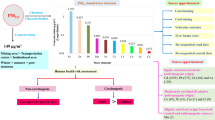
Abstract
This study presents the concentration of submicron aerosol (PM1.0) collected during November, 2009 to March, 2010 at two road sites near the Indian Institute of Technology Delhi campus. In winter, PM1.0 composed 83% of PM2.5 indicating the dominance of combustion activity-generated particles. Principal component analysis (PCA) proved secondary aerosol formation as a dominant process in enhancing aerosol concentration at a receptor site along with biomass burning, vehicle exhaust, road dust, engine and tire tear wear, and secondary ammonia. The non-carcinogenic and excess cancer risk for adults and children were estimated for trace element data set available for road site and at elevated site from another parallel work. The decrease in average hazard quotient (HQ) for children and adults was estimated in following order: Mn > Cr > Ni > Pb > Zn > Cu both at road and elevated site. For children, the mean HQs were observed in safe level for Cu, Ni, Zn, and Pb; however, values exceeded safe limit for Cr and Mn at road site. The average highest hazard index values for children and adults were estimated as 22 and 10, respectively, for road site and 7 and 3 for elevated site. The road site average excess cancer risk (ECR) risk of Cr and Ni was close to tolerable limit (10−4) for adults and it was 13–16 times higher than the safe limit (10−6) for children. The ECR of Ni for adults and children was 102 and 14 times higher at road site compared to elevated site. Overall, the observed ECR values far exceed the acceptable level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An aerosol is a mixture of microscopic solids and liquid droplets suspended in air (Begum et al., 2004). Fine aerosols are denoted as PM2.5 (particulate matter with an aerodynamic diameter less than or equal to 2.5 μm). The chemical composition of fine aerosols poses adverse effects on human health especially in urban cities and plays vital role in climate change (Cheng et al., 2011; Sandhu et al., 2014; Tsai et al., 2012). These particles can easily penetrate into the respiratory system (Chow et al., 1994; Pope III and Dockery, 2006) and can transfer the toxic compounds to bloodstream. Even it has been reported that the chemical composition of fine aerosol is impacting human health, however, the process is uncertain (Pant and Harrison, 2013). A study of Global Burden Disease (GBD) in 2010 has reported six million deaths due to air pollution of particulate matter (PM) in India (Lim et al., 2013). Numerous cardiovascular and respiratory illness studies (Delfino et al., 2008; Godoi et al., 2013; Kloog, 2016; Krall et al., 2016; Yu and Chien, 2016) have revealed the effects of fine aerosol on cardiopulmonary functions. A few studies (Boldo et al., 2011; Brunekreef et al., 2009) showed an increasing trend in long-term exposure can be linked to mortality due to cancer and cardiopulmonary diseases.
Limited studies (Deshmukh et al., 2013; Izhar et al., 2016; Singh and Gupta, 2016) on gravimetric, chemical, and human health risk analysis of the PM1.0 have been reported, while these particles are highly toxic in sizeable doses (Harrison and Yin, 2000); therefore, the toxicity needs to be established to frame the mitigation policy. The PM1.0 are mainly emitted from combustion sources. Urbanization and economic growth of any country is coupled with increasing private vehicle demand, goods transport, and electricity demand thus increasing the emissions, deteriorating the air quality. Therefore, now the air quality and health issues are of utmost concern for population living in urban areas.
Delhi is one of the most polluted urban cities of the world. The city has high population growth rate, economic growth, and ever-increasing demand for transport which is creating excessive pressure on the city’s existing public transport infrastructure. Therefore, an inclination towards private vehicle is understandable and can be depicted through twofold increase in vehicle registration from 3.3 million in year 2000 to 7.4 million in 2013 (delhi.gov.in). In 1998, the Supreme Court of India ordered all public transport vehicles to use compressed natural gas (CNG) as fuel instead of diesel and other hydrocarbons. The introduction of CNG in Delhi is expected to be one of the factors that may result in low emissions and different aerosol composition compared to previously measured (Shridhar et al., 2010). Limited studies can be sighted after introduction of CNG reporting road side PM2.5 (Pant and Harrison, 2013; Shukla and Alam, 2010) and none has focused on PM1.0 which particularly comes from combustion sources such as automobiles.
The availabilty of limited data on aerosol concentration and compostion at road site makes it difficult to assess the human health risk due to heavy traffic flow on congested roads of Delhi. Therefore, the present study focused on measurement of PM1.0 and determination of its chemical constituents including water-soluble inorganic ions and trace elements near major road network around IIT Delhi campus. The paper also presents the PM2.5 concentrations at the same location to understand the relation between PM1.0 and PM2.5 mass concentration. This paper aimed to understand the (1) daily and monthly variation of PM1.0 and chemical constituents, (2) source contribution using principal component analysis (PCA) and multilinear regression (MLR) model, and (3) human health risks associated with exposure to road site.
Material and methods
Sampling location
In order to evaluate the road site aerosol concentration and related carcinogenic and non-carcinogenic health risk around IIT Delhi campus located in South Delhi, the aerosol monitoring was conducted at two gates of the campus. The instrument was mounted at 2-m height above the ground level ~ 1.5 m away from road side (Fig. 1a, b).
Sampling protocol
Air sampling was carried out for 10 h separately for daytime from 8.0 am to 6.0 pm and nighttime from 8 pm to 6 am in November 2009 to March 2010. The 2-h gap in sampling was given due to operational limitations, to avoid excessive heating of the pump, overloading of the filters, and transporting the filters from sampling site to the lab at low temperature to avoid the losses of volatile species. The sampling was carried out 3 days per week throughout the sampling period and a total of 80 samples were collected as per the guidelines of National Ambient Air Quality Standards (NAAQS) (CPCB, 2010). Two single-stage impactors developed and calibrated for PM2.5 and PM1.0 mass collection at the Indian Institute of Technology Kanpur (IITK), India (Chakraborty and Gupta, 2010; Gupta et al., 2011) were used to collect the aerosol mass. The impactors were operated at 15 and 10 liters per minute (LPM) respectively for collection of PM2.5 and PM1.0 on Quartz and Teflon filters. The PM1.0 mass collected on Teflon filters was analyzed for ions and selected trace elements. PM2.5 mass was determine by gravimetric analysis and no chemical characterization was done. Utmost care was taken for filter preparation, sample collection, and gravimetric analysis of the filter papers. The standard procedures (USEPA, 1998; RTI, 2008) were followed for handling and preparation of filters for sampling and analysis. All the filters were subjected to conditioning in a controlled environment at 25 °C temperature and 50% relative humidity for overnight prior and after the sampling. The pre- and post-weights of the filters were recorded using a microbalance (Sartorious CPA2P-F, Germany). Gravimetrically, PM mass was calculated as a difference of pre- and post-weight of filters. The PM concentration was calculated by dividing the difference in pre- and post-weight of filters by the sampled air volume.
Chemical characterization of PM1.0 mass
The Teflon filters were subjected to the analysis of water-soluble inorganic ions such as chloride (Cl−), nitrate (NO3−), sulfate (SO42−), and ammonium (NH4+) and selected trace elements. The Teflon filters were cut into two equally half pieces. One half filter was cut into small fragments and extracted in 20 ml of ultrapure water (ELGA, Purelab Option-Q, UK) using an ultrasonicator (Jasbo Scientific, India) for 30 min. After ultrasonication, the extracts were filtered by 0.22-μm membrane filter (Millipore, 47 mm) and stored in pre-cleaned screw-capped polypropylene vials. All the extracts were stored in a refrigerator till further analysis. The ion analysis was carried out with an ion chromatograph (Dionex ICS 1000, Thermo Fisher, USA) following procedure detailed in Jaiprakash et al. (2017) and in supplementary information [SI] in Section A1.
