Abstract
Although nickel (Ni) frequently enters into water, documents on speciation of the metal and its toxicity to freshwater organisms are scanty. Laboratory experiments, made in this study, with 1.0–5.0 mg/L of Ni revealed that Ni was not quickly removed from water. Application of cubic regression followed by classical optimization technique showed that maximum reduction time (T) of Ni in water ranged between 60 to 65 h. Ninety-six hours of LC50 value of Ni to crustacean Diaptomus forbesi, fish Cyprinus carpio, and worm Branchiura sowerbyi was respectively 5.43, 14.70, and 19.73 mg/L. Normalizing the lethal values and plotting them against time, it was observed that C. carpio was more sensitive than D. forbesi, which was not reflected in the 96-h LC50 values. However, sensitivity of these organisms to Ni was better explained by power regression equation (M = aNib), which exhibited that mortality (M) of D. forbesi and B. sowerbyi increased slowly between 24 to 72 h, increasing sharply at 96 h, while mortality of C. carpio increased steadily every 24 till 96 h. Experiment with 1.0 mg/L of Ni in outdoor vats showed that crustacean zooplankton and worms accumulated high concentration of Ni, while only gut and liver tissues of fish C. carpio accumulated trace and moderately high concentration of Ni, respectively. It is assumed that toxicity of Ni to fish is mediated primarily through gill.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As compared to other toxic metals, evidence on pollution of water by nickel (Ni) and its effects on aquatic organisms are limited. However, the metal frequently enters into natural bodies of water through the effluents discharged from several industries like alloys, electroplating, battery, coal combustion, ore refining (WHO 2005; Cempel and Nikel 2006; Munoz and Costa 2012), and many other anthropogenic sources. As a result, Ni contamination of water is gradually increasing resulting in increasing exposure of living organisms to this metal (Munoz and Costa 2012). Average concentration of Ni in water vary between 2.58 and 11.87 μg/L in India (Anonymous 2014), while globally, it ranges between 1.0 and 10.0 μg/L though the value may be as high as 1000 μg/L in contaminated water (Eisler 1998; Zhou et al. 2016).
Although Ni is considered as an essential micronutrient, essentiality of the element to aquatic organisms is not established (Muyssen et al. 2004). Accordingly, the presence of Ni even in low concentration may be hazardous to aquatic organisms. Though systemic studies on Ni toxicity to aquatic organisms are relatively poor (Hoang et al. 2004), a few literature on acute and chronic toxicity of Ni to fish (Luca et al. 2007; Svecevicius 2010; Shafiq et al. 2012) and aquatic invertebrates (Mohammed et al. 2010; Buttino et al. 2011; Niyogi et al. 2014; Zhou et al. 2016) are available. Ninety-six hours of LC50 values of Ni to some freshwater fish varied between 19.3 to 61.2 mg/L (Svecevicius 2010). The physico-chemical parameters of water like pH, hardness, alkalinity, and dissolved solid could modify toxicity of nickel to fish (Pyle et al. 2002, Hoang et al. 2004). Exposure to 1 mM nickel chloride for 1 h at pH 7.4 and temperature 25 °C affected cellular metabolism of erythrocyte in rainbow trout (Luca et al. 2007). The carp fish Cirrhina mrigala showed reduction in growth when exposed to sublethal concentrations of Ni in water (21.93–29.43 mg/L) along with dietary level of Ni (79.11–88.80 μg/g) for 12 weeks (Shafiq et al. 2012). Nickel has also been found to induce nephrotoxicity, hepatotoxicity, and teratogenesis in invertebrates and vertebrates (Haber et al. 2000; Denkhaus and Salnikow 2002; Vijayavel et al. 2009; De Forest and Schlekat 2013). Exposure to 5.7 mg/L Ni for 10–30 days caused severe damage in the histology of gill of the carp fish Hypophthalmichthys molitrix (Athikesavan et al. 2006). Marine crustaceans have been found to be highly sensitive to Ni (Mohammed et al. 2010; Buttino et al. 2011). More than 94% of nauplii of the calanoid copepod Acartia tonsa died at 0.4 mg/L Ni (Zhou et al. 2016). Common freshwater pulmonate snail Lymnea stagnalis is also highly sensitive to chronic exposure of Ni, EC50 for growth varying between 1.3 and 4.4 μg/L (Schlekat et al. 2010).
