Abstract
Oil spills over seawater and dye pollutants in water cause economic and environmental damage every year. Among various methods to deal oil spill problems, the use of porous materials has been proven as an effective strategy. In recent years, graphene-based porous sorbents have been synthesized to address the shortcomings associated with conventional sorbents such as their low uptake capacity, slow sorption rate, and non-recyclability. This article reviews the research undertaken to control oil spillage using three-dimensional (3D) graphene-based materials. The use of these materials for removal of dyes and miscellaneous environmental pollutants from water is explored and the application of various multifunctional 3D oil sorbents synthesized by surface modification technique is presented. The future prospects and limitations of these materials as sorbents are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil spill accidents occur every year, for example, the Erika spill in France in 1999 and Gulf of Mexico spill in 2010 (Al-Majed et al. 2014). The environmental and water pollution due to oil spills cause a lot of economic and environmental destruction along with health problems. The spilled oil on seawater undergoes various processes: spreading, evaporation, formation of emulsion at the air/water interface, and degradation (Rogowska and Namie 2010). Dyes are pollutants released into receiving waters and rivers which decrease light penetration in water thus decreasing the photosynthetic activity within the water. This reduces the growth of biota (plant and animal life). A large number of dyes are used in the textile industry. The disposal of dye-contaminated water is a serious issue for the textile dyeing and ink-related industries as it possesses the potential to harm human beings as degradation products from dyes may be carcinogenic (Ratna 2012).
Various strategies are used for oil spill cleanup such as in situ burning (Mullin and Champ 2003) or the use of dispersants (Lessard and DeMarco 2000) or sorbent materials (Saleem et al. 2014, 2015, 2018). Booms are also used to physically contain the oil which forms a barrier in the sea around oil slicks. The contained oil can be picked up by skimmers which physically separate oil from water; however, this mechanical method of booms and skimmers is expensive and it is effective only in calm water conditions (Schulze 1998; Al-Majed et al. 2012).
The applications of porous sorbent materials have proven to be an attractive strategy to deal with oil spill problem due to their low cost and efficient oil removal. The conventional sorbents include polypropylene, zeolites, ion exchanges, perlite, organophilic clays, biomass materials, and exfoliated graphite (Adebajo et al. 2003). However, most sorbents suffer from problems such as low sorption capacity and poor recyclability. These issues are well addressed by the use of carbon-based sorbent materials in recent years as they possessed suitable properties for oil uptake such as a porous structure with high surface area and hydrophobic surface.
Carbon has many allotropes depending upon the hybridization on carbon and atomic arrangement. For example, diamond, which is the hardest carbon-based material, is an insulator while layered graphite is soft and is a conductor of electricity (Falcao and Wudl 2007). The sp2 hybridization is present in graphite, graphene, carbon nanotubes, and fullerenes (Georgakilas et al. 2015). Graphene, which was discovered in 2004, is the most recent form of carbon. It has exceptional properties such as large specific surface area, high Young’s modulus, high thermal and electric conductivity, and possesses high optical transmittance. As a result, graphene has been extensively used in various energy and environment applications. For example, the research was directed to make electrodes based on graphene materials for use in energy storage devices (supercapacitors and batteries) (Fan et al. 2015a). The materials have also been used for the photocatalytic degradation of environment pollutants and in gas sensing (Ali Tahir et al. 2016). In water purification, graphene membranes (with fine pores) have been tried with the aim to develop energy efficient/environmentally friendly water filters. Although graphene repels water, rapid water permeation is allowed if narrow pores are made in the graphene sheet. The use of graphdiyne (graphyne with diacetylene groups)-based membranes have also been proposed in water purification (Bartolomei et al. 2014). Further, Carmalin Sophia et al. (2016) reviewed the research on the application of graphene-based materials such as graphene composites and graphene oxide for the removal (adsorption) of traces of water-related pathogenic, micro-organisms, expired antibiotics, heavy metals, etc. which are thrown into water as wastes. To deliver drugs and genes, delivery vehicles based on 3D graphene materials have been reported which have been proved useful in cancer therapy (Wu et al. 2015). Further promising results were obtained in tissue engineering because these materials are porous, biocompatible, and biodegradable and can act as a support/scaffold platform for growth of cells with enhanced oxygenation (Loeblein et al. 2015).
The present article provides a review of the usage of three-dimensional graphene in oil and dye sorption studies as this material possesses high surface area, good mechanical strength, and tunable surface properties which are desirable characteristics of a good sorbent. The removal of miscellaneous environmental pollutants using these materials has also been described. Finally, multifunctional sorbents of 3D graphene synthesized by surface modification are reviewed. The future prospects and limitations of these materials as sorbents have also been discussed. Table 1 provides a comparison on the performance of various 3D carbon materials with graphene for oil sorption application while Fig. 1 presents characterization of 3D graphene.