The other half portion of Teflon filter was subjected to acid digestion with 20 mL of nitric acid (Suprapure, 70% GR grade, Merck) on a hot plate at 180 °C. The digestion was carried out up to dryness. The digest was cooled at room temperature and diluted with ultrapure water and filtered through a 0.22-μm membrane filter. The trace element analysis was carried out following Chakraborty and Gupta (2010), Gupta et al. (2011), and Gupta and Mandariya (2013). Eleven trace elements (Na, K, Mg, Ca, Pb, Zn, Fe, Mn, Cu, Cr, and Ni) were determined using ICP-OES following method detailed in Jaiprakash et al. (2017) Section A1 in SI. Reagents, standards, and quality assurance (QA)/quality control (QC) are also discussed in the sections A2 and A3 in the SI, QA/QC of chemical analysis of collected samples are summarized in Table S1 in the SI.
Mass closure and reconstruction
The PM1.0 and measured chemical components were screened for mass closure as per the procedure described elsewhere (Jaiprakash et al., 2017; Raman et al., 2010 and references cited therein). In this line, first the mass was reconstructed from measured sum of chemical species and deducted from gravimetrically determined PM1.0 to quantify the missing mass (MM), i.e., carbonaceous aerosol and some key crustal elements such as Si, Al, and water content of aerosol. Equation (1) was used to determine MM using reconstructed mass.
where RCM is reconstructed mass (μg m−3) and TMO is the concentration (μg m−3) of trace metal oxide (μg m−3) calculated following Landis et al. (2001) and Olson et al. (2004). The calculation of TMO was done using Eq. (2). Soil is estimated as oxide of Ca and Mg (Malm et al., 1994) using Eq. (3). NaCl is a significant marker of marine aerosol, represented as [Salt]. Due to continental site location, salt is not included in the present work. The missing mass was calculated by subtracting RCM from the gravimetric PM1.0 mass (Eq. 4).
Reconstructed mass was calculated by multiplying their individual concentration with abovementioned conversion factors and tabulated in Table S2 in the SI. Additionally, gravimetrically measured PM1.0 (Fig. S1 in the SI) showed moderate correlation (r2 = 0.75) with slope 2.6 for both sites. It is indicating that the gravimetrically determined PM1.0 mass concentrations were much higher than the sum of the species. Based on the above observations, the MM was observed as 102.4 ± 38.8 μg m−3 (~ 56% of PM1.0 mass) for day and 144.0 ± 35.3 μg m−3 (~ 61% of PM1.0 mass) for night sampling for this study (Table S2 in the SI), which can be recommended that other than trace elements and water-soluble ions the contribution of carbonaceous aerosol could be significant. Therefore, the MM represents unaccounted mass fraction of PM1.0. However, the carbonaceous aerosol contribution can be as high as 25–40% of PM2.5 mass, in Delhi (Kurian, 2011). Further, a study by Gupta and Mandariya (2013) for Kanpur City observed PM1.0 to PM2.5 ratio as ~ 0.85 during winter when carbonaceous aerosols from combustion sources are dominated and ~ 0.65 during summer when dust is dominant constituent. Therefore, we expect the “missing mass” here is largely the combination of carbonaceous aerosol and dust, which depends on season and source activities.
Source identification using PCA/MLR
Principal component analysis-multilinear regression (PCA-MLR) was used to establish a relationship among various water-soluble ions and selected trace elements of PM1.0 for the possible source identification without any specific assumptions about the number or nature of the sources following the method of Thurston and Spengler (1985). In brief, the chemical characterization data along with MM were screened for quality assessment. The missing values and values below detection limit of a given species were replaced respectively with geometric mean and half of the method detection limit (MDL) of species itself. In the present study, MM was also included as a species. PCA was performed using the statistical package SPSS, version 19.0. For detecting major pollutant sources, 25 varimax rotations were used to redistribute the variance and calculate factor loading. To quantify the contributions of sources and apportionment of PM1.0, the absolute principal component score (APCS) method was approached. This APCS was coupled with MLR for quantifying source contribution followed by Larsen and Baker (2003) as depicted by Eq. (5).
where X i is PCA factor score as independent variables, and dependent variable, y, is the total PM1.0. If the independent and dependent variables are “normally standardized,” after normalization regression, coefficients are represented as B, with an intercept (b) is 0 in Eq. (6).
where Z is the normalized deviates of PM1.0, and factor score X i have a mean 0 and standard deviation of 1. The linear multiple regression was performed using Excel. The coefficients thus obtained were multiplied with APCS score to predict the PM1.0 mass. The regression plot between measured and predicted PM1.0 mass concentration showed strong correlation (R2 = 0.85). Thus, the contribution of factors to PM1.0 mass concentration was estimated.
Human health risk assessment
Human health risk was assessed on the basis of observed mean concentrations of particulate-bound trace elements and it depends on specific locations and its exposure route, i.e., ingestion, dermal, and inhalation. However, human health is assumed to be exposed to insignificant amount of trace elements in PM1.0 via ingestion and dermal contact because trace element of PM1.0 can enter the body via inhalation (Huang et al. 2014). Thus, this study only examined health risks associated with exposure of trace elements through inhalation only.
Exposure dose
The risk associated with PM1.0 exposure was calculated following the method used (Singh and Gupta, 2016) to govern the exposure dose in terms of life time average daily dose (ADDinh in mg kg−1 day−1) of aerosol. This can be expressed as follows:
where C is the species concentration (μg m−3), IR is the rate of air inhalation as 10 m3 day−1 for children and 20 m3 day−1 for adults, CF is a unit correction factor as 0.001, EF is the relative exposure frequency (days year−1), ED is the exposure duration during lifetime (years), BW is the body weight assumed as 15 kg for children and 70 kg for adult, and AT n is the average time (days). ADD was calculated according to the Human Health Evaluation Manual (Part A), supplemental guidance for inhalation risk assessment (Part F) (USEPA, 2004 and 2009). All values used in calculation are summarized in Table S3 in the SI.
Non-carcinogenic risk
Non-carcinogenic risk is represented by hazard quotient (HQ) and hazard index (HI), which can be used to estimate for non-carcinogenic risk of trace elements. Once ADD due to inhalation was calculated using Eq. (5), an HQ can be determined by Eq. (8).
where RfDinh is the reference exposure level for the human population adopted from USEPA (United States Environmental Protection Agency) (2015). The details are provided in Table S3 in the SI. If HQ > 1, i.e., average daily dose is greater than reference value, then the expected adverse effect on human health is due to inhalation exposure, whereas if HQ < 1, it indicates no adverse effect on health.
HI is the sum of all hazard quotient inhalation exposure. The HI was calculated as the sum of HQ of individual element for inhalation route. The HI < 1 indicates no risk from non-carcinogens, while HI > 1 indicates significant non-carcinogenic risk. HI was calculated as given in Eq. (9) following the method of Zheng et al. (2010).
where i = first to nth element.
Excess cancer risk
Excess cancer risk (ECR) can be estimated in terms of incremental probability of developing cancer over a lifetime from magnitude of total exposure to potential carcinogen to the human beings. The ECR can be expressed as follows (USEPA 2011):
where C is the average concentration of element species (μg m−3), ET is the exposure time (12 h day−1), EF is the exposure frequency (days year−1), ED is exposure duration average time, 24 years, and AT n is average time for carcinogen (AT n = 70 years × 365 days × 24 h day−1). IUR is the inhalation unit risk (μg/m3)−1 of the element, obtained from the USEPA database, Integrated Information Risk System (https://www.epa.gov/iris and listed in Table S3 in the SI). The range of excess cancer risk has been recommended by the USEPA (United States Environmental Protection Agency) (2015) for public health. The acceptable value is 10−6 for a protection and 10−4 is likely to be tolerable risk. In the present study, the excess cancer risk was estimated only for Pb, Cr, and Ni out of 11 trace elements which are well-known carcinogens that pose risk to inhalation pathway (IRIS 1995).
Risk apportionment
The source-specific risk was calculated as the sum of cancer risks of selected species having unit risk as followed by Wu et al. (2009).
where X ip is the average concentration of i species in source p and IUR i is the unit risk of species i. The IUR were obtained from USEPA (2011) and Pb, Cr, and Ni were included in source-specific risk.
