Abstract
The generation of biogenic manganese oxides (BMnOx) by Microbacterium sp. CSA40, and As(III) removal efficiency and mechanism by BMnOx were investigated in this study. The propagation and growth of Microbacterium sp. CSA40 was conducted in half-strength Luria Broth with 10 mg/L Mn(II), then high concentration of Microbacterium sp. CSA40 was added to PYG medium making its OD600 = 0.9 ± 0.05 for BMnOx generation. The initial Mn(II) concentrations, excessively oligotrophic condition, and pH had great influence on generation of BMnOx by Microbacterium sp. CSA40. An appropriate Mn(II) concentration (50 mg/L) was obtained for generation of BMnOx, and higher or lower Mn(II) concentration would interfere Mn(II) oxidization performance. Mn(II) oxidation ability performed best in weak alkaline conditions and would be restricted in an excessively oligotrophic condition. As(III) oxidization and As(V) adsorption proceed simultaneously by BMnOx, what is more, more than 90% of total As was removed by 0.5 g/L BMnOx. During the application process, no Mn(II) was released in the solution, that is, BMnOx retained its ability for Mn(II) oxidization caused by activity of Microbacterium sp. CSA40. Therefore, BMnOx would be a pollution-free, cost-effective, and high-efficiency material for As(III) treatment in groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater contamination by arsenic, which is known as a human carcinogen, is a serious problem worldwide (Guo et al. 2017; Paul et al. 2015; Wang et al. 2017). Among the four oxidation states of arsenic species, As(III) and As(V) are the predominant forms in aqueous environments. However, As(III) is more harmful than As(V) due to its higher toxicity, solubility, and mobility (Shih et al. 2015; Tian et al. 2015; Wang et al. 2015; Yang et al. 2014a). The oxidizing form of As(III) to As(V) is an essential pretreatment to reduce toxicity and to enhance the removal efficiency for most arsenic removal technologies (Mohora et al. 2014; Wang et al. 2014).
Numerous methods have been developed to oxidize As(III) to As(V) in aquatic system, such as chemical oxidation (Sorlini and Gialdini 2010; Zaw and Emett 2002), photocatalysis (Dutta et al. 2005; Xu et al. 2007), and biological techniques (Silver and Phung 2005; Weeger et al. 1999; Yang et al. 2014b). Some of the methods are effective on the oxidation of As(III); however, these technologies typically suffer from high costs and undesirable byproduct generation (Qi et al. 2015; Shrestha et al. 2008; Wang et al. 2014). What is more, subsequent steps are required to remove As(V) which is transformed from As(III), leading to extra operation costs and complexity for additional processing. Hence, As(III) oxidization and As(V) adsorption proceeding simultaneously would be an optimal way to treat arsenic-contaminated groundwater.
Manganese oxides have a wide distribution in the natural environment (Abu Hasan et al. 2012; Zhou et al. 2015), including water, soil, and sediment. Most of Mn oxides in natural environment are believed to be biogenic, because microbes can accelerate the oxidation of Mn(II) by several orders of magnitude through enzymatic catalytic reaction (Li et al. 2016; Mayanna et al. 2015; Tu et al. 2014a). In addition, biogenic manganese oxides (BMnOx) can oxidize various substances (e.g., steroid hormones, biocides, ciprofloxacin, U(IV), and Ce(III)) due to its high redox potential (Furgal et al. 2015; Tang et al. 2014; Tu et al. 2014b; Wang et al. 2013), and many heavy metal ions, such as Pb(II), Cd(II), Zn(II), and Hg(II), could be adsorbed by BMnOx (Boonfueng et al. 2009; Iyer et al. 2012; Rashidi Nodeh et al. 2016; Tani et al. 2004; Toner et al. 2006; Villalobos et al. 2004). Thus, As(III) could be oxidized and subsequent As(V) could be adsorbed by BMnOx, which might be a perfect material for As(III) remediation in groundwater.