The main objectives of the present study are to determine persistence of Ni in water with respect to time after treatment of water with environmentally relevant concentrations of Ni, lethal concentration of Ni to selected species of crustacea, oligochaet worms, and fish and pattern of bioaccumulation of Ni in these aquatic organisms through experiments conducted in outdoor earthen vats. We used quadratic and cubic regression in an attempt to explain the non-linear variation in concentration of Ni in water with respect to time and correlate acute toxicity of Ni to aquatic organisms with the change in concentration of the metal in water. Deposition of Cu in water was best explained by cubic regression, while mortality of organisms with respect to time could be explained by linear regression (Ghosh et al. 2016). Various regression equations are used to explain variation of toxicants on living organisms with respect to several independent variables. But judgment to choose the type of regression depends on the best-fitted curve and corresponding R2 value.
Materials and methods
Experimental design
Three experiments were conducted, two in the laboratory and one in outdoor earthen vats. Experiments to study the pattern of Ni deposition in water and acute toxicity bioassays for fish were made in the laboratory in 20-L glass aquaria each containing 10 L of water. Acute toxicity bioassays for crustacean and oligochaet worms were carried out in 300 ml beakers each holding 250 ml of water. Experiments on bioaccumulation of Ni were made in outdoor earthen vats (0.098 m3 space each). Deep tube-well water stored in an overhead tank (dissolved oxygen 7.50 ± 0.30 mg/L; free CO2 0.30 ± 0.01 mg/L, pH 7 15 ± 0.12, alkalinity 125 ± 12 mg/L as CaCO3 and hardness 150 ± 8.0 mg/L as CaCO3) was used as test medium for all the experiments. The test vials were arranged as per randomized block design so that there were three replicates for each of the concentrations of Ni and control tested. Nickel (II) sulfate hexahydrate (NiSO4, 6H2O; Purity ≥ 97.0%) salt procured from MERCK (Mumbai, India) was used for Ni treatment. The salt was dissolved in double distilled water to make a stock solution of 1000 mg/L Ni. Necessary amount from this stock solution was added to the test water to achieve desired concentration of Ni.
Experiments on deposition of Ni
Based on concentrations of Ni determined in natural freshwater bodies in India and assumptions of actual discharges from the sources, three concentrations of Ni (1.0, 2.0, and 5.0 mg/L) were used for experiments on deposition of Ni. The aquaria were filled with water and kept undisturbed for 24 h before making the above treatments and a control with appropriate replications as mentioned above. Water samples were collected by siphoning from mid depth of each aquarium after 1, 6, 12, 24, 48, 72, and 96 h of treatment for the determination of Ni. The water samples were filtered and the filtrates were digested by strong nitric acid following the procedures of APHA (1995). The digested samples were diluted with double distilled water. A blank was prepared from double distilled water using the above method. Levels of Ni in the digested samples were determined in flame atomic absorption spectrophotometer (AAS) (Spectra AA 240, Agilent Technologies) against the blank after calibration with Varian standards of known concentrations.
The data generated were used to fit quadratic and cubic regression using the method of least square, and results were compared to recognize the best one that govern the variation of Ni concentration against time for each treatment. After selection of best-fit regression equation, the classical optimization technique was applied to determine the optimal rate of change of Ni concentration with respect to time and identify time at which variation was maximum.