Results and discussions
Daily and monthly variation of PM1.0
The Student t test conducted on concentration data from site I and site II for both PM1.0 and PM2.5 showed no significant difference at 95% confidence in all the sampling months. Therefore, these data were to be merged from two sites for further investigation. The daily average PM2.5 concentrations in all the sampling days were factor of 3–6 higher than NAAQS (60 μg m−3) indicated the severity of particulate pollution at road site which has direct implications to high exposure and related health effect. In addition, the occurrence of PM2.5 showed similar trend as PM1.0 (Fig. S2a in the SI) and PM1.0 to PM2.5 ratio was as high as 0.83 ± 0.06 and showed little day to day variability rendering that the submicron particles from anthropogenic activities dominated the aerosol burden rather than airborne road dust during sampling period (Fig. S2b in the SI). Another important point is the PM2.5 and PM1.0 concentrations at night were always higher than the daytime. Unexpectedly, high pollution at night could be because of secondary aerosol formation from NO X , SO2, and vapor phase organic compounds emitted from heavy-duty diesel trucks which are allowed inside the city during nighttime. As the PM1.0 to PM2.5 ratios were high throughout the sampling period, we investigated the role of combustion activities in aerosol burden by characterizing the PM1.0 mass for water-soluble ions and trace elements. Therefore, hereafter this manuscript focuses on PM1.0 concentration and composition.
The monthly average PM1.0 concentrations in day and nighttime were estimated as 201 ± 40 and 231 ± 40 μg m−3, respectively, with highest concentration observed in December 2009 as daytime average 125 ± 38 μg m−3 and nighttime average 194 ± 51 μg m−3. The lowest concentration was observed in March 2010 (Fig. 2). The day and nighttime concentration in December 2009 was significantly higher than March 2010 at 95% confidence interval (pday = 0.003, pnight = 0.0008). The standard deviation around mean was low in all the months, indicating low day to day variability. This consistency can be speculated as an indication of no radical changes in source activity during sampling period near the receptor site. In winter, 6 days in December and January were associated with heavy foggy events when average PM1.0 concentrations were observed as 213 ± 55 μg m−3 during day and 249 ± 31 μg m−3 at night, which were higher than concentration of PM1.0 observed during clear days as 158 ± 56 μg m−3 during day and 238 ± 5 μg m−3 at night (Fig. 2). During winter nights, the atmosphere remains calm allowing low or no dispersion and cold (12 ± 4 °C) and mostly inversion condition prevails which accompanied with high relative humidity (78 ± 12%) favors fog-smog-fog cycle. Such atmosphere instigates both fog and secondary aerosol formation and accumulation near ground surface. The breakdown of the thermal inversion due to high temperature during daytime induces the dilution of such particles in the lower atmosphere; however, the temperature still remains lower at ground than at higher altitude causing pollution to confine near ground. Secondly, the traffic activity increases during day provide more primary pollutants and precursors of aerosol further enhancing the pollution load. Therefore, in contrast to elevated site in Jaiprakash et al. (2017), near ground site did not show significant difference during day and nighttime concentration during foggy period. The chemistry of fog and secondary aerosol formation has been explained well in Jaiprakash et al. (2017) for the same sampling period.
The PM1.0 concentrations reported in this study were 24 to 37% higher in all the months except in December 2009 and January 2010 compared to average concentration of PM1.0 reported by Jaiprakash et al. (2017) for urban background site at 30 m height above ground inside IIT Delhi campus. This clearly indicated that during period of October, November, and March, the pollution near ground was higher than those at high altitude. Overall the average (20 h) PM1.0 concentrations observed in present study were significantly higher (p < 0.001) at road site compared to elevated site in Jaiprakash et al. (2017) (Table 1). Similarly, the monthly average PM1.0 concentrations at a road observed in present work were also considerably higher by 42 to 78% than values reported by Gupta and Mandariya (2013) for the same time period for residential cum institutional area at IIT Kanpur. Such high differences in PM1.0 concentration in the months of November 2009 and February 2010 were probably due to difference in meteorological conditions, emission activities in two cities, and also the concentration measured in the present work was near the road side while the concentration reported by Gupta and Mandariya (2013) was from urban background site.
Monthly variation of ions
All the PM1.0 samples were analyzed for SO42−, NO3−, Cl−, F−, and NH4+. Fluoride (F−) was not found in all the samples. Monthly average day and night concentration of ions NH4+, Cl−, NO3−, and SO42− for the period of November 2009 to March 2010 is presented in Fig. 3. The highest daytime concentration of ammonium (NH4+) was observed as 12 ± 3 μg m−3 in January 2010, while lowest concentration was observed as 5 ± 3 μg m−3 in March 2010. However, such trend was not observed for nighttime NH4+ concentration. Overall, the concentration of NH4+ varied in the range of 10–12 μg m−3 during night. An average highest concentration of Cl− was observed as 15 ± 4 and 17 ± 8 μg m−3 and for NO3− as 34 ± 11 and 42 ± 5 μg m−3 during day and night, respectively, in December 2009, while highest SO42− concentrations as 24 ± 12 and 29 ± 2 μg m−3 at day and night respectively were observed in January 2010 (Fig. 3). Generally, nighttime concentrations were higher due to further processing of gas and vapor to secondary aerosol as discussed before and in Jaiprakash et al. (2017). The overall average concentrations of SO42− and NO3− for road site were significantly higher (\( {\mathrm{p}}_{{\mathrm{SO}}_{4^{2-}}} \) < 0.00 and \( {\mathrm{p}}_{{\mathrm{NO}}_{3^{-}}} \)< 0.04) than the average values reported by Jaiprakash et al. (2017) for an elevated site (Table 1). The possible reason could be particles emitted from vehicles remain confined near the ground and also the precursor gases (SO2 and NO x ) have contributed to higher rate of secondary aerosol formation as compared elevated (Table 1). The average ratios of NO3−/SO42− found in the range of 1.5–2.7 and were 50–166% higher than ratio reported by Jaiprakash et al. (2017) for winter season which clearly revealed that vehicle emissions dominated near ground pollutant concentrations. Interestingly, road site NH4+ and Cl− average concentrations were considerably lower than average values reported by Jaiprakash et al. (2017) for elevated site (Table 1). The possible reason could be NH4+ and Cl− species are not dominantly produced from tail pipe (Shukla et al., 2017) and road dust suspension at road site.
The ion balance could not be established due to unavailability of results of cations such as K+, Na+, Ca2+, and Mg2+. Therefore, an equivalent ratio of NH4+/(Cl− + SO42− + NO3−) is presented in Fig. S3 in the SI for both day and night. An equivalent ratio of 1.0 indicates the neutralization of HNO3, HCl, and H2SO4 by NH3 in the atmosphere (Gupta and Mandariya 2013). However, the results revealed that the equivalent ratio varied as low as 0.3 indicating insufficient ammonia for secondary aerosol conversion to 1.0 sufficient to completely convert all acidic compounds to aerosol during daytime. While in nighttime this range 0.2–1.4 (Fig. S3 in the SI) indicated the excess of ammonia by 40% in some of the nights (Rajeev et al. 2016). Previous study by Updyke et al. (2012) has reported that reactions of secondary aerosol compounds such as isoprene, α-pinene, limonene, α-cedrene, α-humulene, farnesene, pine leaf essential oils, and cedar leaf essential oils with NH3 resulting in production of light-absorbing “brown carbon” compounds. The study by Singh and Gupta (2016) has also reported the excess NH4+ combines with water-soluble organic carbon. Therefore, probably the excess ammonia was associated with water-soluble organic carbon and was extracted along with other water-soluble inorganic ions. During November 2009 to March 2010, especially daytime aerosol was dominated by acidic nature with higher concentration of Cl− + SO42− + NO3−, while in December 2009 to February 2010, nights favor alkaline nature of aerosol with high concentration of NH4+ due to low temperature and high relative humidity.