Although BMnOx has attracted a great attention in the recent years because of its high oxidation ability and adsorption capacity, there are still some challenges that need to be faced. For instance, in growth medium, Mn(II)-oxidizing organisms are easily contaminated by other bacterium, and in generation medium, several days will be taken for an adaptation and growth process before oxidizing Mn(II) (Tu et al. 2014b; Watanabe et al. 2013). However, few researches have focused on these issues. In this work, a Mn(II)-oxidizing bacteria named Microbacterium sp. CSA40, isolated from waste water, was used for generation of BMnOx. For reducing contamination by other bacterium, half-strength Luria Broth containing Mn(II) was applied as the growth medium. In addition, for shortening adaptation and growth process, high concentration of Microbacterium sp. CSA40 was added to BMnOx generation medium (PYG medium (OD600 = 0.9 ± 0.05)). Furthermore, BMnOx occurs as a complex of manganese oxide particles embedded in microbial biomass (Sasaki et al. 2014); different organisms and culture conditions potentially affect the composition and construction of BMnOx. Hence, a variety of different parameters including Mn(II) concentration, nutrition condition, and pH were performed to explore generation mechanism of BMnOx. On the other hand, BMnOx was conducted to treat a simulated As(III)-contaminated underground water. The removal efficiency and mechanism of As(III) by BMnOx have been investigated through the detection of arsenic species transformation and total As sequestration.
Materials and methods
Mn(II)-oxidizing bacterial strain
A Mn(II)-oxidizing bacteria was isolated from waste water containing large amounts of Mn(II), located in Beijing, China. According to the physicochemical and 16S rRNA characterizations, the bacteria was identified and named as Microbacterium sp. CSA40 (GenBank accession number, KX289438.1). CSA40 is a Gram-positive, rod-shaped bacteria.
Growth of bacteria and generation of biogenic manganese oxides (BMnOx)
A nutrient medium (half-strength Luria Broth, that is 5.00 g/L peptone, 2.50 g/L yeast extract, and 5.00 g/L NaCl) containing 10 mg/L Mn(II) was used for cell propagation and growth. And, a peptone yeast extract glucose (PYG) medium was used for BMnOx generation. The composition of PYG was 0.25 g/L peptone, 0.25 g/L yeast extract, 0.25 g/L glucose, 0.50 g/L MgSO4·7H2O, 0.06 g/L CaCl2·2H2O, and 2.38 g/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer. Half-strength Luria Broth and PYG were autoclaved at 120 °C for 15 min before use.
For propagation and growth, Microbacterium sp. CSA40 was transferred to a 250-mL Erlenmeyer flask containing 100 mL of half-strength Luria Broth with 10 mg/L Mn(II)(aq). After incubation for 16 h (exponential period, Fig. S1) at 28 °C and 160 rpm, harvested Microbacterium sp. CSA40 was washed twice by sterile water and centrifuged under freezing condition in a sterile environment, then a certain amount of Microbacterium sp. CSA40 was transferred to 100 mL PYG medium which was performed in a 250-mL Erlenmeyer flask, making the OD600 value 0.90 ± 0.05. The pH of PYG medium was adjusted with NaOH and HNO3, and HEPES was used as buffer. The initial Mn(II) concentration in PYG was in the range of 5–100 mg/L. Mn(II) stock solution was prepared with MnCl2. The mixture was incubated at 28 °C and 160 r/min for generation of BMnOx. The pH, generated BMnOx concentration, density of the bacteria, and concentration of Mn(II) of the mixture were analyzed at predetermined reaction times.
Effect of different parameters on BMnOx generation
The effect of initial Mn(II) concentration, pH, and an excessively oligotrophic condition were investigated in order to achieve the optimal conditions of BMnOx generation and explore its generation mechanism. For initial Mn(II) concentration assays, initial Mn(II) concentrations were set as 5, 10, 20, 30, 40, 50, and 100 mg/L to evaluate Mn(II) oxidation performance at different Mn(II) concentrations. An excessively oligotrophic condition was obtained by PYG diluted 20 times to explore the influence of Mn(II) oxidation ability by nutrition condition. The pH values were adjusted to 5.0, 6.0, 7.0, 7.5, and 8.0, then 2.38 g/L HEPES was supplemented as buffer.
Characterization of BMnOx
After acclimation for 15 days, the flasks were stood for 30 min to make BMnOx particles subsided. Then, the mixture was centrifuged (5000×g) to obtain the particles. The obtained particles were resuspended and washed three times with sterile deionized distilled (DD) water to remove the organic matters and salts. The obtained particles were freeze-dried and stored at 4 °C before further characterization. Scanning electron microscope (SEM) coupled with dispersive X-ray analysis (EDX) were used to investigate size, morphology, and element distribution of BMnOx with a SEM/EDX analyzer (Hitachi SU-8020, JAPAN). The elemental valence state of BMnOx was obtained by an X-ray photoelectron spectrometer (ULVAC-PHI, JAPAN), using a Mg Ka X-ray source (1253 eV) and a base pressure of 3 × 10−9 Torr in the analytical chamber. The powder X-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance (Bruker AXS, Karlsruhe, Germany).
