Abstract
Experiments with environmentally relevant concentrations of Cu in glass aquaria revealed that Cu was quickly removed from water. Cubic regression of Cu concentration against time showed that maximum rate of removal was around 69.34–72.11 h irrespective of treatment. The 96 h LC50 value of Cu was respectively 0.18, 0.19 and 0.35 mg/L for fish Cyprinus carpio, crustacean Diaptomus forbesi and worm Branchiura sowerbyi. Normalizing the lethal values and plotting them against time it was observed that there was sharp differences in mortality over time between the organisms and 96 h lethal values could misrepresent susceptibility of the organisms to Cu. Treatment of 0.1 mg/L of Cu in water resulted in accumulation of 10.57, 4.38, 1.46 and 2.44 µg/g of Cu, respectively in sediment, worm, crustacean zooplankton and whole body of fish. But, Cu deposited in high concentrations in gut and liver of fish indicating that Cu was principally accumulated through food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Influx of metals from terrestrial sources into aquatic environment in India has tremendously increased in recent years due to rapid progress in industrialization and urbanization (Javed and Usmani 2013). Freshwater ponds used for culturing fish are also not spared from this influx (Raychaudhuri et al. 2008). Some of these metals are highly toxic, persistent in the aquatic ecosystem and tend to magnify through food chain. The main objective of the present study is to determine kinetics of deposition of Cu in water, acute toxicity of Cu to selected freshwater organisms and pattern of bioaccumulation of Cu in selected species of worms, crustacean zooplankton and fish through experiments conducted in laboratory.
Copper (Cu) is a common metallic pollutant of water and is highly toxic to fish (Kiyani et al. 2013) and aquatic invertebrates (Brown et al. 2004). In India, Cu is frequently detected in river water from trace (0.43 ppb) to high (1.4 mg/L) concentration (Singh et al. 2012; Ghorade et al. 2014). Areas influenced by mining activities (Pandey et al. 2007) and industrial discharges (Tewari et al. 2015) show higher level of Cu in water. Chemical controls of algal bloom in water also contribute to contamination of water by Cu (Kumar and Sinha 2014).
Adequate literatures are not available to relate toxicity of copper to aquatic organisms with the ambient concentration of the metal in water or to the concentration at which the metal is discharged into water from different sources. Attempts were made in this study to address these issues.
Materials and Methods
Three separate experiments were conducted in this study. These included kinetics of deposition, acute toxicity and bioaccumulation of Cu. Deep tube-well water stored in an overhead tank (dissolved oxygen 7.25 ± 0.24 mg/L; free CO2 0.25 ± 0.01 mg/L, pH 7.12 ± 0.15, alkalinity 125 ± 12 mg/L as CaCO3 and hardness 140 ± 8.0 mg/L as CaCO3) was used as test medium for all the experiments. Copper(II) sulphate pentahydrate (CuSO4⋅5H2O; Purity ≥99.0 %) salt procured from MERCK, Mumbai, India was used for Cu treatment. The salt was dissolved in double distilled water to make a stock solution of 1000 mg/L Cu. Necessary amount from this stock solution was added to the test water to achieve desired concentration of Cu.
Experiments to study kinetics of Cu deposition in water and acute toxicity bioassays for fish were made in the laboratory in 20 L glass aquaria (38 × 25 × 25.5 cm) each containing 10 L of water. Acute toxicity bioassays for crustacean and oligochaet worms were carried out in 300 mL beakers each holding 250 mL of water. Experiments on bioaccumulation of Cu were made in outdoor field (12:12 h dark-light photoperiod) in bowl shaped earthen vats, each with 0.098 m3 space (surface radius = 0.33 m, maximum height = 0.36 m) and stocked with 60 L of water and 5 kg of uncontaminated soil (pH 7.2 ± 0.2, organic carbon 1.72 ± 0.10 %, available N 12.23 ± 0.22 mg/100 g, available P 7.25 ± 0.05 mg/100 g). The test vials were arranged as per randomized block design so that there were three replicates for each of the concentrations of Cu and control tested.