Monthly variations of trace metals
Monthly day and night variations of trace metals are shown in Fig. 4. On an average daytime highest metal concentration was observed as 24 ± 7 μg m−3 in December 2009 and lowest as 18.5 ± 3.5 μg m−3 in November 2009. Similarly, in nighttime, the highest metal concentration as 25.5 ± 10 μg m−3 was observed in February 2010 and lowest concentration as 18 ± 3.0 μg m−3 in January 2010. Interestingly, among all the tested trace elements, high concentration of Ca was observed as 9.2 ± 2.1 μg m−3 during daytime in January 2010 and as 12 ± 1.4 μg m−3 during nighttime in February 2010 (Fig. 4a). Similarly, high concentrations of Fe as 2.7 ± 1.0 and 2.6 ± 1.0 μg m−3 and Mg as 2.7 ± 0.9 and 2.6 ± 0.9 μg m−3 were observed during day and nighttime, respectively, in December 2009 (Fig. 4a).
Paired t test was conducted for trace metals and shown in Table 1. Overall, average concentrations of Na, K, Ca, and Fe were considerably higher (pNa < 0.43, pK < 0.12, pCa < 0.004, pFe < 0.00) compared to average values reported by Jaiprakash et al. (2017), while road site average concentrations of Mg were lower compared to the elevated site. The average values of non-carcinogenic metals of Ni, Zn, Mn, and Cr (except Pb and Cu) were also significantly higher (pNi < 0.00, pZn < 0.01, pMn < 0.01, pCr < 0.00) than the average values reported by Jaiprakash et al. (2017) for the elevated site.
Crustal elements dominate the coarse size fraction of aerodynamic particle size between 2.5 and 10 μm (Chow et al., 2004). It shall be noted that these elements were higher in winter season, when the stable atmosphere free from turbulence resulted in low mixing height and accumulation of re-suspended road dust contributed the crustal elements. Similar trends were also found in studies carried out at road site sampling by Pant et al. (2017).
The Na- and K-dominated aerosol loading was observed especially in November 2009 and March 2010 (Fig. 4b). In daytime, the average highest concentrations of K and Na were 3.6 ± 0.6 and 4.0 ± 1.5 μg m−3, respectively, in November 2009 and March 2010. During nighttime, the highest concentrations of K and Na were 4.4 ± 2.9 and 5.2 ± 3.3 μg m−3 in December 2009 and March 2010. The main sources of K would be wood burning and coal combustion in winter months, whereas both crustal origin Na and K were deposited on the leaves of several road site plant/shrubs and they will re-entrained air upon the combustion of these shrubs and plants for space heating/cooking, especially in winter period (Gupta and Mandaria 2013; Mehta et al. 2009).
Comparatively higher daytime and nighttime concentrations of Ni were observed as 3.3 ± 0.3 and 3.4 ± 0.3 μg m−3 in November 2009. The concentration of Pb and Zn was higher in December 2009 and January 2010 compared to November and March due to the same reason of accumulation of pollutants near ground in winter. Artaxo et al. (1999) and Aatmeeyata et al. (2009) reported that the possible sources of Pb and Zn could be wear and tear of engines, wearing of vehicle tire, and road dust. Other minor trace elements such as Mn, Cr, and Cu (Fig. 4b) were dominant in March 2010. These may be contributed by vehicles exhaust and waste burning (Chakraborty and Gupta 2010).
Source identification using PCA/MLR
The PCA by varimax rotation with Kaiser normalization was used to identify the possible sources of PM1.0 using SPSS software (IBM, version 19.0). This technique is used for dimension reduction, which reduces the number of random variables based on eigenvalue. Varimax rotation with Kaiser normalization is especially used for originating maximum explained variance. In present study, a total of 63 samples and 16 species (MM, trace elements and ions) were used in PCA analysis. The factor loadings N > 0.5 were considered for the clear appearance of factor. The factor loading with eigenvalue > 1, explaining 67% of total variance, is presented in Table 2. PCA resolved six principal components (PCs) and their factor score is presented in Fig. 5, which are arranged in descending order on the basis their contribution.
Secondary aerosol was first extracted component explaining maximum loading of NO3−, SO42−, MM, and Cl−, and it accounted 17% of total variance. The high emissions of nitrogen oxides from fuel or oil combustion could be the possible reason of high nitrate than sulfate, which play an important role in secondary aerosol formation (Jaipraksh et al. 2017; Cheng et al., 2010; Shukla et al., 2017). In addition, the sulfur content could be as high as 50 ppm in low grade BSII and BS-III diesel used in heavy-duty vehicles in other states which are allowed to travel inside the city during night.
The second extracted component was road dust which showed maximum loading of Fe, Mg, and Zn (Table 2 and Fig. 5) and contributed 14% of total variance. The Fe and Zn are predominant from brake/tire wear of vehicles (Pant and Harrison, 2013, 2012; Sahu et al., 2011; Song et al., 2012). Tires of light and heavy motor vehicles contain natural rubber polymers such as styrene-butadiene and polyisoprene in which Zn is used to facilitate speed. The deterioration of tires contributes Zn in road dust. High Fe concentration can be attributed to wear and tear of brake lining. Therefore, Zn and Fe are dominant species in road dust. The moderate loading of Mg with Fe may originate from road surface wear. Kupiainen et al. (2003) also reported these metals in road dust originated from Ca2(MgFe)4Al(Si7Al)O22(OHF)2 in tar. High Mg concentrations in road cement have been reported in many studies (Lawrence et al., 2013; Vega et al., 2001).
Third component was dominated by Na with moderate contribution of Ca, Cl−, and Mg, which explained 13% of total variance (Table 2 and Fig. 5). This could be from biogenic and geogenic material deposited and mixed with road dust and smoke (Thorpe and Harrison, 2008).
The fourth component was dominated by Cu and Pb with moderate loading of SO42−, which accounted for 8% of total variance (Table 2). The maximum loadings of Pb and Cu are presented in Fig. 5, which could be contributed from vehicular emissions in urban roads and high traffic volume. The Cu/Zn ratios observed in the present work were 0.41 ± 0.21 during day and 0.47 ± 0.24 at night are higher than Cu/Zn ratios for gasoline (0.04–0.35) and diesel (0.06–0.22) fuel combustion in engine reported by Jaiprakash and Habib (2017) and Pulles et al. (2012), while the present values were lower than the ratio 1.05 ± 0.13 reported inside tunnel and close to the values reported for urban background 0.31 ± 0.28 by Pant et al. (2017). This suggests that along with traffic the non-combustion sources also contributed considerably amount of Cu, also suggested by Gietl et al. (2010) and Gioia et al. (2010) that Cu and Pb come dominantly from wear of tire rather than engine combustion.
A similar magnitude (8%) of explained variance was accounted for the fifth source, which was dominated by K with moderate loading of Ca and Ni (Fig. 5 and Table 2). Biomass burning is a key source of K in the atmosphere. For various types of agricultural residue combustion, Andreae et al. (1998) reported K/Na ratio as 0.6–2.9. Similar ratios as 0.6 to 2.5 were also obtained in the present study. This confirms high loading of K at road site may originate from biomass or residue burning for heating purpose.
The sixth component was exceptionally loaded with NH4+ and explained 7% of total variance (Fig. 5 and Table 2). Various studies suggested that emissions from gasoline are an important contributor of NH3 (Burgard et al., 2006; Kean et al., 2003; Liu et al., 2014). Typically, in Delhi and other cities, the on-road fleet is characterized by high traffic volume of gasoline vehicles because of preference to high fuel efficiency vehicles over fuel cost (Jaiprakash and Gazala, 2017, b; Sadavarte and Venkataraman, 2014). Recent tunnel studies by Nordin et al. (2013) and Platt et al. (2013) demonstrated that emissions from light duty gasoline vehicles (LDGVs) dominated the formation of secondary ammonia during idling condition. The source apportionment of present study is in agreement with the majority of the previous studies conducted on road site pollution. Our findings clearly indicated that the major contributions of toxic metals in PM1.0 are from road dust, tire or brake wear, engine lining wear along with vehicle exhaust, fuel combustion, and biomass burning.
In the next step, the absolute principal component scores (APCS) were calculated after standard normalization following procedure explained by Larsen and Baker (2003), and thus, these were regressed against measured PM1.0 concentration. The MLR was observed as Eq. (12):
where Z is model-predicted PM1.0 concentration, \( {\upsigma}_{{\mathrm{PM}}_{1.0}} \) and \( {\upmu}_{{\mathrm{PM}}_{1.0}} \) are mean concentration and standard deviation of measured PM1.0, and X1…X6 are APCSs.