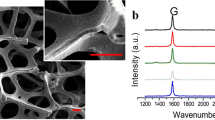
Characterization of three-dimensional (3D) graphene. a, b SEM micrographs of 3D-GFs at low and high magnification; the inset shows an enlarged view of 3D-GF skeleton surface. c Typical Raman spectrum of 3D-GFs. Reproduced from Li et al. (2013). Copyright 2013 Nature Publishing Group
Synthesis of graphene and graphene oxide
At the beginning, i.e., in 2004, the mechanical exfoliation (also called “scotch tape”) method was used to synthesize graphene; however, various methods are now available for the preparation of graphene (Fig. 2a). Graphene synthesis methods can be divided into bottom-up graphene methods (chemical vapor deposition and epitaxial growth on SiC) and top-down graphene methods (exfoliation of graphite or graphite derivatives—mechanical, chemical, or ultrasonic exfoliation) (Bhuyan et al. 2016).
Graphene oxide GO is prepared by the Hummers method by the oxidation of graphite with oxidizing agents such as KMnO4 and NaNO3 in H2SO4 (strong oxidizing agents) (Hummers and Offeman 1958); however, an improved Hummers method (Fig. 2b) was reported in 2010 which uses KMnO4/H2SO4/H3PO4 as an oxidizing agent (Marcano et al. 2010).
3D graphene-based structures preparation methods
The preparation methods generally fall into two categories: template-directed methods and self-assembly methods (Żelechowska et al. 2014; Yang et al. 2015b).
Template-directed methods
In these methods, the templates used are metal foams (Ni porous hard template), mesoporous silica, and hard spheres of polystyrene. Three-dimensional graphene structures are usually prepared via chemical vapor deposition method.
Chen et al. prepared graphene foam (an interconnected flexible network of graphene) using the CVD method (template-directed chemical vapor deposition) in which nickel foam is chosen as a porous metal template on which graphene is grown by the decomposition of a precursor gas CH4 at 1000 °C. Afterwards, the nickel was etched away (Chen et al. 2010, 2011). After Chen’s pioneering work, several researches appeared to prepare graphene-based 3D structures using metal template-directed chemical vapor deposition and such materials have been prepared by adding a number of chemicals with the aim to obtain or create functionalized nano-architectures. The added chemicals include, for example, metal nanoparticles such as Pt nanoparticles, multi-walled carbon nanotubes (CNTs), and metal oxides such as MnO2 (Cao et al. 2013) and Fe3O4 (Luo et al. 2013).
Sha et al. (2016) combined traditional powder metallurgy and chemical vapor deposition to obtain 3D mesoporous graphene foams. The prepared structures possessed high conductivity, mechanical robustness, and also were recoverable after being compressed (Sha et al. 2016).
Furthermore, to get 3D highly porous composites with the desired pore size and branch size, Ni template was synthesized instead of using commercial Ni template foam which has a large pore size (about 200–500 μm) and wide branch size (about 50 μm) (Żelechowska et al. 2014). In one experiment, the facial electrodeposition method was used to obtain Ni mesh/film with pore and branch sizes much smaller than those of a commercial Ni-foam template. The pore size of 5–10 μm and branch length of about 5 μm were obtained. The Ni films so obtained were then used by the CVD method to prepare ultrathin 3D graphene foam with dimensions similar to that of the template used in the CVD method (Xia et al. 2014; Żelechowska et al. 2014).
Self-assembly methods
Graphite oxide is obtained by the oxidation of graphite and then graphene oxide, GO, is formed by the exfoliation of graphite oxide. Chemical reduction of the GO dispersion in aqueous medium leads to the formation of free-standing graphene hydrogels, and this method is called hydrothermal gelation. During the reduction reaction, the hydrophilic GO is converted to hydrophobic reduced graphene oxide (RGO) (Żelechowska et al. 2014) (see Fig. 3).
The reduction can be done by various reducing agents such as by sodium ascorbate (Sheng et al. 2011). The prepared hydrogels were characterized by X-ray diffraction (XRD), rheological tests, electrical conductivity measurements, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and scanning electron microscopy (SEM). The reduction (deoxygenation) of graphene oxide by hydrazine and by a heat reduction method have also been reported (Gao et al. 2010).
The GO hydrogels can also be obtained from graphene oxide aqueous dispersion by other methods: by the addition of cross-linkers such as divalent (Ca2+, Mg2+, Cu2+), trivalent metal (Cr3+, Fe3+) ions, small quaternary ammonium salts such as cetyl trimethyl ammonium bromide (Bai et al. 2011), and DNA (Xu et al. 2010). The hydrogels based on graphene oxide and DNA possess dye loading capacity, high self-healing property, and high mechanical strength (Xu et al. 2010). Freeze drying has also been reported to obtain GO hydrogels (Bi et al. 2012; Xia et al. 2014).
Applications of 3D graphene-based architectures in oil and dye sorption studies
The ideal sorbent should have high sorption capacity, fast sorption speed, high surface area, and should be strong (possesses good mechanical strength). Furthermore, to decrease the cost of the material, it should be recyclable (see Fig. 4). It has been found that superhydrophobic (when the contact angle with the surface is over 150°) and superoleophilic materials have excellent oil absorption capabilities (Gupta and Tai 2016).