As(III) oxidation and absorption experiments
The As(III) oxidation and absorption activities of the BMnOx were tested at ambient temperature. The simulated As(III) wastewater was prepared by dissolving NaAsO2 into DD water at As(III) concentration of 1000 μg/L. BMnOx particles were harvested after 15 days and washed three times with sterile DD water. Then, different dosages (0.125, 0.250, and 0.500 g/L) of BMnOx particles were dispersed into simulated As(III) wastewaters and the pH was adjusted to 7 ± 0.05 with 0.1 M NaOH or 0.1 M HCl. And, the mixture solutions were mechanically stirred at an agitation speed of 100 rpm. At predetermined time, the samples were taken and filtered through a 0.22-μm poly-(vinylidene fluoride) membrane for analysis.
Analytical methods
The amount of BMnOx was confirmed using leucoberbelin blue (LBB) (Krumbein and Altmann 1973), which forms a blue color that absorbs at 620 nm after interaction with Mn(III)/Mn(IV). The microbial biomass was measured by a UV spectrophotometer (U-3010, Hitachi, Japan) at 600 nm, and 200 μM ascorbate was added to dissolve the generated BMnOx particles, so the potential possible interference of BMnOx was removed. And, pH was measured with a pH meter (PHS-3C, Shanghai INESA Scientific Instrument Co., Ltd., China). Mn(II) and total arsenic in the solutions were measured using inductively coupled plasma optical emission spectrometry (ICP-OES 710, Agilent, USA). The concentrations of As(III) and As(V) in the supernatant were analyzed by HPLC-atomic fluorescence spectrometry (HPLC-AFS, PSA, USA), and total As sorption was calculated from the difference between the concentration of dissolved As(III) and As(V) before and after the experiment. The surface excess (q) of total As on BMnOx was calculated using the following equation (Eq.1):
where q t(TAs)(μg/g) is the surface excess of total As on BMnOx at time t; c 0 and c t (μg/L) are the total As concentrations, a sum of As(III) and As(V), at the initial time and at time t in the solution, respectively; v is the volume (L) of solution; and m is the mass (g) of BMnOx. Samples were diluted with DD water when it was necessary to achieve a measureable concentration range.
Results and discussion
Growth of bacteria and BMnOx generation
During the growth stage of Microbacterium sp. CSA40, a nutrition medium was helpful for propagation and growth of bacterium; thereby, half-strength Luria Broth was applied as growth medium. Furthermore, the previous research had reported that the concentration of Mn(II) above 10 μM was toxic to majority of bacterium and then could inhibit their growth (Chapnick et al. 1982), so a medium containing high Mn(II) concentration was commonly used as a method to isolate Mn(II)-oxidizing bacteria (Cerrato et al. 2010; LY et al. 2001; Nealson 2006). To obtain pure bacterial strain of Microbacterium sp. CSA40, Mn(II) was added into the medium to reduce contamination by the other bacterium. In this study, different concentrations of Mn(II) (0, 10, and 100 mg/L) in half-strength Luria Broth were carried out to explore the effect of CSA 40 growth (Fig. S1), indicating that half-strength Luria Broth medium with 10 mg/L Mn(II) was the optimal medium to enlarge culture of CSA40 because there was no apparent influence on its growth at 10 mg/L Mn(II). Moreover, in the growth process, the Mn(II) in half-strength Luria Broth medium could not be oxidized by CSA40 because bacterial Mn(II)-oxidizing ability was restricted under such a eutrophic condition (Akob et al. 2014; Fuller and Bargar 2014).
In the generation stage of BMnOx, a lag-phase and reaction period were required after being inoculated for BMnOx formation (Meng et al. 2009; Pei et al. 2013), and a positive correlation was proved between Mn(II) oxidation rates and the spore concentrations (Toyoda and Tebo 2016). Therefore, in our study, high concentration of Microbacterium sp. CSA40 was added to the PYG medium (OD600 = 0.90 ± 0.05), and oxidation of Mn(II) could be observed on the first day (Figs. 1 and 2), which is earlier than in the other researches. The formation of BMnOx was observed after 42 h by Acremonium sp. strain KR21-2 (Tanaka et al. 2010) and 48 h by Shewanella putrefaciens CN-32 and Shewanella loihica PV-4 (Wright et al. 2016). Nevertheless, after 15 days, a maximum amount of BMnOx was obtained in the PYG medium (Fig. S2).