Acute toxicity bioassay
The 96-h static bioassays were conducted following the procedure of APHA (1995) to determine LC50 values of Ni for the crustacean Diaptomus forbesi, oligochaet worms Branchiura sowerbyi and the teleost fish Cyprinus carpio. The crustaceans and the worms were collected from the local unpolluted water bodies and fingerlings of the fish C. carpio (L = 3.47 ± 0.28 cm; W = 525 ± 30 mg) were procured from a local fish farm. The test organisms were acclimatized in the laboratory conditions for 96 h and were stocked at 10 individuals of crustacean or worms in 300 ml beaker and at 5 individuals of fish in 20 L aquarium. For control and each treatment of Ni, three replicates were maintained. After 1 h of treatment, water samples were collected from each test vial to determine actual concentration of Ni in water by AAS. Mortality of the organisms was recorded every 24 h and dead organisms were removed immediately. Lethal concentration at which 50% animals died (LC50) was calculated by computer programme of probit analysis (EPA Ver. 1.5) based on probit analysis of Finney (1971). The mortality patterns of the test organisms were also evaluated by normalizing the lethal values and plotting them against time. Finally, power regressions were calculated to get a comparative view about the mortality rate with respect to Ni concentration.
Experiments on bioaccumulation of Ni
Six outdoor earthen vats were used for this experiment. Each vat was filled with 60 L of water and 5 kg of uncontaminated soil (pH 7.2 ± 0.2, organic carbon 1.72 ± 0.10%, available N 12.23 ± 0.22 mg/100 g, available P 7.25 ± 0.05 mg/100 g). The vats were then stocked with sufficient quantity of crustacean zooplankton and oligochaet worms collected from unpolluted sources and were conditioned for 15 days for the planktons and worms to grow. Three vats were then treated with 1.0 mg/L of Ni and three vats were kept as controls. The concentration of Ni selected for this experiment was approximately 20% of the LC50 value of Ni for D. forbesi, the most sensitive species tested in the present study. Samples of water were carefully collected from each vat after 96 h of exposure by high density polyethylene (HDPE) bucket and passed through a plankton net made up of bolting silk no. 25 (mesh size 64 μm) to collect the crustacean zooplankton. Sediment samples were collected from each vat by a hand dredger and were spayed in a plastic tray to collect worms. Sediment samples were then dried to constant weight in oven at 98 ± 2 °C. Collected samples were preserved by the methods described by Guhathakurta and Kaviraj (2004). Each vat was then stocked with ten fingerlings of the carp fish C. carpio and was kept in the vat for another 96 h. Fish samples were collected after 96 h of exposure, i.e., 192 h of the whole experiment. All samples collected from the experimental vats were immediately digested or preserved for the determination of Ni.
Water samples were digested in strong nitric acid as described above. The sediment soils were digested in nitric acid and hydrochloric acid (Guhathakurta and Kaviraj 2000). Fish samples were dissected to collect gill, liver, gut, kidney, and muscle tissues. The tissues were digested in nitric acid, sulphuric acid, and perchloric acid (Guhathakurta and Kaviraj 2004). Ni in the digested samples was determined in AAS using blank and standards as described above. Precision and accuracy of the analytical methods were checked by analyses of a standard reference material of Ni in aqueous solution (NIST, SRM no. 3136) and soil samples spiked by Ni (Nafde et al. 1998). Adopted analytical procedures yielded 96 ± 2% recovery of Ni from the SRM and spiked samples tested. Detection limit of Ni in the instrument was found at 0.01 mg/L.
Results
Deposition of Ni in water
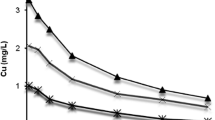
Deposition of Ni in water with respect to time has been presented in Fig. 1. Dissolved Ni in water decreased slowly with the period of exposure. The concentration of Ni after 96 h of exposure was 0.65, 1.29, and 4.12 mg/L respectively for the treatments 1.0, 2.0, and 5.0 mg/L thereby showing reduction of only 17.64, 35.02, and 34.90% of Ni in water after 96 h in respective treatment.
Acute toxicity
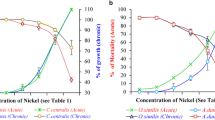
LC50 values of Ni with 95% confidence limit for the three species of aquatic organisms have been presented in Table 1. The values were determined based on nominal concentration of Ni. Ninety-six hours of LC50 values of Ni for the three test organisms ranged from 5.43 to 19.73 mg/L. The crustacean D. forbesi was most susceptible followed by the fish C. carpio and the oligochaet worm B. sowerbyi.