Based on concentrations of Cu determined in natural freshwater bodies in India and assumptions of actual discharges from the sources, three concentrations of Cu (1.5, 2.5 and 3.5 mg/L) were used for experiments on deposition of Cu. The aquaria were filled with water and kept undisturbed for 24 h before making the above treatments and a control with appropriate replications as mentioned above. Water samples were collected by siphoning from mid depth of each aquarium after 1, 6, 12, 24, 48, 72 and 96 h of treatment for determination of Cu. The water samples were filtered and the filtrates were digested by strong nitric acid following the procedures of APHA (1995). The digested samples were diluted with double distilled water. A blank was prepared from double distilled water using the above method. Levels of Cu in the digested samples were determined in flame atomic absorption spectrophotometer (Spectra AA 240, Agilent Technologies) against the blank after calibration with Varian standards of known concentrations. The data generated were used to fit cubic regression using the method of least squares to determine Cu concentration against time for each treatment. The classical optimization technique was applied to determine the optimal rate of change of Cu concentration with respect to time and the time at which the rate was optimal.
The 96 h static bioassays were conducted following the procedure of APHA (1995) to determine LC50 values of Cu for the crustacean Diaptomus forbesi, oligochaet worms Branchiura sowerbyi and the teleost fish Cyprinus carpio. The crustaceans and the worms were collected from the local unpolluted water bodies and fingerlings of the fish C. carpio (L = 3.47 ± 0.28 cm; W = 525 ± 30 mg) were procured from a local fish farm. The test organisms were acclimatized in the laboratory conditions for 96 h and were stocked at ten individuals of crustacean or worms in 300 mL beaker and at five individuals of fish in 20 L aquarium. For control and each treatment of Cu, three replicates were maintained. After 1 h of treatment, water samples were collected from each test vial to determine actual concentration of Cu in water by flame atomic absorption spectrophotometer (AAS). Mortality of the organisms was recorded every 24 h and dead organisms were removed immediately. Lethal concentration at which 50 percent animals died (LC50) was calculated by computer programme of probit analysis (EPA Ver. 1.5) based on probit analysis of Finney (1971). The mortality patterns of the test organisms were also evaluated by normalizing the lethal values and plotting them against time. Finally, the linear regression curves were calculated to get a comparative view about the mortality rate with respect to Cu concentration.
Experiments on bioaccumulation of Cu were made in six bowl shaped earthen vats. The vats were stocked with sufficient quantity of crustacean zooplankton and oligochaet worms collected from unpolluted sources and were conditioned for 15 days for the planktons and worms to grow. Three vats were then treated with a sub-lethal concentration of Cu (0.1 mg/L) and three vats were kept as controls. The sub-lethal concentration selected was based on 50 % of the LC50 value of nominal concentration of Cu for the crustacean D. forbesi and the teleost fish C. carpio. After 96 h of exposures, samples of water were carefully collected from at least 10 cm above bottom soil of each vat by a 1 L high density polyethylene (HDPE) bucket and passed through a plankton net made up of bolting silk no.25 (mesh size 64 μm) to collect the crustacean zooplankton. Sediment samples were collected from each vat by a hand dredger and were spread in a HDPE tray to collect worms. Sediment samples were then dried to constant weight in oven at 98 ± 2°C. Collected samples were preserved by the methods described by Guhathakurta and Kaviraj (2004). Then each vat was stocked with ten fingerlings of the fish C. carpio and was kept in the vat for another 96 h in order to determine accumulation of Cu from ambient concentration of Cu and food organisms. Fish samples were collected after 96 h of its exposure, i.e., 192 h of the whole experiment. All samples collected from the experimental vats were immediately digested or preserved for determination of Cu.
Water samples were digested in strong nitric acid as described above. The sediment soils were digested in nitric acid and hydrochloric acid (Guhathakurta and Kaviraj 2000). Fish samples were divided into two groups. From one part of the sample, whole fish was digested while fish from another part of the sample were dissected to collect gill, liver, gut, kidney and muscle tissues. The whole fish and each tissue samples were digested in nitric acid, sulphuric acid and perchloric acid (Guhathakurta and Kaviraj 2004). Cu in the digested samples was determined in flame AAS using blank and standards as described above. Precision and accuracy of the analytical methods were checked by analyses of a standard reference material of Cu in aqueous solution (NIST, SRM No 3114) and soil samples spiked by Cu (Nafde et al. 1998). Adopted analytical procedures yielded 96 ± 2 % recovery of Cu from the SRM and spiked samples tested. Detection limit of Cu in the instrument was found at 0.01 mg/L.