The regression coefficients were multiplied by source contribution matrix and the total sum was considered as model-predicted PM1.0 mass for the six sources. The predicted PM1.0 mass showed strong correlation with measured PM1.0 (r2 = 0.85 with slope 0.99, see Fig. S4 in the SI). After PCA-MLR, the species concentration from each sources was obtained and listed in Table 3 and the mean contribution of sources was obtained by 100 × (X i /ΣX i ). Thus, the mean contributions of sources were estimated as 56, 3, 20, 3, 9, and 9%, respectively, from secondary aerosol, brake/tire wear, road dust, vehicle exhaust, biomass burning, and secondary ammonia.
Human health risk assessment
Naturally, inhalation is a primary route of direct exposure to particle bound metals of PM1.0. An average daily dose (ADDinh) and non-carcinogenic risk of six trace metals (Cr, Cu, Mn, Ni, Pb, Zn) in PM1.0 for children and adults were estimated for road site and urban background elevated site. The concentrations of elements measured during same time period at elevated site have been reported in Jaiprakash et al. (2017). ECR and source-specific risk for children and adults were also estimated for road site and elevated site for Cr (VI) (1/7 of total Cr), Ni, and Pb.
Average daily dose from inhalation route
The average daily dose from inhalation route (ADDinh) values for children and adult is presented in Fig. S5a, b in the SI. At road site, the (ADDinh) for children and adults was higher than the elevated site (p = 3.4E-07) at 95% confidence interval. Among metals, the (ADDinh) of Ni was found to be highest as 8.19E-04 and 3.51E-04, for children and adults, respectively, at road site and it was 11 times higher than the dose for elevated site (p = 8.9E-11). The ADDinh of other metals for road site was found to be two to three times higher than average dose estimated for elevated site. The (ADDinh) both for children and adults for road site and elevated site respectively followed the order of Zn > Ni > Pb > Mn > Cr > Cu and Pb > Zn > Cu > Mn > Ni > Cr. It is important to note that for both sites the ADDinh for children is two times higher than for adults (Fig. S5a, b in the SI). The risk was calculated in this study on the basis of particle-bound metals not for others toxic compound such as polycyclic aromatic hydrocarbons (PAHs).
Non-carcinogenic risk
The HQs and HIs for children and adults exposed near road site and elevated site are presented in Fig. 6a, b. Overall non-carcinogenic health risks for children were two times higher than those for adults at road and elevated sites due to higher inhalation rate for children compared to adult. Among the elements, the average HQs for children and adults decreased in the following order: Mn > Cr > Ni > Pb > Zn > Cu both at road and elevated site. For the road site, the HQ of Ni was more than 11 times higher than elevated site (p = 8.9E-11), whereas other metals such as Mn, Cr, and Zn were two to three times higher than elevated site. For children, the mean HQs were observed in safe level for Cu, Ni, Zn, and Pb, while the HQs for Cr (5.6) and Mn (14) exceeded the safe level (Fig. 6a, b) at road site. The average highest HI value was 22 and 10 for children and adults from road site, while lowest values 7 and 3 were estimated for elevated site. It should be noted that the potential health risk due to long-term exposure to vehicle exhaust emissions and re-suspended road dust and emissions from wear and tear of vehicle parts is threat to pedestrians, people working in commercial establishments near roads, and residents living nearby.
A similar study at the urban background site of Kanpur City, India (Singh and Gupta, 2016) reported non-carcinogenic risk of PM1.0 due inhalation of Cr, Mn, and Ni as 27–127 times lower than risk calculated for road site and 2–16 times lower risk estimated for elevated site in the present work. This indicates a severity of risk from toxic metals in Delhi City compared to Kanpur City. The aerosol composition and sources in Kanpur and Delhi are different, resulting in very different level of metal concentration and related health risk. It should be noted that the vehicle population in Delhi is 6.8 times higher than Kanpur (MORTH 2013), and vehicles are major source of Cr, Mn, and Ni.
Excess cancer risk
The ECRs of PM1.0-bound metals due to inhalation were calculated for the both monitoring sites (road and elevated), which are shown in Fig. 7a, b. The total ECR values for adults and children were estimated as 30 × 10−6 and 121 × 10−6 for road site which were four to five times higher than total ECR estimated for elevated site. For the road site, the mean ECRs for adults and children from Cr, Ni, and Pb ranged from 2 × 10−6 to 64 × 10−6 and 0.5 × 10−6 to 16 × 10−6, respectively, while for elevated site, the values between 0.6 × 10−6 and 24 × 10−6 were estimated for adults and 0.5 × 10−6 to 16 × 10−6 for children. The road site average ECR risk of Cr and Ni was close to tolerable limit (10−4) for adults (Fig. 7a) and it was 13–16 times higher than the safe limit (10−6) for children (Fig. 7b). No such trend was observed for Ni at elevated site for both adults and children. The ECR value of Cr for road site was three times higher than elevated site for both adults and children. Importantly, in the present work, the ECR of Ni was a factor of 102 and 15 higher at road site for adults and children compared to elevated site. Oppositely, the ECR of Pb was 16% higher at elevated site compared to road site for both adults and children.
The Pb comes from break lining and battery recycling process (Aatmeeyata, et al. 2009; Jaiprakash et al. 2017). The heavy traffic on major ring road around IIT Delhi and a large scale glass industry in Haryana (MSME 2013) and electroplating and battery processing units in NCR may have contributed to high level of Pb and related health risk at elevated site. In the present work, the ECRs from Pb for both children and adult at road and elevated sites respectively are 11 and 13 times higher than previous work reported by Izhar et al. (2016) for PM1.0 bound Pb for Kanpur City. Overall, the observed ECR values far exceed the acceptable level and it is of great concern to inhabitants (both adults and children) of the capital of India. An increased lung cancer mortality may occur among population residing close to road side in Delhi due to inhalation of particulate-bound trace elements.
ECR in the present work was also compared to previous studies and shown in Table S4 in the SI. A study at urban background site of Kanpur City, India (Izhar et al., 2016) reported six to seven times lower than ECR values than those observed at road site for adults and children, while another study by Singh and Gupta (2016) estimated incremental life time cancer risk (ILCR) using cancer slope factor, and reported value is 5–12 times higher than the present work. In Delhi City, recent studies (Khanna et al., 2015; Khillare and Sarkar, 2012) reported ECR value for adults at kerbsite and residential site for inhalation of PM2.5 and PM10. In the present work, average ECR value is three times higher than ECR value reporetd by Khanna et al. (2015) while 1.6 times lower than those reported by Khillare and Sarkar (2012). One study at mining the site of Dhanbad, India (Jena and Singh, 2017) observed high ECR value, which is 24 times higher for adults and 1000 times higher for children compared to road site ECR estimated in the present work. A study by Bari and Kindzierski (2016) reported ECR value for urban loaction of Canada, which is 120 times lower than the present work for road site. In another study at a semi-urban site, Malaysia Khan et al. (2016) accounted ECR value is 32 times lower than the present work.
Source-specific risk
In the present work, risk was also estimated by sources of PM1.0 to determine the source-specific risk for understanding which source may be prone to high risk in Delhi City. Recent studies (Bari and Kindzierski, 2017; Khan et al., 2016; Li et al., 2015; Liao et al., 2015) have used this approach for estimating the source-specific risks. Source-specific risk of PM1.0 due to inhalation exposure for Delhi City was shown in Fig. S6 in the SI. Mean excess cancer risk from different sources resolved using PCA was estimated in the range of 4 × 10−6 to 95 × 10−6 and all risk values were greater than 1 × 10−6 (Fig. S6 in the SI). It shall be noted that that secondary aerosol and secondary ammonia contributed 56 and 9% to PM1.0 mass, while they contributed less carcinogenic risk as 11 and 9%, respectively. In contrast, biomass burning only contributed 9% of PM1.0 but accounted 44% of carcinogenic risk. Road dust along with brake/tire wear and vehicle exhaust was other dominant contributor to carcinogenic risk (36%) to public health.