Bioaccumulation of Ni
Concentration of Ni in water, sediment, crustacean plankton, and oligochaet worms after 96 h of treatment with 1.0 mg/L Ni in water is shown in Table 2. Although reduction of Ni in water after 96 h of treatment was more than that observed in laboratory aquaria, the crustacean plankton accumulated high concentration of Ni. Deposition of Ni in sediment was rather low and the bottom organism B. sowerbyi also accumulated moderate amount of Ni. Accumulation of Ni in different tissues is shown in Table 3. In control fish, Ni was detected in low quantity only in gill tissues. After 96 h of treatment with 1.0 mg/L, Ni was detected at moderately high concentration in gill and at trace concentration in gut. Concentration of Ni in liver, kidney, and muscle tissues was below detection limit of the instrument.
Discussion
Deposition of Ni
Heavy metals do not persist in water for long and settle down quickly over sediment. This was evident from the experiments with heavy metals like Cd and Cu which showed 72 to 97% reduction from their initial concentration after 96 h of exposure (Ghosal and Kaviraj 2002; Ghosh et al. 2016). Results of the present study indicate that Ni remains in water for relatively longer period. Only 17 to 35% reduction in the concentration of Ni in water was found after 96 h of exposure to 1.0 to 5.0 mg/L Ni. We applied quadratic and cubic regression equations between concentration of Ni in water and time of observation to explain the pattern of deposition of Ni in water (Table 4). The curves drawn from these equations (Fig. 2) as well as the R2 value (Table 4) exhibited that the cubic regression was best fitted for representing the contamination pattern.
Now, differentiating the cubic regression equation (Table 4), the rate of change of Ni in water (dNi/dT) was computed, and by using classical optimization technique, the time required for maximum reduction (T) of Ni in water was derived for each treatment (Table 5). It was revealed that the time required for maximum reduction of Ni ranged between 60 to 65 h. The higher the concentration of Ni, the lower was the time required for maximum reduction.
Speciation of metal in water varies from metal to metal and is influenced by several physical, chemical, and biological factors (Brown and Markich 2000; Paquin et al. 2000, 2002). Speciation of Ni in water is also dependent upon the solubility of the salt of Ni, pH of water, and presence of organic ligands in water (FAO 1984). Most of the salts of Ni, except nickel sulphide, is highly soluble in water and precipitates less. Ni also forms stable complexes with amino acids, ethylenediaminetetraacetic acid (EDTA), nitrilotriacetic acid (NTA), etc. (FAO 1984). However, the presence of these organic ligands in the laboratory aquarium water is negligible. In the present study, pH of the experimental water was also near neutral (7.15 ± 0.12). Figure 3 demonstrates that even under such condition, Ni precipitated less and only 17 to 35% reduction in the concentration of Ni in water was revealed.
Acute toxicity
Ninety-six hours of LC50 value of C. carpio as observed in the present study is close to that of rainbow trout Onchorhynchus mykiss (Brix et al. 2004; Svecevicius 2010), but lower than those of three spined stickle back Gasterosteus aculeatus, roach Rutilus rutilus, perch Perca fluviatilis, and dace Leuciscus leuciscus (Svecevicius 2010). Physiological differences between species render differences in their susceptibility to metals. It was observed in the present study that susceptibility of Ni to C. carpio changed sharply with time. Svecevicius (2010) also observed that susceptibility of rainbow trout changed with exposure duration, though change in susceptibility to G. aculeatus, R. rutilus, P. fluviatilis, and L. leuciscus did not exhibit such time-dependent change in susceptibility to Ni. Hoang et al. (2004) observed that the susceptibility of fathead minnow Pimephales promelas to Ni changed with age of the fish as well as with hardness, alkalinity, pH, and natural organic matter of water.
Mechanism of toxicity of Ni to aquatic organisms is not completely known. Respiratory trouble resulting from damages in gill is probably the primary reason for toxicity of Ni to fish (Pane et al. 2004; Athikesavan et al. 2006). Oxidative damages to DNA and proteins and inhibition of cellular antioxidant defenses have been observed in fish exposed to Ni (Eisler 1998). Oxidative stress has also been observed in erythrocytes of rainbow trout exposed to 1 mM nickel chloride for 1 h at pH 7.40 and 25 °C (Luca et al. 2007).
