verstraete W (2011) Biogenic metals for the oxidative and reductive removal of pharmaceuticals, biocides and iodinated contrast media in a polishing membrane bioreactor. Water Res 45(4):1763–1773
Francis GA, Co EM, Tebo BM (2001) Enzymatic manganese (II) oxidation by a marine-proteobacterium. Appl Environ Microbiol 67(9):4024–4029
Fuller CC, Harvey JW (2000) Reactive uptake of trace metals in the hyporheic zone of a mining-contaminated stream, Pinal Creek, Arizona. Environ Sci Technol 34(7):1150–1155
Furgal KM, Meyer RL, Bester K (2015) Removing selected steroid hormones, biocides and pharmaceuticals from water by means of biogenic manganese oxide nanoparticles in situ at ppb levels. Chemosphere 136:321–326
Han W, Zhong C, Liang L, Sun Y, Guan Y, Wang L, Li J (2014) Electrochemical degradation of triazole fungicides in aqueous solution using TiO2-NTs/SnO2-Sb/PbO2 anode: experimental and DFT studies. Electrochim Acta 130:179–186
Hasan HA, Abdullah SRS, Kamarudin SK, Kofli NT (2015) Effective curves of completing simultaneous ammonium and manganese removal in polluted water using a biological aerated filter. J Ind Eng Chem 30:153–159
Hastings D, Emerson S (1986) Oxidation of manganese by spores of a marine Bacillus: kinetic and thermodynamic considerations. Geochim Cosmochim Ac 50(8):1819–1824
Jia Y, Aagaard P, Breedveld GD (2007) Sorption of triazoles to soil and iron minerals. Chemosphere 67(2):250–258
Jiang J, Pang S, Ma J (2009) Oxidation of triclosan by permanganate (Mn(VI)): importance of ligands and in situ formed manganese oxides. Environ Sci Technol 43(21):8326–8331
Jiang J, Gao Y, Pang S, Lu XT, Zhou Y, Ma J, Wang Q (2014) Understanding the role of manganese dioxide in the oxidation of phenolic compounds by aqueous permanganate. Environ Sci Technol 49(1):520–528
Jiang XB, Shen JY, Lou S, Mu Y, Wang N, Han WQ, Sun XY, Li JS, Wang LJ (2016) Comprehensive comparison of bacterial communities in a membrane-free bioelectrochemical system for removing different mononitrophenols from wastewater. Bioresource Technol 216:645–652
Karolina M, Furgal RL, Meyer KB (2015) Removing selected steroid hormones, biocides and pharmaceuticals from water by means of biogenic manganese oxide nanoparticles in situ at ppb levels. Chemosphere 136:321–326
Kay JT, Conklin MH, Fuller CC, O’Day PA (2001) Process of nickel and cobalt uptake by a manganese oxide forming sediment in Pinal Creek, Globe Mining District, Arizona. Environ Sci Technol 35(24):4719–4725
Kinnberg K, Holbech H, Petersen GI, Bjerregaard P (2007) Effects of the fungicide prochloraz on the sexual development of zebrafish (Danio rerio). Comp Biochem Phys C 145(2):165–170
Klausen J, Haderlein SB, Schwarzenbach RP (1997) Oxidation of substituted anilines by aqueous MnO2: effect of co-solutes on initial and quasi-steady-state kinetics. Environ Sci Technol 31(9):2642–2649
Laha S, Luthy RG (1990) Oxidation of aniline and other primary aromatic amines by manganese dioxides. Environ Sci Technol 24:363–373
Liang J, Bai Y, Hu C, Qu J (2016) Cooperative Mn(II) oxidation between two bacteria strains in an aquatic environment. Water Res 89:252–260
Liu XD, Chen Y, Zhang X, Jiang XB, Wu SJ, Shen JY, Sun XY, Li JS, Lu LD, Wang LJ (2015) Aerobic granulation strategy for bioaugmentation of a sequencing batch reactor (SBR) treating high strength pyridine wastewater. J Hazard Mater 295:153–160