Effect of initial Mn(II) concentrations, excessively oligotrophic condition, and pH on BMnOx generation
Effect of initial Mn(II) concentrations
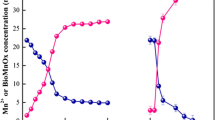
Figure 2 illustrated the time-course curves of microbial biomass, pH, Mn(II) concentration, and generated BMnOx under different initial Mn(II) concentrations within 15 days. The pH of the medium was kept at 6.8–8.0 in the entire process. In the pH scope, the autocatalytic abiotic Mn(II) oxidation by oxygen in the air and alkaline changes is kinetically inhibited (Aly 1983; Larsen et al. 1999; Rosson and Nealson 1982). Meanwhile, OD600 value reduced as time passed; the higher the concentration of Mn(II) was, the greater the OD600 value declined, indicating microbial biomass decreased gradually during generation of BMnOx. As BMnOx maintained the cell morphology inside with Mn oxides adhering tightly outside the bacterial capsular (Sasaki et al. 2014), the generation of BMnOx has been accompanied inevitably by the decrease of biomass of Microbacterium sp. CSA 40. When the maximum amount of BMnOx was obtained, the OD600 should be the lowest value. The difference variation trend of OD600 was observed between nutrient-rich medium (such as half-strength Luria Broth, in Fig. S1) and nutrient-less medium (such as PYG, in Fig. 2). Two reasons led to the decline of OD600 value in the PYG: the bacterium precipitated by manganese oxide accumulation on their surface; in the less rich medium, there are fewer organic compounds to chelate the Mn(II), so it might be more toxic to bacterium in the nutrient-less medium than in the nutrient-rich medium under the same Mn(II) concentration (Nealson 2006). Hence, the different results between Figs. S1 and 2 were caused by the difference of the two media.
The final concentrations of the generated BMnOx were 1.7, 3.7, 6.9, 15.1, 30.6, and 11.8 mg/L when the initial Mn(II) concentrations were 5, 10, 20, 40, 50, and 100 mg/L, respectively. A proportional increase of BMnOx concentration was observed with initial Mn(II) concentration increased from 5 to 50 mg/L; however, when the initial Mn(II) concentration was up to 100 mg/L, the concentration of manganese oxide reduced obviously. Consequently, 50 mg/L Mn(II) as an appropriate concentration was obtained for Microbacterium sp. CSA40 to generate BMnOx, and a lower Mn(II) concentration could not provide sufficient manganese source, while a higher Mn(II) concentration could be toxic to the bacteria.
Effect of excessively oligotrophic condition
An excessively oligotrophic condition was obtained by PYG diluted 20 times. The effect of excessively oligotrophic condition on generation of BMnOx is shown in Fig. 3. The variation tendency of pH and OD600 value were almost the same as in the PYG medium. The range of pH was between 6.8 and 8.0, and OD600 value decreased gradually.
The capacity of Mn(II) adsorbed on the BMnOx and cell surfaces are shown in Fig. S3. The quantity of Mn(II) adsorbed on the BMnOx and cell surfaces was very little, so the removal of Mn(II) could primarily depend on the BMnOx generation. However, over 90% of Mn(II) remained in the medium and the concentration of BMnOx was 3.1 mg/L after 15 days, which is far less than that in the PYG medium. The tremendous differences in BMnOx concentration showed that an excessively oligotrophic condition restricted the Mn(II) oxidation ability of Microbacterium sp. CSA40. Tebo et al. found that Mn(II) oxidation bacterium grew well in the nutritious medium, but their Mn(II) oxidation ability was restricted completely (Tebo et al. 2004); nevertheless, the excessively oligotrophic medium also depresses the Mn(II) oxidation ability significantly.
Effect of pH on BMnOx generation
The effect of pH on Mn(II) oxidation was examined in PYG medium at 50 mg/L initial Mn(II) concentration and displayed in Fig. 4. The concentration of BMnOx increased with pH value increased from 5.0 to 7.5 and then decreased at pH = 8.0 (Fig. 4a). The generated BMnOx was 5.3 and 14.2 mg/L when pH was 5.0 and 6.0, respectively. And at pH = 7.5, BMnOx generation amount was 60.6 mg/L, which is twice the amount at pH = 8.0. As could be seen in Fig. 4b, under the condition of pH = 5.0 and 6.0, the residuals of Mn(II) were 89 and 68%, and at the optimum pH = 7.5, more than 90% of Mn(II) was removed after 15 days. These results indicate that the Mn(II)-oxidizing activity of Microbacterium sp. CSA40 was strongly influenced by pH value, and slight alkaline conditions were favorable for Mn(II) oxidation. The similar result was reported by Su et al., that high Mn(II) oxidase activity was obtained in the pH range of 7.2 to 8.0, and activity was low at pH values below 7.0 (Su et al. 2014). The pH effect of biological Mn(II) oxidation rate should be attributed to the activity of enzymes affected by pH change (Adams and Ghiorse 1987; Cerrato et al. 2010).