Results of the present study indicated that the freshwater crustacean D. forbesi was most sensitive to Ni followed by the carp fish C. carpio and the oligochaet worm B. sowerbyi. Hunt et al. (2002) also observed marine crustacean Mysidopsis intii as more sensitive (96 h LC50 = 0.15 mg/L) to Ni than the marine fish Atherinops affinis (96 h LC50 = 26.56 mg/L). Hatching success of the marine calanoid copepod Acartia tonsa was reduced only in 0.63 mg/L NiCl2, while significant mortality of their nauplii was found only in 0.1 mg/L of NiCl2 (Zhou et al. 2016). In the present study, B. sowerbyi was found least sensitive to Ni among the three animals tested. Toxicity of a metal to aquatic organisms is dependent on the pattern of its speciation in water and bioavailability to aquatic organisms. Precipitation, adsorption, and complexion of metals to inorganic and organic compounds present in water influence speciation and bioavailability of metals in water. B. sowerbyi was found more sensitive to cadmium than the fish C. carpio when water was free of compost manure (Ghosal and Kaviraj 2002). But sensitivity changed with the increase in concentration of compost manure added, and finally, the sensitivity was reversed after addition of a certain level of compost manure. In our experiment with copper, we observed that initially B. sowerbyi was more sensitive to Cu than the copepod D. forbesi and the fish C. carpio. But sensitivity changed with time, and after 96 h, the worm became least sensitive to copper (Ghosh et al. 2016). Results of the present study revealed that Ni precipitated slowly over sediment thereby making the metal biologically less available to the bottom organism B. sowerbyi. In order to compare the susceptibility of the three test organisms to Ni, we first normalized the lethal values of Ni and then plotted against time (Fig. 4). It was observed that lethal values of Ni sharply decreased with the increase of time for the fish C. carpio, while the lethal values decreased moderately for the crustacean D. forbesi and slowly for the worm B. sowerbyi. The curve indicated that C. carpio was more sensitive than D. forbesi, which was not reflected in the 96 h LC50 values. Further, we computed power regression equations (M = aNib), where M was mortality of the test organisms and Ni was the concentration of Ni treated (Table 6). The corresponding power regression curves (Fig. 5) plotted from the equation for each species separately revealed that Ni-induced mortality pattern varied from species to species and depended on exposure period. Mortality increased with the increase of exposure period. But for D. forbesi and B. sowerbyi, mortality increased slowly between 24 to 72 h, thereafter increased sharply at 96 h. In contrast to this, mortality of the fish C. carpio exposed to Ni steadily increased every 24 h during the exposure period.
Bioaccumulation of Ni
Rate of removal of Ni from water after 96 h of treatment in outdoor earthen vat experiment was more than that of laboratory experiment. The environment in outdoor vats was different from that of laboratory aquaria. The bottoms of the vats were filled with soil and plenty of planktons grew in the vats which together contributed to increase in concentration of particulate matters in water. Particulate matters tend to absorb and remove Ni from water (Cempel and Nikel 2006). The zooplankton accumulated high concentration of Ni in the present study. This was possible due to retention of Ni in water as observed in the laboratory experiment without any plankton organisms. Oligochaet worms also accumulated high concentration of Ni. Interestingly, Ni was detected only in gill in moderately high concentration. In liver, kidney, and muscle, Ni concentration could not be detected, while in gut, Ni was detected in trace quantity, indicating that accumulation of Ni through food chain was negligible. Fish harvested from Karasu River, Turkey, also exhibited trace amount of Ni in gill, liver, and muscle although moderate to high concentration of Ni (37 to 88 μg/L) was recorded in the river water (Kalkan et al. 2015). Average level of Ni in fish harvested from Persian Gulf was 0.059 μg/g dry weight, and it was assumed that there was little risk in consumption of such fish at the rate of 4–7 kg/d by adult man (Malakootian et al. 2016).