Results and Discussion
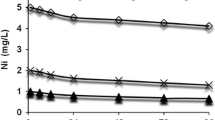
Deposition of Cu in water in respect of time has been presented in Fig. 1. Dissolved Cu in water gradually decreased with the period of exposure. The concentration of Cu after 96 h of exposure was 0.04, 0.43 and 0.66 mg/L, respectively for the treatments 1.5, 2.5 and 3.5 mg/L thereby showing 2.7 %, 17.2 % and 18.9 % of Cu in water after 96 h in respective treatments.
By normalizing the data of deposition of Cu with respect to time and then fitting the data in cubic regression equation the following relationships were obtained between concentration of Cu and time of observation (Fig. 2a–c):
Now by differentiating Eq. (1) with respect to time (T), the rate of change for concentration for treatment of 1.5 mg/L of Cu was computed as \({{Y}_{1}}=\frac{dCu}{dT}=-0.7311+2\times 0.2291T-3\times 0.02643{{T}^{2}}\). This equation was again differentiated with respect of time and by solving \(\frac{d{{Y}_{1}}}{dT}=0\), we obtained time as T = 2.88939 days or 69.34 h. Because, \({{\frac{{{d}^{2}}{{Y}_{1}}}{d{{T}^{2}}}}_{T=2.88939}}=-6\times 0.02643<0\), i.e., Y1 (treatment of 1.5 mg/L Cu) had maximum reduction at 69.34 h. By applying similar approach on Eq. (2), it was observed that Y2 (treatment of 2.5 mg/L Cu) had maximum reduction at T = 2.90632 or 69.75 h. Finally, from Eq. (3) it was observed that Y3 (treatment of 3.5 mg/L Cu) had maximum reduction at T = 3.00421 days or 72.11 h.
LC50 values of Cu with 95 % confidence limit for the three species of aquatic organisms have been presented in Table 1. The values were determined based on nominal concentration as well as on actual concentration of Cu determined after 1 h of exposure. There was no mortality of any organism in control. The 96 h LC50 values of Cu for the three test organisms ranged from 0.20 to 0.39 mg/L of nominal concentration and 0.18–0.35 mg/L of actual 1 h concentration of Cu in water, thereby showing only 10 % difference in LC50 values between nominal and actual concentration. All the test organisms were highly sensitive to Cu, worm being relatively more tolerant than other two organisms.
The 96 h LC50 value of Cu for the crustacean Mysis sp (1.44 mg/L) and the teleost fish Mugil cephalus (4.4 mg/L) recorded by Zyadah and Abdel-Baky (2000) are slightly higher than the LC50 values of Cu for the crustacean D. forbesi and the fish C. carpio recorded in the present study, while 48 h EC50 of Cu to the crustacean Daphnia similis (0.013–0.046 mg/L) and fish Danio rerio (0.038–0.095 mg/L) (Filho et al. 2004) are much lower than those recorded in the present study. While sensitivity of the aquatic organisms to Cu varies from species to species, it is influenced strongly by the physicochemical parameters including pH, water hardness and dissolved organic matter (Kim et al. 2001; Kiyani et al. 2013). Using species sensitivity distribution curve by log-logistic model Xin et al. (2015) observed that invertebrate taxa were more sensitive to Cu than vertebrates. But results of the present study revealed that there was no significant difference in 96 h LC50 values between the fingerlings of teleost fish C. carpio and the crustacean D. forbesi. The fish species was even more susceptible to Cu than the oligochaet worm B. sowerbyi. However, relative susceptibility of the three organisms to Cu changed with exposure period. Toxicity of metals to aquatic organisms depends on bioavailability of the metal and mode of its entry into the body. Playle et al. (1992) observed that adult fathead minnows (Pimephales promelas) had the ability to modify its local chemical microenvironment at their gill surface and regulate accumulation and toxicity of Cu. It is not known if small fry of C. carpio (mean weight 0.52g ± 0.03) used in the present study had such regulation. Ability to regulate accumulation of Cu from solution is not uniform among crustaceans. Though certain species of cladocerans (Choueri et al. 2009) can efficiently regulate Cu in their body ability of D. forbesi or copepods, in general, to regulate Cu is not known.