Sources emitting large amount of trace metals contributed high health risk compared to those whose emission are rich in secondary aerosol precursors. The present work did not include toxic species such as PAHs, organic molecules identified by USEPA (2015) that may pose to more potential risk to human health from the road site in Delhi City. This limitation can further be assessed and a complete health risk from hazardous air toxic species in urban cities in India should be reported.
Conclusion
This study presented the concentration of submicron aerosol (PM1.0 and PM2.5) collected during October 2009 to March 2010 at two road sites near the Indian Institute of Technology Delhi campus. Very high average concentration of aerosol crossing limit of 60 μg m−3 pointed the severity of particulate pollution at road site which has direct implications to high exposure and related health effect. The formation of secondary aerosol from precursor gases dominated the aerosol burden; however, considerable contributions from road dust, engine exhaust, and wear and tear of engine and tire were observed using PCA. The trace elements contributed from engine combustion, road dust, engine, and tire wear imposed very high non-carcinogenic and excess carcinogenic risk for adults and children near road compared to high altitude. The ECRs of Cr and Ni at road site were close to tolerable limit for adults but significantly higher than the safe limit for children. Overall, the observed ECR values far exceed the acceptable level and it is of great concern to inhabitants of the capital of India. An increased lung cancer mortality may occur among population residing close to road side in Delhi due to inhalation of particulate-bound carcinogenic trace elements. Source and risk apportionment using all available toxic species including volatile organic compounds is recommended for improving understanding on potential risk posed by various sources in urban areas. However, our findings offer reasonably strong baseline information for understanding current major sources affecting road site and elevated site PM1.0 levels in Delhi.
References
Aatmeeyata, Kaul DS, Sharma M (2009) Traffic generated non-exhaust particulate emissions from concrete pavement: a mass and particle size study for two-wheelers and small cars. Atmos Environ 43(35):5691–5697. https://doi.org/10.1016/j.atmosenv.2009.07.032
Andreae, O.M., Annegran, T.W., Beer, H., Cachier, J., Le Canut, H., Elbert, P., Maenhaut, W., Salma, I., Wienhold, F.G., Zenker, T., 1998. Airborne studies of aerosol emissions from savanna fires in southern Africa: 2. Aerosol chemical composition, Journal of geophysical research. William Byrd Press for John Hopkins Press
Artaxo P, Oyola P, Martinez R (1999) Aerosol composition and source apportionment in Santiago de Chile. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms 150(1-4):409–416. https://doi.org/10.1016/S0168-583X(98)01078-7
Bari A, Kindzierski WB (2016) Fine particulate matter PM2.5 in Edmonton, Canada: source apportionment and potential risk for human health. Environ Pollut 218:219–229. https://doi.org/10.1016/j.envpol.2016.06.014
Bari MA, Kindzierski WB (2017) Concentrations, sources and human health risk of inhalation exposure to air toxics in Edmonton, Canada. Chemosphere 173:160–171. https://doi.org/10.1016/j.chemosphere.2016.12.157
Begum BA, Kim E, Biswas SK, Hopke PK (2004) Investigation of sources of atmospheric aerosol at urban and semi-urban areas in Bangladesh. Atmos Environ 38(19):3025–3038. https://doi.org/10.1016/j.atmosenv.2004.02.042
Boldo E, Linares C, Lumbreras J, Borge R, Narros A, García-Pérez J, Fernández-Navarro P, Pérez-Gómez B, Aragonés N, Ramis R, Pollán M, Moreno T, Karanasiou A, López-Abente G (2011) Health impact assessment of a reduction in ambient PM2.5 levels in Spain. Environ Int 37(2):342–348. https://doi.org/10.1016/j.envint.2010.10.004
Brunekreef, B., Beelen, R.M.J., Hoek, G., Schouten, L.J., Bausch-Goldbohm, S., Fischer, P., Armstrong, B., Hughes, E., Jerrett, M., van den Brandt, P., 2009. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res Rep Health Eff Inst Mar, 5–71-89
Burgard DA, Bishop GA, Stedman DH, Gessner VH, Daeschlein C (2006) Remote sensing of in-use heavy-duty diesel trucks. Environ Sci Technol 40(22):6938–6942. https://doi.org/10.1021/es060989a
Chakraborty A, Gupta T (2010) Chemical characterization and source apportionment of submicron (PM1) aerosol in Kanpur Region, India. Aerosol Air Qual Res 10:433–445. https://doi.org/10.4209/aaqr.2009.11.0071
Cheng Y, Lee SC, Ho KF, Chow JC, Watson JG, Louie PKK, Cao JJ, Hai X (2010) Chemically-speciated on-road PM2.5 motor vehicle emission factors in Hong Kong. Sci Total Environ 408(7):1621–1627. https://doi.org/10.1016/j.scitotenv.2009.11.061
Cheng Y, Zou SC, Lee SC, Chow JC, Ho KF, Watson JG, Han YM, Zhang RJ, Zhang F, Yau PS, Huang Y, Bai Y, Wu WJ (2011) Characteristics and source apportionment of PM1.0 emissions at a roadside station. J Hazard Mater 195:82–91. https://doi.org/10.1016/j.jhazmat.2011.08.005
Chow JC, Watson JG, Fujita EM, Lu Z, Lawson DR, Ashbaugh LL (1994) Temporal and spatial variations of PM2.5 and PM10 aerosol in the Southern California air quality study. Atmos Environ 28(12):2061–2080. https://doi.org/10.1016/1352-2310(94)90474-X
Chow JC, Watson JG, Kuhns H, Etyemezian V, Lowenthal DH, Crow D, Kohl SD, Engelbrecht JP, Green MC (2004) Source profiles for industrial, mobile, and area sources in the big bend regional aerosol visibility and observational study. Chemosphere 54(2):185–208. https://doi.org/10.1016/j.chemosphere.2003.07.004
CPCB, 2010. Central pollution control board, air quality monitoring, emission inventory and source apportionment study for Indian cities, National Summary Report The Central Pollution Control Board, New Delhi, India
Delfino, R.J., Staimer, N., Tjoa, T., Gillen, D., Kleinman, M.T., Sioutas, C., Cooper, D., 2008. Research | children’s health personal and ambient air pollution exposures and lung function decrements in children with asthma, 550–558. doi:https://doi.org/10.1289/ehp.10911
Deshmukh DK, Deb MK, Mkoma SL (2013) Size distribution and seasonal variation of size-segregated particulate matter in the ambient air of Raipur city, India. Air Qual Atmos Heal 6(1):259–276. https://doi.org/10.1007/s11869-011-0169-9
Gietl JK, Lawrence R, Thorpe AJ, Harrison RM (2010) Identification of brake wear particles and derivation of a quantitative tracer for brake dust at a major road. Atmos Environ 44(2):141–146. https://doi.org/10.1016/j.atmosenv.2009.10.016
Gioia SMCL, Babinski M, Weiss DJ, Kerr AAFS (2010) Insights into the dynamics and sources of atmospheric lead and particulate matter in São Paulo, Brazil, from high temporal resolution sampling. Atmos Res 98(2-4):478–485. https://doi.org/10.1016/j.atmosres.2010.08.016
Godoi RHM, Godoi AFL, de Quadros LC, Polezer G, Silva TOB, Yamamoto CI, van Grieken R, Potgieter-Vermaak S (2013) Risk assessment and spatial chemical variability of PM collected at selected bus stations. Air Qual Atmos Heal 6(4):725–735. https://doi.org/10.1007/s11869-013-0210-2
Gupta T, Jaiprakash, Dubey S (2011) Field performance evaluation of a newly developed PM2.5 sampler at IIT Kanpur. Sci Total Environ 409(18):3500–3507. https://doi.org/10.1016/j.scitotenv.2011.05.020
Gupta T, Mandariya A (2013) Sources of submicron aerosol during fog-dominated wintertime at Kanpur. Environ Sci Pollut Res 20(8):5615–5629. https://doi.org/10.1007/s11356-013-1580-6