Conclusions
It is concluded from this study that Ni is not quickly precipitated once it enters into water. Cubic regression is a powerful tool to explain the deposition of Ni in water. As Ni persists in water for long period, pelagic organisms like crustacean zooplankton remain at risk to exposure of Ni. However, sensitivity of aquatic organisms to Ni is better explained by power regression between mortality of organisms and concentration of Ni in water as compared to 96 h LC50 value. The gill of the fish Cyprinus carpio is a sensitive tissue and can accumulate high concentration of Ni from water when the fish is exposed to 1.0 mg/L of Ni. But transfer of Ni from gill to liver, kidney, and muscle tissues of the fish is too little to detect within 96 h of exposure. Gut also accumulates trace amount of Ni within this period, indicating that transfer of Ni through gut wall is also negligible within 96 h. It is assumed from this study that toxicity of Ni to fish is mediated primarily through the gill tissues.
References
Anonymous (2014) Status of trace and toxic metals in Indian rivers. River data directorate, planning and development organization. Ministry of Water Resource, Govt. of India, New Delhi
APHA (1995) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, DC
Athikesavan S, Vincent S, Ambrose T, Velmurugan B (2006) Nickel induced histopathological changes in the different tissues of freshwater fish, Hypophthalmichthys molitrix (Valenciennes). J Environ Biol 27(2):391–395
Brix KV, Keithly J, Deforest DK, Laughlin J (2004) Acute and chronic toxicity of nickel to rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 23:2221–2228
Brown PL, Markich SJ (2000) Evaluation of the free ion activity model of metal-organism interaction: extension of the conceptual model. Aquat Toxicol 51:177–194
Buttino I, Pellegrini D, Romano G, Hwang JS, Liu TM, Sartori D, Sun CK, Macchia S, Ianora A (2011) Study of apoptosis induction using fluorescent and higher harmonic generation microscopy techniques in Acartia tonsa nauplii exposed to chronic concentrations of nickel. Hydrobiologia 27(S2):97–104
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15(3):375–382
De Forest DK, Schlekat CE (2013) Species sensitivity distribution evaluation for chronic nickel toxicity to marine organisms. Integr Environ Assess 9:580–589
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity and carcinogenicity. Crit Rev Oncol Hematol 42:35–56
Eisler R (1998) Nickel hazards to fish, wildlife, and invertebrates: a synoptic review. Contaminant Hazard Reviews Report 34, Biological Science Report USGS/BRD/BSR-1998-0001. Patuxent Wildlife Research Centre, US Geological Survey, Laurel
FAO (1984) Water quality criteria for European freshwater fish, report on nickel and freshwater fish. EIFAC Technical Paper No. 45. Rome, FAO
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, London
Ghosal TK, Kaviraj A (2002) Combined effects of cadmium and composted manure to aquatic organisms. Chemosphere 46(7):1099–1105
Ghosh A, Kaviraj A, Saha S (2016) Kinetics of deposition, acute toxicity and bioaccumulation of copper in some freshwater organisms. Bull Environ Contam Toxicol 97:820–825
Guhathakurta H, Kaviraj A (2000) Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackishwater ponds of Sundarban, India. Mar Pollut Bull 40(11):914–920
Guhathakurta H, Kaviraj A (2004) Effects of salinity and mangrove detritus on desorption of metals from brackish water pond sediment and bioaccumulation in fish and shrimp. Acta Hydrochim Hydrobiol 32(6):411–418
Haber LT, Erdreicht L, Diamond GL, Maier AM, Ratney R, Zhao Q, Dourson ML (2000) Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharmacol 3:210–230
Hoang TC, Tomasso JR, Klaine SJ (2004) Influence of water quality and age on nickel toxicity to fathead minnows (Pimephales promelas). Environ Toxicol Chem 23(1):86–92