Characterization of BMnOx
SEM images of BMnOx after lyophilization are shown in Fig. 5a. BMnOx exhibited as a fibrous precipitate and manganese oxides particles accumulated on the bacterium cell surface and had micrometer-scale sizes, which was a typical morphology for biogenic manganese oxide. Biogenic manganese oxides still maintained their cell morphology with Mn oxides adhering tightly to the bacterial capsular (Fig. 5b). EDS analysis (Fig. 5c) showed BMnOx contained C, O, and Mn as its main components; it indicated that BMnOx occurs as a complex of manganese oxide nanoparticles embedded in microbial biomass rather than Mn oxides only. As shown in Fig. S4, the XRD spectrum indicated that the BMnOx existed in an amorphous form.
As seen in survey XPS spectrum (Fig. 6a), four main peaks corresponding to Mn 2p1/2 (654.0 eV), Mn 2p3/2 (642.3 eV), O 1s (530.0 eV), and C 1s (285 eV.0) were observed on the surface of BMnOx (Islam et al. 1996; Raj et al. 2010). The Mn 2p region consisted of a spin-orbit doublet with Mn 2p1/2 and Mn 2p3/2. Figure 6b showed the region and curve-fitted XPS spectra of Mn 2p. To obtain further insight into the chemical bonds on the surface of BMnOx, curve-fitting of Mn 2p3/2 peak was performed using XPS Peak 4.1 software. The peak at 642.3 eV was decomposed into two peaks (Beyreuther et al. 2006; Nesbitt and Banerjee 1998): a peak at 642.2 eV corresponding to Mn(IV) (84.14%) and a peak at 641.7 eV due to Mn(III) (15.86%), respectively. The result concluded that BMnOx contained C, O, and Mn, which was accordant to the result of EDX. Mn was composed of Mn (IV) and Mn (III), the two valence states existing in the ratio of 5.31:1, and the average oxidation valence of the Mn was 3.84.
As(III) oxidation and absorption by BMnOx particles
In order to evaluate the As(III) removal performance and mechanism by BMnOx particles, the initial concentration of As(III) was fixed at 1000 μg/L whereas the amounts of the BMnOx were 0.125, 0.250, and 0.500 g/L. Figure 7 showed the As(III) and As(V) concentrations in the solution. As could be seen, the oxidation of As(III) took place at all concentration gradients of BMnOx because of high Mn average oxidation valence (3.84), proving that the removal of As(III) was not only by the decrease of As(III) in the solution but also by the subsequent detection of As(V) throughout time of reaction. The residual As(III) concentrations in liquid phase after reaction for 18 h were 496.2 ± 14.9, 216.2 ± 14.6, and 46.2 ± 3.4 μg/L, when BMnOx dosages were 0.125, 0.250, and 0.500 g/L. In addition, the subsequent As(V) concentrations in solutions after 18 h were 90.7 ± 8.2, 110.7 ± 9.6, and 50.7 ± 3.4 μg/L. In total, more than 90% of total As was removed from the simulated wastewater by 0.500 g/L BMnOx. As BMnOx dosages were 0.125, 0.250, and 0.500 g/L, the saturated adsorption amounts of total As were 3304.8 ± 90.3, 2692.4 ± 166.1, and 1806.2 ± 169.2 μg/g (Fig. 8). In the reaction, BMnOx was the electron acceptor for As(III) oxidation, resulting in the reduction of Mn(III/IV) to Mn(II) (Eq. 2). However, no released Mn(II) was detected in the solution (data not shown), indicating BMnOx remains active for Mn(II) oxidization during the application process, and released Mn(II) could be oxidized to Mn(III/IV). Therefore, BMnOx was a pollution-free, cost-effective, and high-efficiency material for As(III) treatment:
Based on our results, a hypothesized mechanism was obtained for the removal of As(III) by BMnOx: at the beginning, some of the free As(III) species were adsorbed on the surface of BMnOx, and the BMnOx itself has a strong oxidation ability; hence, most of the As(III) species were oxidized to As(V) at the same time on the surface, resulting in the reduction of Mn(III/IV) to Mn(II), and released Mn(II) was oxidized to Mn(III/IV) because of the remaining activity for Mn(II) oxidization in BMnOx. As a result, As(V) was adsorbed by BMnOx or released into the water system. In one word, subsequent removal of total arsenic can be attributed to the sorption of As(V) onto BMnOx.