Harrison RM, Yin J (2000) Particulate matter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ 249(1-3):85–101. https://doi.org/10.1016/S0048-9697(99)00513-6
Huang M, Wang W, Chan CY, Cheung KC, Man YB, Wang X, Wong MH (2014) Contamination and risk assessment (based on bio-accessibility via ingestion and inhalation) of metal (loid) s in outdoor and indoor particles from urban centers of Guangzhou, China. Sci Total Environ, 479, 117-124. https://doi.org/10.1016/j.scitotenv.2014.01.115
IRIS (Integrated Risk Assessment System), 1995. United States Environmental Protection Agency, www.epa.gov/IRIS/
Izhar S, Goel A, Chakraborty A, Gupta T (2016) Annual trends in occurrence of submicron particles in ambient air and health risk posed by particle bound metals. Chemosphere 146:582–590. https://doi.org/10.1016/j.chemosphere.2015.12.039
Jaiprakash, Gazala H (2017) Chemical and optical properties of PM2.5 from on-road operation of light duty vehicles in Delhi city. Sci Total Environ 586:900–916. https://doi.org/10.1016/j.scitotenv.2017.02.070
Jaiprakash, Singhai A, Habib G, Raman RS, Gupta T (2017) Chemical characterization of PM1.0 aerosol in Delhi and source apportionment using positive matrix factorization. Environ Sci Pollut Res 24(1):445–462. https://doi.org/10.1007/s11356-016-7708-8
Jena S, Singh G (2017) Human health risk assessment of airborne trace elements in Dhanbad. India Atmos Pollut Res J 8(3):490–502. https://doi.org/10.1016/j.apr.2016.12.003
Kean AJ, Harley RA, Kendall GR (2003) Effects of vehicle speed and engine load on motor vehicle emissions. Environ Sci Technol 37(17):3739–3746. https://doi.org/10.1021/es0263588
Khan MK, Latif MT, Saw WH, Amil N, Nadzir MSM, Sahani M, Tahir NM, Chung JX (2016) Fine particulate matter in the tropical environment: monsoonal effects, source apportionment, and health risk assessment fine particulate matter in the tropical environment: monsoonal effects, source apportionment, and health risk assessment. Atmos Chem Phys 16(2):597–617. https://doi.org/10.5194/acp-16-597-2016
Khanna, I., Khare, M., Gargava, P., 2015. Health risks associated with heavy metals in fine particulate matter: a case study in Delhi City, India. 72–77
Khillare PS, Sarkar S (2012) Atmospheric pollution research airborne inhalable metals in residential areas of Delhi. India: Distrib Source Apportionment and Health Risks 3:46–54. https://doi.org/10.5094/APR.2012.004
Kloog I (2016) Fine particulate matter (PM2.5) association with peripheral artery disease admissions in northeastern United States. Int J Environ Health Res 26(5-6):572–577. https://doi.org/10.1080/09603123.2016.1217315
Krall, J.R., Mulholland, J.A., Russell, A.G., Balachandran, S., Winquist, A., Tolbert, P.E., Waller, L.A., Sarnat, S.E., 2016. Associations between source-specific fine particulate matter and emergency department visits for respiratory disease in four U.S. cities. doi:https://doi.org/10.1289/EHP271
Kupiainen K, Tervahattu H, Raisanen M (2003) Experimental studies about the impact of traction sand on urban road dust composition. Sci Total Environ 308(1-3):175–184. https://doi.org/10.1016/S0048-9697(02)00674-5
Kurian, A. J., (2011) Chemical characterization of aerosol in Delhi: identification and quantification of sources sing positive matrix factorization. M.Tech. Thesis, IIT Delhi
Landis MS, Norris GA, Williams RW, Weinstein JP (2001) Personal exposures to PM2.5 mass and trace elements in Baltimore, MD, USA. Atmos Environ 35(36):6511–6524. https://doi.org/10.1016/S1352-2310(01)00407-1
Larsen RK, Baker JE (2003) Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods. https://doi.org/10.1021/es0206184
Lawrence S, Sokhi R, Ravindra K, Mao H, Douglas H, Bull ID (2013) Source apportionment of traffic emissions of particulate matter using tunnel measurements. Atmos Environ 77:548–557. https://doi.org/10.1016/j.atmosenv.2013.03.040
Li Z, Yuan Z, Li Y, Lau AKH, Louie PKK (2015) Characterization and source apportionment of health risks from ambient PM10 in Hong Kong over 2000 and 2011. Atmos Environ 122:892–899
Liao HT, Chou CCK, Chow JC, Watson JG, Hopke PK, Wu CF (2015) Source and risk apportionment of selected VOCs and PM2.5 species using partially constrained receptor models with multiple time resolution data. Environ Pollut 205:121–130. https://doi.org/10.1016/j.envpol.2015.05.035
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Ross Anderson H, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Shen Chen J, Tai-Ann Cheng A, Christine Child J, Cohen A, Ellicott Colson K, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Ray Dorsey E, Driscoll T, Edmond K, Eltahir Ali S, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Gerry Fowkes FR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Dean Hosgood H III, Hosgood H III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y-H, Khatibzadeh S, Khoo J-P, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Kent Lin J, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Memish ZA, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafi ah K, Mokdad AA, Morawska L, Mozaff arian D, Murphy T, Naghavi M, Neal B, Nelson PK, Miquel Nolla J, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Arden Pope C III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KRC, Stapelberg NJ, Steenland K, Stöckl H, Jacob Stovner L, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Lennert Veerman J, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray LCJ, Ezzati M, Lim SS, Flaxman AD, Andrews MPH KG, Atkinson CB, Carnahan EB, Colson BA, Engell BA, Freedman GB, Freeman BA, Gakidou MK, Jasrasaria E, Lozano RB, Mallinger MPH R, Mokdad L, Murphy AA, Naghavi T, Roberts M, Rosenfeld MPH TB, Sanman LC, Straney EB, Murray LL, Vos CJ, Charlson MPH T, Page F, Lopez A, Blore AD, Norman JD, Hall R, Veerman AW, Khatibzadeh JL, Shi S, Danaei P, Ding G, ScD EL, Giovannucci E, Laden ScD F, Lin AB, Lu JK, Micha YM, Mozaff arian R, Rao D, Salomon MB, Singh JA, White GM, MA RA, Adair-Rohani MPH H, Chafe MPH Z, Smith KR, Tran Ma JH, George S (2013) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. https://doi.org/10.1016/S0140-6736(12)61766-8
Liu EF, Yan T, Birch G, Zhu YX (2014) Pollution and health risk of potentially toxic metals in urban road dust in Nanjing, a mega-city of China. Sci Total Environ 476:522–531. https://doi.org/10.1016/j.scitotenv.2014.01.055
Malm WC, Sisler JF, Huffman D, Eldred RA, Cahill TA (1994) Spatial and seasonal trends in particle concentration and optical extinction in the United States. J Geophys Res 99(D1):1347–1370. https://doi.org/10.1029/93JD02916
Mehta B, Venkataraman C, Bhushan M, Tripathi SN (2009) Identification of sources affecting fog formation using receptor modeling approaches and inventory estimates of sectoral emissions. Atmos Environ 43(6):1288–1295. https://doi.org/10.1016/j.atmosenv.2008.11.041
MORTH (2013) Road Transport Year Book 2012-13. Ministry of Road Transport and Highways, New Delhi
MSME (2013) Ministry of Micro Small and Medium Enterprises, annual report 2013–14. http://msme.gov.in/WriteReadData/DocumentFile/ANNUALREPORT-MSME-2013-14P.pdf
Nordin EZ, Eriksson AC, Roldin P, Nilsson PT, Carlsson JE, Kajos MK, Hellén H, Wittbom C, Rissler J, Löndahl J, Swietlicki E, Svenningsson B, Bohgard M, Kulmala M, Hallquist M, Pagels JH (2013) Geoscientific instrumentation methods and data systems secondary organic aerosol formation from idling gasoline passenger vehicle emissions investigated in a smog chamber. Atmos Chem Phys 13(12):6101–6116. https://doi.org/10.5194/acp-13-6101-2013