Results of the present study indicated that D. forbesi could regulate Cu up to 48h more efficiently than the C. carpio fry and the B. sowerbyi. Accordingly, D. forbesi was more tolerant than either C. carpio or B. sowerbyi up to 48 h. As actual mechanism of toxicity of Cu to the three test organisms used in the present study is not generally known we applied a different technique to compare mortality pattern of the three aquatic organisms with respect to time. The lethal values of Cu (based on 1 h actual concentration) were normalized and plotted against time (Fig. 3). It was observed that lethal values of Cu sharply decreased with the increase of time for the crustacean D. forbesi, while the lethal values decreased moderately for the fish C. carpio and slowly for the worm B. sowerbyi. Accordingly, D. forbesi became more sensitive to Cu at 96 h than either B. sowerbyi or C. carpio. Although such difference in sensitivity between D. forbesi and B. sowerbyi was discernible from 96 h LC50 values the difference between D. forbesi and C. carpio was not reflected in LC50 values.
As maximum reduction in the concentration of Cu in water was found between 69.34 ~ 72.11 h, the mortality data were normalized and linear regression curves were drawn for 48, 72 and 96 h (Fig. 4). It was revealed that mortality rate at 72 h was higher compared to 48 and 96 h exposure period for D. forbesi and C. carpio, but the difference in mortality of B. sowerbyi among the three exposure periods was not substantial.
In the bioaccumulation experiment, Cu was found to partition differentially between abiotic and biotic components of the experimental medium (Table 2). After 96 h of exposure, concentration of Cu in water was only 0.017 ± 0.002, which was lower than the permissible level of Cu in surface water prescribed by Bureau of Indian Standards (Anonymous 2012). But Cu deposited over bottom sediment and the concentration became as high as 10.57 ± 0.98 mg.kg−1. However, increase in concentration of Cu in sediment does not necessarily increase toxicity of Cu to oligochaet worms because sediment bound Cu is not always bioavailable to these organisms (Ankley et al. 1994). Critical body residue of Cu in which 50 % of reduction in survival or autotomy or reproduction occurred in the oligochaet Tubifex tubifex was 2–11 times higher in sediment than water only medium (Mendez-Fernandez et al. 2013). Based on LC50 values of some sediment organisms Roman et al. (2007) calculated “No Observed Effect Concentration” (NOEC) of Cu in the sediment that produced little effects to sediment organisms and it ranged between 3 and 47 mg/kg of the sediment. Based on this NOEC value, sediment concentration of Cu in the present study appeared to render little risk to worms, which accumulated 4.38 ± 1.12 µg/g Cu during 96 h exposure. Accumulation of Cu in planktonic organisms and whole body of fish were also only 1.46 ± 0.51 and 2.44 ± 0.20 µg/g respectively, indicating that food chain concentration of Cu was not prominent.
However, Cu accumulated in high concentration in gut and liver (Table 3) indicating that Cu accumulation in C. carpio was principally through food. Possibility of depuration of Cu from these tissues was minimum, because fish samples were immediately preserved after collection from the experimental vats. This was one of the possible reasons of high concentration of Cu in these tissues. High concentration of Cu in liver indicates potential of detoxification of Cu in this tissue (Nazi et al. 2014). Copper often accumulates and becomes toxic to aquatic organisms at concentrations slightly over the level in which it is required in the body.
It is concluded from the present study that irrespective of concentration at which Cu is evaluated in laboratory test water, the time required for its maximum reduction in concentration range between 69 and 72 h. Relative sensitivity of the aquatic organisms C. carpio, D. forbesi and B. sowerbyi to Cu change with exposure period and 96 h LC50 value alone is not adequate to compare their sensitivity to Cu. Cubic regression of the data of Cu deposition in water and normalization of the mortality data followed by linear regression can better explain the pattern of Cu retention in water and its toxicity to aquatic organisms. The bioaccumulation experiment indicates that the fish C. carpio probably accumulates Cu mostly from food, but food chain concentration of the metal is not evident from this experiment.