Conclusion
This study used a Mn(II)-oxidation bacteria (Microbacterium sp. CSA40) isolated from metal-contaminated water. The generation of biogenic manganese oxides by Microbacterium sp. CSA40 and its application in the removal of As(III) in groundwater were investigated. There were several primary outcomes from this study: (1) 10 mg/L Mn(II) in half-strength Luria Broth reduced contamination by other bacteria, and a high initial bacteria concentration shortened adaptation and growth process for BMnOx generation process. (2) Initial Mn(II) concentrations, nutritional condition, and pH of the PYG had great influence on the generation of BMnOx by Microbacterium sp. CSA40. An appropriate Mn(II) concentration (50 mg/L) was obtained for generation of BMnOx, and higher or lower Mn(II) concentration would interfere Mn(II)oxidization performance. The rate of Mn(II) oxidation was very slow at pH values less than 6.00; slight alkaline conditions were favorable for Mn(II) oxidizing. Mn(II)-oxidation ability would be restricted in an excessively oligotrophic condition. (3) As(III) oxidization and As(V) adsorption proceed simultaneously by BMnOx without Mn(II) being released. The removal of arsenic in the groundwater by BMnOx would be a pollution-free, cost-effective, and high-efficiency treatment.
References
Abu Hasan H, Sheikh Abdullah SR, Tan Kofli N, Kamarudin SK (2012) Effective microbes for simultaneous bio-oxidation of ammonia and manganese in biological aerated filter system. Bioresour Technol 124:355–363
Adams L, Ghiorse WC (1987) Characterization of extracellular Mn2+−oxidizing activity and isolation of an Mn2+−oxidizing protein from Leptothrix discophora SS-1. J Bacteriol 169:1279–1285
Akob DM, Bohu T, Beyer A, Schäffner F, Händel M, Johnson CA, Merten D, Büchel G, Totsche KU, Küsel K (2014) Identification of Mn(II)-oxidizing bacteria from a low-pH contaminated former uranium mine. Appl Environ Microbiol 80:5086–5097
Aly, Osman M (1983) Chemistry of water treatment. Butterworth, 101–112 pp
Beyreuther E, Grafström S, Eng LM, Thiele C, Dörr K (2006) XPS investigation of Mn valence in lanthanum manganite thin films under variation of oxygen content. Phys Rev B 73:155425
Boonfueng T, Axe L, Yee N, Hahn D, Ndiba PK (2009) Zn sorption mechanisms onto sheathed Leptothrix discophora and the impact of the nanoparticulate biogenic Mn oxide coating. J Colloid Interface Sci 333:439–447
Cerrato JM, Falkinham Iii JO, Dietrich AM, Knocke WR, McKinney CW, Pruden A (2010) Manganese-oxidizing and -reducing microorganisms isolated from biofilms in chlorinated drinking water systems. Water Res 44:3935–3945
Chapnick SD, Moore WS, Nealson KH (1982) Microbially mediated manganese oxidation in a freshwater lake. Limnol Oceanogr 27:1004–1014
Dutta PK, Pehkonen S, Sharma VK, Ray AK (2005) Photocatalytic oxidation of arsenic (III): evidence of hydroxyl radicals. Environ Sci Technol 39:1827–1834
Fuller CC, Bargar JR (2014) Processes of zinc attenuation by biogenic manganese oxides forming in the hyporheic zone of Pinal Creek, Arizona. Environ Sci Technol 48:2165–2172
Furgal KM, Meyer RL, Bester K (2015) Removing selected steroid hormones, biocides and pharmaceuticals from water by means of biogenic manganese oxide nanoparticles in situ at ppb levels. Chemosphere 136:321–326
Guo H, Zhang D, Ni P, Cao Y, Li F (2017) Hydrogeological and geochemical comparison of high arsenic groundwaters in inland basins, PR China. Procedia Earth Planet Sci 17:416–419
Islam MN, Ghosh T, Chopra K, Acharya H (1996) XPS and X-ray diffraction studies of aluminum-doped zinc oxide transparent conducting films. Thin Solid Films 280:20–25
Iyer A, Del-Pilar J, King’ondu CK, Kissel E, Garces HF, Huang H, El-Sawy AM, Dutta PK, Suib SL (2012) Water oxidation catalysis using amorphous manganese oxides, octahedral molecular sieves (OMS-2), and octahedral layered (OL-1) manganese oxide structures. J Phys Chem C 116:6474–6483