Olson DS, Norris GA, Landis MS, Vette AF (2004) Chemical characterization of ambient particulate matter near the World Trade Center: elemental carbon, organic carbon, and mass reconstruction. Environ Sci Technol 38(17):4465–4473. https://doi.org/10.1021/es030689i
Pant P, Harrison RM (2013) Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: a review. Atmos Environ 77:78–97. https://doi.org/10.1016/j.atmosenv.2013.04.028
Pant P, Harrison RM (2012) Critical review of receptor modelling for particulate matter: a case study of India. Atmos Environ 49:1–12. https://doi.org/10.1016/j.atmosenv.2011.11.060
Pant P, Shi Z, Pope FD, Harrison RM (2017) Characterization of traffic-related particulate matter emissions in a road tunnel in Birmingham. trace metals and organic molecular markers, UK, pp 117–130. https://doi.org/10.4209/aaqr.2016.01.0040
Platt SM, El Haddad I, Zardini AA, Clairotte M, Astorga C, Wolf R, Slowik JG, Temime-Roussel B, Marchand N, Ježek I, Drinovec L, Močnik G, Möhler O, Richter R, Barmet P, Bianchi F, Baltensperger U, Prévôt ASH (2013) Geoscientific instrumentation methods and data systems secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber. Atmos Chem Phys 13(18):9141–9158. https://doi.org/10.5194/acp-13-9141-2013
Pope CA III, Dockery DW (2006) Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc 56(6):709–742. https://doi.org/10.1080/10473289.2006.10464485
Pulles T, Denier van der Gon H, Appelman W, Verheul M (2012) Emission factors for heavy metals from diesel and petrol used in European vehicles. Atmos Environ 61:641–651. https://doi.org/10.1016/j.atmosenv.2012.07.022
Rajeev P, Rajput P, Gupta T (2016) Chemical characteristics of aerosol and rain water during an El Nino and PDO influenced Indian summer monsoon. Atmos Environ 145:192–200. https://doi.org/10.1016/j.atmosenv.2016.09.026
Raman RS, Ramachandran S, Rastogix N (2010) Source identification of ambient aerosols over an urban region in western India. J Environ Monit 12(6):1330–1340. https://doi.org/10.1039/b925511g
RTI (2008) Research Triangle Institute (RTI), Standard Operating Procedure for Particulate Matter Gravimetric Analysis
Sadavarte P, Venkataraman C (2014) Trends in multi-pollutant emissions from a technology-linked inventory for India: I. Industry and transport sectors. Atmos Environ 99:353–364. https://doi.org/10.1016/j.atmosenv.2014.09.081
Sahu LK, Kondo Y, Miyazaki Y, Pongkiatkul P, Kim Oanh NT (2011) Seasonal and diurnal variations of black carbon and organic carbon aerosols in Bangkok. J Geophys Res Atmos 116(D15):1–14. https://doi.org/10.1029/2010JD015563
Sandhu K, Singh A, Vigyan Kendra K (2014) Impact of climatic change on human health. Indian Res J Ext Edu 14:36–48
Shridhar V, Khillare PS, Agarwal T, Ray S (2010) Metallic species in ambient particulate matter at rural and urban location of Delhi. J Hazard Mater 175(1-3):600–607. https://doi.org/10.1016/j.jhazmat.2009.10.047
Shukla A, Alam M (2010) Assessment of real world on-road vehicle emissions under dynamic urban traffic conditions in Delhi. Int J Urban Sci 14(2):207–220. https://doi.org/10.1080/12265934.2010.9693677
Shukla PC, Gupta T, Labhsetwar NK, Agarwal AK (2017) Trace metals and ions in particulates emitted by biodiesel fuelled engine. Fuel 188:603–609. https://doi.org/10.1016/j.fuel.2016.10.059
Singh DK, Gupta T (2016) Source apportionment and risk assessment of PM1 bound trace metals collected during foggy and non-foggy episodes at a representative site in the Indo-Gangetic plain. Sci Total Environ 550:80–94. https://doi.org/10.1016/j.scitotenv.2016.01.037
Song S, Wu Y, Jiang J, Yang L, Cheng Y, Hao J (2012) Chemical characteristics of size-resolved PM 2.5 at a roadside environment in Beijing, China. Environ Pollut 161:215–221. https://doi.org/10.1016/j.envpol.2011.10.014
Thorpe A, Harrison RM (2008) Sources and properties of non-exhaust particulate matter from road traffic: a review. Sci Total Environ 400(1-3):270–282. https://doi.org/10.1016/j.scitotenv.2008.06.007
Thurston GD, Spengler JD (1985) A quantitative assessment of source contribution to inhalable particulate matter pollution in Metropolitan Boston. Atmos Environ 19(1):9–25. https://doi.org/10.1016/0004-6981(85)90132-5
Tsai JH, Lin JH, Yao YC, Chiang HL (2012) Size distribution and water soluble ions of ambient particulate matter on episode and non-episode days in southern Taiwan. Aerosol Air Qual Res 12:263–274. https://doi.org/10.4209/aaqr.2011.10.0167
Updyke KM, Nguyen TB, Nizkorodov SA (2012) Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors. Atmos Environ 63:22–31. https://doi.org/10.1016/j.atmosenv.2012.09.012
USEPA (U.S. Environmental Protection Agency), (1998) Quality assurance guidance document 2.12: monitoring PM2.5 in ambient air using designated reference or class I equivalent methods. National Exposure Research Laboratory, Research Triangle Park, NC
USEPA (U.S. Environmental Protection Agency), (2004). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment). Office of Superfund Remediation and Technology Innovation, Washington, D.C.
USEPA (U.S. Environmental Protection Agency), (2009). Risk assessment guidance for superfund volume I: human health evaluation manual (part F, supplemental guidance for inhalation risk assessment). Office of Superfund Remediation and Technology Innovation, Washington, D.C.
USEPA, (2011). Risk assessment guidance for superfund. In: Part, A. (Ed.), Human health evaluation manual; part E, supplemental guidance for dermal risk assessment; part F, supplemental guidance for inhalation risk assessment, I.
USEPA (United States Environmental Protection Agency), (2015). User's guide/technical background document for US EPA region 9's RSL (Regional Screening Levels) tables. http://www.epa.gov/region9/superfund/prg/
Vega E, Mugica V, Reyes E, Anchez G, Chow JC, Watson JG (2001) Chemical composition of fugitive dust emitters in Mexico City. Atmos Environ 35(23):4033–4039. https://doi.org/10.1016/S1352-2310(01)00164-9
Wu CF, Wu SY, Wu YH, Cullen AC, Larson TV, Williamson J, Liu LJ (2009) Cancer risk assessment of selected hazardous air pollutants in Seattle. Environ Int 35(1):516–522. https://doi.org/10.1016/j.envint.2010.06.006
Yu H-L, Chien L-C (2016) Short-term population-based non-linear concentration–response associations between fine particulate matter and respiratory diseases in Taipei (Taiwan): a spatiotemporal analysis. J Expo Sci Environ Epidemiol 26(2):197–206. https://doi.org/10.1038/jes.2015.21
Zheng N, Liu J, Wang Q, Liang Z (2010) Science of the total environment health risk assessment of heavy metal exposure to street dust in the zinc smelting district. Northeast of China 408(4):726–733. https://doi.org/10.1016/j.scitotenv.2009.10.075
Acknowledgements
The authors gratefully acknowledge the financial support of IIT Delhi through seed grant for the completion of this project. The authors also acknowledge the contribution of M. Tech. colleague (Amrita Singhai) and for his participation in sample collection and chemical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 136 kb)
Rights and permissions
About this article
Cite this article
Prakash, J., Lohia, T., Mandariya, A.K. et al. Chemical characterization and quantitativ e assessment of source-specific health risk of trace metals in PM1.0 at a road site of Delhi, India. Environ Sci Pollut Res 25, 8747–8764 (2018). https://doi.org/10.1007/s11356-017-1174-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-1174-9