References
Ankley GT, Leonard EN, Mattson VR (1994) Prediction of bioaccumulation of metals from contaminated sediments by the oligochaete, Lumbriculus variegates. Water Res 28(5):1071–1076
Anonymous (2012) Indian standard for drinking water specification (2nd revision). Bureau of Indian Standards, New Delhi
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, DC
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jonesa MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66:267–278
Choueri RB, Gusso-choueri PK, Grac MD¸ Mela AG, Lombardi AT, Vieira AAH (2009) The influence of cyanobacterium exudates on copper uptake and toxicity to a tropical freshwater cladoceran. J Plankton Res 31(10):1225–1233
Filho ECDO, Lopes RM, Paumgartten FJR (2004) Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 56:369–374
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, London
Ghorade IB, Lamture SV, Patil SS (2014) Assessment of heavy metal content in Godavari river water. Int J Res Appl Nat Soc Sci 2(6):23–26
Guhathakurta H, Kaviraj A (2000) Heavy metal concentration in water, sediment, Shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackishwater ponds of Sundarban, India. Marine Poll Bull 40(11):914–920
Guhathakurta H, Kaviraj A (2004) Effects of salinity and mangrove detritus on desorption of metals from brackish water pond sediment and bioaccumulation in fish and shrimp. Acta Hydrochim Hydrobiol 32(6):411–418
Javed M, Usmani N (2013) Assessment of heavy metal (Cu, Ni, Fe, Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. Springer Plus 2:390
Kim SD, Gu MB, Allen HE (2001) Physicochemical factors affecting the sensitivity of Ceriodaphnia dubia to copper. Environ Monit Asses 70:105–116
Kiyani V, Hosynzadeh M, Ebrahimpour M (2013) Investigation acute toxicity some of heavy metals at different water hardness. Int J Adv Biol Biom Res 1(2):134–142
Kumar B, Sinha A (2014) Microcystis toxic blooms in fish culture ponds and their biological and chemical control. Int J Sci Technicol Res 3(3):398–410
Mendez-Fernandez L, Martınez-Madrid M, Rodriguez P (2013) Toxicity and critical body residues of Cd, Cu and Cr in the aquatic oligochaete Tubifex tubifex (Müller) based on lethal and sublethal effects. Ecotoxicology 22(10):1445–1460
Nafde AS, Kondawar VK, Hasan MZ (1998) Precision and accuracy control in the determination of heavy metals in sediment and water by atomic absorption spectrophotometry. J Indian Assoc Environ Manage 25:83–91
Nazi A, Ismail A, Kamrani E, Sohrabi T (2014) Correlation of MT levels in livers and gills with heavy metals in wild tilapia (Oreochromis mossambicus) from Klang river, Malaysia. Bull Environ Contam Toxicol 92:674–679
Pandey PK, Sharma R, Roy M, Pandey M (2007) Toxic mine drainage from Asia’s biggest copper mine at Malanjkhand, India. Environ Geochem Health 29:237–248
Playle RC, Gensemer RW, Dixon DW (1992) Copper accumulation on gills of fathead minnows: influence of water hardness, complexation and pH of the gill micro-environment. Environ Toxicol Chem 11(3):381–391
Raychaudhuri S, Mishra M, Salodkar S, Sudarshan M, Thakur AR (2008) Traditional aquaculture practice at East Calcutta wetland: the safety assessment. Am J Environ Sci 4(2):173–177
Roman YE, De Schamphelaere KAC, Nguyen LT, Janssen CR (2007) Chronic toxicity of copper to five benthic invertebrates in laboratory-formulated sediment: sensitivity comparison and preliminary risk assessment. Sci Total Environ 387(1–3):128–140
Singh L, Choudhary SK, Singh PK (2012) Status of heavy metal concentration in water and sediment of river Ganga at selected sites in the middle Ganga plain. Int J Res Chem Environ 2:236–243
Tewari MK, Bajpai S, Dewangan UK, Tamrakar RK (2015) Assessment of heavy metal concentrations in surface water sources in an industrial region of central India. Karbala International. J Mod Sci 1:9–14
Xin Z, Wenchao Z, Zhenguang Y, Yiguo H, Zhengtao L, Xianliang Y, Xiaonan W, Tingting L, Liming Z (2015) Species sensitivity analysis of heavy metals to freshwater organisms. Ecotoxicology 24:1621–1631
Zyadah MA, Abdel-Baky TE (2000) Toxicity and bioaccumulation of copper, zinc, and cadmium in some aquatic organisms. Bull Environ Contam Toxicol 64:740–747
Acknowledgments
Partial research grants received from University of Kalyani through URS and PRG fellowship to AG and personal research grant to AK are thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, A., Kaviraj, A. & Saha, S. Kinetics of Deposition, Acute Toxicity and Bioaccumulation of Copper in some Freshwater Organisms. Bull Environ Contam Toxicol 97, 820–825 (2016). https://doi.org/10.1007/s00128-016-1953-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1953-x