Krumbein WE, Altmann HJ (1973) A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol Mar Res 25:347–356
Larsen EI, Sly LI, McEwan AG (1999) Manganese(II) adsorption and oxidation by whole cells and a membrane fraction of Pedomicrobium sp. ACM 3067. Arch Microbiol 171:257–264
Li C, Wang S, Du X, Cheng X, Fu M, Hou N, Li D (2016) Immobilization of iron- and manganese-oxidizing bacteria with a biofilm-forming bacterium for the effective removal of iron and manganese from groundwater. Bioresour Technol 220:76–84
LY S, La Duc MT, Grundl TJ, Nealson KH (2001) Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ Microbiol 3:10–18
Mayanna S, Peacock CL, Schäffner F, Grawunder A, Merten D, Kothe E, Büchel G (2015) Biogenic precipitation of manganese oxides and enrichment of heavy metals at acidic soil pH. Chem Geol 402:6–17
Meng Y-T, Zheng Y-M, Zhang L-M, He J-Z (2009) Biogenic Mn oxides for effective adsorption of Cd from aquatic environment. Environ Pollut 157:2577–2583
Mohora E, Rončević S, Agbaba J, Tubić A, Mitić M, Klašnja M, Dalmacija B (2014) Removal of arsenic from groundwater rich in natural organic matter (NOM) by continuous electrocoagulation/flocculation (ECF). Sep Purif Technol 136:150–156
Nealson KH (2006) The manganese-oxidizing bacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes: volume 5: Proteobacteria: alpha and beta subclasses. Springer New York, New York, pp 222–231
Nesbitt H, Banerjee D (1998) Interpretation of XPS Mn (2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am Mineral 83:305–315
Paul D, Kazy SK, Banerjee TD, Gupta AK, Pal T, Sar P (2015) Arsenic biotransformation and release by bacteria indigenous to arsenic contaminated groundwater. Bioresour Technol 188:14–23
Pei Y, Chen X, Xiong D, Liao S, Wang G (2013) Removal and recovery of toxic silver ion using deep-sea bacterial generated biogenic manganese oxides. PLoS One 8:e81627
Qi J, Zhang G, Li H (2015) Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresour Technol 193:243–249
Raj AME, Victoria SG, Jothy VB, Ravidhas C, Wollschläger J, Suendorf M, Neumann M, Jayachandran M, Sanjeeviraja C (2010) XRD and XPS characterization of mixed valence Mn 3 O 4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl Surf Sci 256:2920–2926
Rashidi Nodeh H, Wan Ibrahim WA, Ali I, Sanagi MM (2016) Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As(III) and As(V) species from environmental water samples. Environ Sci Pollut Res 23:9759–9773
Rosson RA, Nealson KH (1982) Manganese binding and oxidation by spores of a marine bacillus. J Bacteriol 151:1027–1034
Sasaki K, Yu Q, Momoki T, Kaseyama T (2014) Adsorption characteristics of Cs+ on biogenic birnessite. Appl Clay Sci 101:23–29
Shih Y-J, Huang R-L, Huang Y-H (2015) Adsorptive removal of arsenic using a novel akhtenskite coated waste goethite. J Clean Prod 87:897–905
Shrestha RA, Lama B, Joshi J, Sillanpää M (2008) Effects of Mn(II) and Fe(II) on microbial removal of arsenic (III). Environ Sci Pollut Res 15:303–307
Silver S, Phung LT (2005) Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl Environ Microbiol 71:599–608
Sorlini S, Gialdini F (2010) Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res 44:5653–5659
Su J, Deng L, Huang L, Guo S, Liu F, He J (2014) Catalytic oxidation of manganese(II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res 56:304–313
Tanaka K, Tani Y, Takahashi Y, Tanimizu M, Suzuki Y, Kozai N, Ohnuki T (2010) A specific Ce oxidation process during sorption of rare earth elements on biogenic Mn oxide produced by Acremonium sp. strain KR21-2. Geochim Cosmochim Acta 74:5463–5477
Tang Y, Webb SM, Estes ER, Hansel CM (2014) Chromium(iii) oxidation by biogenic manganese oxides with varying structural ripening. Environ Sci : Processes Impacts 16:2127–2136