McKeown DA, Post JE (2001) Characterization of manganese oxide mineralogy in rock varnish and dendrites using X-ray absorption spectroscopy. Am Mineral 86(5–6):701–713
Meerburg F, Hennebel T, Vanhaecke L, Verstraete W, Boon N (2012) Diclofenac and 2-anilinophenylacetate degradation by combined activity of biogenic manganese oxides and silver. Microb Biotechnol 5(3):388–395
Miyata N, Tani Y, Maruo K, Tsuno H, Sakata M, Iwahori K (2006) Manganese (IV) oxide production by Acremonium sp. strain KR21-2 and extracellular Mn (II) oxidase activity. Appl Environ Microb 72(10):6467–6473
Miyata N, Tani Y, Sakata M, Iwahori K (2007) Microbial manganese oxide formation and interaction with toxic metal ions. J Biosci Bioeng 104(1):1–8
Murray KJ, Tebo BM (2007) Gr(III) is indirectly oxidized by the Mn(II)-oxidizing bacterium Bacillus sp. strain SG-1. Environ Sci Technol 41(2):528–533
Ou CJ, Shen JY, Zhang S, Mu Y, Han WQ, Sun XY, Li JS, Wang LJ (2016) Coupling of iron shavings into the anaerobic system for enhanced 2,4-dinitroanisole reduction in wastewater. Water Res 101:457–466
Post JE (1999) Manganese oxide minerals: crystal structures and economic and environmental significance. Proc Natl Acad Sci U S A 96(7):3447–3454
Remucal CK, Ginder-Vogel M (2014) A critical review of the reactivity of manganese oxides with organic contaminants. Environ Sci: Processes Impacts 16(6):1247–1266
Sasaki K, Matsuda M, Hirajima T, Takano K, Konno H (2006) Immobilization of Mn (II) ions by a Mn-oxidizing fungus Paraconiothyrium sp-like strain at neutral pHs. Mater Trans 47(10):2457–2461
Schulze B, Schubert US (2014) Beyond click chemistry-supramolecular interactions of 1,2,3-triazoles. Chem Soc Rev 43(8):2522–2571
Spiro TG, Bargar JR, Sposito G, Tebo BM (2009) Bacteriogenic manganese oxides. Accounts Chem Res 43(1):2–9
Stone AT (1987) Reductive dissolution of manganese (III/IV) oxides by substituted phenols. Environ Sci Technol 21(10):979–988
Tang H, Zheng C, Ren X, Liu J, Liu N, Lv JG, Zhou YJ (2013) Synthesis and biological evaluation of novel triazole derivatives as antifungal agents. Chinese Chem Lett 24(3):219–222
Tani Y, Miyata N, Iwahori K, Soma M, Tokuda SI, Seyama H, Theng BK (2003) Biogeochemistry of manganese oxide coatings on pebble surface in the Kikukawa River System, Shizuoka, Japan. Appl Geochem 18(10):1541–1554
Tebo BM, Bargar JR, Ciement BG, Dick GJ, Murray KJ, Parker D, Webb SM (2004) Biogenic manganese oxides: properties and mechanisms of formation. Annu Rev Earth Planet Sci 32:287–328
Terners TA, Joss A, Siegrist H (2004) Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ Sci Technol 38(20):392A–399A
Tu J, Yang Z, Hu C, Qu J (2014) Characterization and reactivity of biogenic manganese oxides for ciprofloxacin oxidation. J Environ Sci 26(5):1154–1161
Van Wassbergen LG, Hildebrand M, Tebo BM (1996) Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J Bacteriol 178(12):3517–3530
Villalobos M, Toner B, Bargar J, Sposito G (2003) Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochim Cosmochim Acta 67(14):2649–2462
Villalobos M, Lanson B, Manceau A, Toner B, Sposito G (2006) Structural model for the biogenic Mn oxide produced by Pseudomonas putida. Am Mineral 91(4):489–502