Hunt JW, Anderson BS, Phillips BM, Tjeerdema RS, Puckett HM, Stephenson M, Tucker DW, Watson D (2002) Acute and chronic toxicity of nickel to marine organisms: implications for water quality criteria. Environ Toxicol Chem 21(11):2423–2430
Kalkan H, Şişman T, Kılıç D (2015) Assessment of heavy metal bioaccumulation in some tissues of Leuciscus cephalus from Karasu River, Erzurum-Turkey. Austin J Environ Toxicol 1:1004
Luca GD, Gugliotta T, Parisi G, Romano P, Geraci A, Romano O, Scuteri A, Romano L (2007) Effects of nickel on human and fish red blood cells. Biosci Rep 27:265–273
Malakootian M, Mortazavi MS, Ahmadi A (2016) Heavy metals bioaccumulation in fish of southern Iran and risk assessment of fish consumption. Environ Health Eng Manag 3(2):61–68
Mohammed EH, Wang GZ, Jiang JL (2010) The effects of nickel on the reproductive ability of three different marine copepods. Ecotoxicology 19:911–916
Munoz A, Costa M (2012) Elucidating the mechanisms of nickel compound uptake: a review of particulate and nano-nickel endocytosis and toxicity. Toxicol Appl Pharm 260(1):1–16
Muyssen BTA, Brix KV, DeForest DK, Janssen CR (2004) Nickel essentiality and homeostasis in aquatic organisms. Environ Rev 12(2):113–131
Nafde AS, Kondawar VK, Hasan MZ (1998) Precision and accuracy control in the determination of heavy metals in sediment and water by atomic absorption spectrophotometry. JIAEM 25:83–91
Niyogi S, Brix KV, Grosell M (2014) Effects of chronic waterborne nickel exposure on growth, ion homeostasis, acid-base balance, and nickel uptake in the freshwater pulmonate snail, Lymnaea stagnalis. Aquat Toxicol 150:36–44
Pane EF, Haque A, Wood CM (2004) Mechanistic analysis of acute, Ni-induced respiratory toxicity in the rainbow trout (Oncorhynchus mykiss): an exclusively branchial phenomenon. Aquat Toxicol 69:11–24
Paquin PR, Santore RC, KB W, Kavvadasa CD, Toro DMD (2000) The biotic ligand model: a model of the acute toxicity of metals to aquatic life. Environ Sci Pol 3(1):175–182
Paquin PR, Zoltay V, Winfield RP, Wu KB, Mathew R, Santore RC, Toro DMD (2002) Extension of the biotic ligand model of acute toxicity to a physiologically-based model of the survival time of rainbow trout (Oncorhynchus mykiss) exposed to silver. Comp Biochem Physiol C 133:305–343
Pyle GG, Swanson SM, Lehmkuhl DM (2002) The influence of water hardness, pH and suspended solids on nickel toxicity to larval fathead minnows (Pimephales promelas). Water Air Soil Pollut 133:215–226
Schlekat CE, Genderen EV, De Schamphelaere KAC, Antunes PMC, Rogevich EC, Stubblefield WA (2010) Cross-species extrapolation of chronic nickel biotic ligand models. Sci Total Environ 408:6148–6157
Shafiq A, Abdullah S, Javed M, Hayat S, Batool M (2012) Effect of combined exposure of water-borne and dietary nickel on the growth performance of Cirrhina mrigala. J Anim Plant Sci 22(2):425–430
Svecevicius G (2010) Acute toxicity of nickel to five species of freshwater fish. Pol J Environ Stud 19(2):453–456
Vijayavel K, Gopalakrishnan S, Thiagarajan R, Thilagam H (2009) Immunotoxic effects of nickel in the mud crab Scylla serrata. Fish Shellfish Immun 26(1):133–139
WHO (2005) Guidelines for drinking water quality, nickel in drinking water. World Health Organization, Geneva
Zhou C, Vitiello V, Casals E, Puntes VF, Iamunno F, Pellegrini D, Changwen W, Benvenuto G, Buttino I (2016) Toxicity of nickel in the marine calanoid copepod Acartia tonsa: nickel chloride versus nanoparticles. Aquat Toxicol 170:1–12
Funding
This research was funded by University Research Scholarship (URS) to AG by the University of Kalyani, W.B., India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ghosh, A., Kaviraj, A. & Saha, S. Deposition, acute toxicity, and bioaccumulation of nickel in some freshwater organisms with best-fit functions modeling. Environ Sci Pollut Res 25, 3588–3595 (2018). https://doi.org/10.1007/s11356-017-0628-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0628-4