Tani Y, Ohashi M, Miyata N, Seyama H, Iwahori K, Soma M (2004) Sorption of Co(II), Ni(II), and Zn(II) on biogenic manganese oxides produced by a Mn-oxidizing fungus, strain KR21-2. J Environ Sci Health A Tox Hazard Subst Environ Eng 39:2641–2660
Tebo BM, Bargar JR, Clement BG, Dick GJ, Murray KJ, Parker D, Verity R, Webb SM (2004) Biogenic manganese oxides: properties and mechanisms of formation. Annu Rev Earth Planet Sci 21:287–328
Tian H, Shi Q, Jing C (2015) Arsenic biotransformation in solid waste residue: comparison of contributions from bacteria with arsenate and iron reducing pathways. Environ Sci Technol 49:2140–2146
Toner B, Manceau A, Webb SM, Sposito G (2006) Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm. Geochim Cosmochim Acta 70:27–43
Toyoda K, Tebo BM (2016) Kinetics of Mn(II) oxidation by spores of the marine Bacillus sp. SG-1. Geochim Cosmochim Acta 189:58–69
Tu J, Yang Z, Hu C, Qu J (2014a) Characterization and reactivity of biogenic manganese oxides for ciprofloxacin oxidation. J Environ Sci (China) 26:1154–1161
Villalobos M, Bargar J, Sposito G (2004) Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environ Sci Technol 39:569–576
Wang Z, Lee S-W, Kapoor P, Tebo BM, Giammar DE (2013) Uraninite oxidation and dissolution induced by manganese oxide: a redox reaction between two insoluble minerals. Geochim Cosmochim Acta 100:24–40
Wang Z, Bush RT, Sullivan LA, Chen C, Liu J (2014) Selective oxidation of arsenite by peroxymonosulfate with high utilization efficiency of oxidant. Environ Sci Technol 48:3978–3985
Wang YH, Li P, Dai XY, Zhang R, Jiang Z, Jiang DW, Wang YX (2015) Abundance and diversity of methanogens: potential role in high arsenic groundwater in Hetao plain of Inner Mongolia, China. Sci Total Environ 515–516:153–161
Wang Y, Xie X, Ma T, Pi K, Su C, Liu Y, Li J (2017) Remediation of high arsenic aquifers by learning from the nature. Procedia Earth Planet Sci 17:13–16
Watanabe J, Tani Y, Chang J, Miyata N, Naitou H, Seyama H (2013) As(III) oxidation kinetics of biogenic manganese oxides formed by Acremonium strictum strain KR21-2. Chem Geol 347:227–232
Weeger W, Lievremont D, Perret M, Lagarde F, Hubert J-C, Leroy M, Lett M-C (1999) Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. Biometals 12:141–149
Wright MH, Farooqui SM, White AR, Greene AC (2016) Production of manganese oxide nanoparticles by Shewanella species. Appl Environ Microbiol 82:5402–5409
Xu T, Cai Y, O'Shea KE (2007) Adsorption and photocatalyzed oxidation of methylated arsenic species in TiO2 suspensions. Environ Sci Technol 41:5471–5477
Yang J-S, Kim Y-S, Park S-M, Baek K (2014a) Removal of as(III) and as(V) using iron-rich sludge produced from coal mine drainage treatment plant. Environ Sci Pollut Res 21:10878–10889
Yang L, Li X, Chu Z, Ren Y, Zhang J (2014b) Distribution and genetic diversity of the microorganisms in the biofilter for the simultaneous removal of arsenic, iron and manganese from simulated groundwater. Bioresour Technol 156:384–388
Zaw M, Emett MT (2002) Arsenic removal from water using advanced oxidation processes. Toxicol Lett 133:113–118
Zhou D, Kim D-G, Ko S-O (2015) Heavy metal adsorption with biogenic manganese oxides generated by pseudomonas putida strain MnB1. J Ind Eng Chem 24:132–139
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No.413731291008712). The authors thank the editor and anonymous reviewers for their insightful comments, which strengthened this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 626 kb).
Rights and permissions
About this article
Cite this article
Liang, G., Yang, Y., Wu, S. et al. The generation of biogenic manganese oxides and its application in the removal of As(III) in groundwater. Environ Sci Pollut Res 24, 17935–17944 (2017). https://doi.org/10.1007/s11356-017-9476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9476-5