Wang W, Shao Z, Liu Y, Wang G (2009) Removal of multi-heavy metals using biogenic manganese oxides generated by a deep-sea sedimentary bacterium-Brachybacterium sp. strain Mn32. Microbiology 155(6):1989–1996
Wang B, Liu X, Zhang X, Zhang J, Song H, Li Z (2011) Synthesis, structure and biological activity of novel 1,2,4-triazole mannich bases containing a substituted benzylpiperazine moiety. Chem Biol Drug Des 78(1):42–49
Watanabe N, Horikoshi S, Kawasaki A, Hidaka H, Serpone N (2005) Formation of refractory ring-expanded triazine intermediates during the photocatalyzed mineralization of the endocrine disruptor amitrole and related triazole derivatives at UV-irradiated TiO2/H2O interfaces. Environ Sci Technol 39(7):2320–2326
Webb SM, Dick GJ, Bargar JR, Tebo BM (2005) Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II). P Natl Acad Sci USA 102(15):5558–5563
Wu S, Yue Q, Qi Y, Gao B, Han S, Yue M (2011) Preparation of ultra-lightweight sludge ceramics (ULSC) and application for pharmaceutical advanced wastewater treatment in a biological aerobic filter (BAF). Bioresour Technol 102(3):2296–2300
Wu H, Shen J, Wu R, Sun X, Li J, Han W, Wang L (2016) Biodegradation mechanism of 1H-1,2,4-triazole by a newly isolated strain Shinella sp. NJUST26. Sci Rep 6:29675
Xu L, Xu C, Zhao M, Qiu Y, Sheng GD (2008) Oxidative removal of aqueous steroid estrogens by manganese oxides. Water Res 42(20):5038–5044
Zhang H, Huang C (2003) Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ Sci Technol 37(11):2421–2430
Zhang H, Huang C (2005) Oxidative transformation of fluoroquinolone antibacterial agents and structurally related amines by manganese oxides. Environ Sci Technol 39(12):4474–4483
Zhang H, Chen W, Huang C (2008) Kinetic modeling of oxidation of antibacterial agents by manganese oxides. Environ Sci Technol 42(15):5548–5554
Zhang Y, Zhu H, Szewzyk U, Geissen SU (2015) Removal of pharmaceuticals in aerated biofilters with manganese feeding. Water Res 72:218–226
Zhou H, Pan H, Xu J, Xu W, Liu L (2016) Acclimation of a marine microbial consortium for efficient Mn(II) oxidation and manganese containing particle production. J Hazard Mater 304:434–440
Zimmermann SG, Wittenwiler M, Hollender J, Krauss M, Ort C, Siegrist H, von Gunten U (2011) Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment: micropollutant oxidation, by-product formation and disinfection. Water Res 45(2):605–617
Zou Z, Meng M, Zha Y (2009) Surfactant-assisted synthesis, characterizations, and catalytic oxidation mechanisms of the mesoporous MnOx-CeO2 and Pd/MnOx-CeO2 catalysts used for CO and C3H8 oxidation. J Phys Chem C 114(1):468–477
Acknowledgements
This work was financed by the Major Project of Water Pollution Control and Management Technology of China (No. 2012ZX07101-003-001), National Natural Science Foundation of China (No. 51478225), and Natural Science Foundation of Jiangsu Province (BK20151485).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Electronic supplementary material
ESM 1
(DOCX 1915 kb)
Rights and permissions
About this article
Cite this article
Wu, R., Wu, H., Jiang, X. et al. The key role of biogenic manganese oxides in enhanced removal of highly recalcitrant 1,2,4-triazole from bio-treated chemical industrial wastewater. Environ Sci Pollut Res 24, 10570–10583 (2017). https://doi.org/10.1007/s11356-017-8641-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8641-1