The oil sorption materials with good sorption capacity include activated charcoal (have a fire hazard, pore-clogging issues), polypropylene, and polyurethane (possesses non-biodegradable issue). Now with the advent of nanotechnology, many nanomaterials have been synthesized with better sorption capacities to clean up the oil spills. Some materials include membranes such as nanowire membranes (Yuan et al. 2008), aerogels such as nanocellulose aerogels (Juuso et al. 2011), sponges such as carbon nanotube CNT sponges and CNT/polydimethylsiloxane (PDMS) sponge (Wang and Lin 2013), and porous boron nanosheets (Lei et al. 2013). Many 3D graphene-based porous structures have been synthesized for use in oil and dye sorption studies, and energy storage devices. Here, in this article, we will concentrate and discuss the use of 3D graphene materials in sorption studies particularly related to oil spill cleanups and removal of dye and miscellaneous pollutants from water (see Tables 2 and 3).
Oil sorption studies
To control oil spills, various types of booms (floating nets/barriers) are used. Other methods to clean oil spills include mechanical cleaning by the use of skimmers (Broje and Keller 2006), microbial treatment which involves the degradation of oil by microorganisms (Zahed et al. 2010), by the use of chemical dispersants (usually applied for the use in aircraft or boats) which decrease the interfacial tension at the oil/water interface thereby dispersing the oil as fine droplets from the surface into the bulk water (Kujawinski et al. 2011), in situ burning of oil slicks/films (Buist et al. 2011), and by the use of magnetically driven floating foams (Calcagnile et al. 2012). Absorption is one of the most effective approaches to clean oil spills (Chu and Pan 2012). The reasons include low operational costs and ready availability of absorbent materials (Bayat et al. 2005). Graphene-based three-dimensional materials possess various suitable properties for oil cleanup, hence various works utilizing the benefits of this material for oil sorption are summarized in this section.
He et al. prepared reduced graphene oxide GO foams by three different freezing methods which were characterized using various techniques such as scanning electron microscopy (SEM), Fourier-transform infrared (FTIR) spectroscopy, and X-ray diffraction (XRD). The oil absorption capacity of reduced graphene oxide (RGO) foams was higher than GO foams. The absorption capacity values of RGO foams prepared by unidirectional freeze-drying method were more than 100 g/g for five tested oils (olive oil, lubrication oil, pump oil, diesel, petrol/gasoline). The highest value of 122 g/g for olive oils was obtained. The absorption capacity values obtained in this study are higher than previous studies, and therefore it can be concluded that RGO foams are good absorbents for organic liquids (He et al. 2013) (see Fig. 5).
Oil sorption of 3D graphene showing that it can selectively uptake up to 60–160 g/g of various oil and solvents from water for 50 cycles without significant change in sorption capacity. Reproduced from Liu et al. (2013). Copyright American Chemical Society 2013
Melamine sponges coated with graphene nanosheets and PDMS were prepared and the resulting graphene nanosheets coated with melamine sponges possessed superhydrophobic and superoleophilic properties. The sponges showed excellent absorption capacity from 54 to 165 times their weight for different oils (soybean oil, motor oil, pump oil, used pump oil). The water contact angle on graphene nanosheets was 132° which increased to 160° (a superhydrophobic material) on graphene-coated nanosponges. The absorption capacity for oils decreased by about 20 times their weight after the first cycle due to the presence of residual oils in the graphene-coated sponges which could not be removed by mechanical squeezing, but the absorption capacity for organic solvents (methanol, ethanol, acetone, hexane, DMF, chloroform) remained constant even up to five cycles. It was suggested that the prepared materials in this study can be used for the large-scale cleanup of organic pollutants and oil spills (Nguyen et al. 2012).
Zhao et al. reported the formation of a self-assembled 3D nitrogen-doped graphene-based framework which can be termed as a graphene aerogel. The material is ultralight (density about 2 mg/cm3 which is comparable to the density of air at 1.2 mg/cm3) with high absorption capacities of about 200–600 times its weight for oils (olive oil) and organic solvents (ethanol, DMSO, toluene, gasoline). The framework was prepared from graphite oxide and pyrrole C 4 H 4 NH. The presence of pyrrole prevents the re-stalking of graphene oxide layers leading to the formation of a porous network. The material possessed strong thermal stability and could sustain up to 600 °C in the air. Furthermore, it was suggested that material hopefully can be used as an electrode material for supercapacitors (Zhao et al. 2012b).
Polyurethane sponges coated with reduced graphene oxide were prepared. The characterization of these materials was done by instrumental techniques such SEM, XRD, and FTIR. The prepared sponges showed high absorption capacity for organic liquids from 80 to 160 g/g. Further excellent reusability was seen even after 50 cycles (Liu et al. 2013). A spongy graphene, SG, was prepared by the reduction of graphene oxide followed by shape molding. The SG was found to absorb petroleum products (pump oil, kerosene), fats (soybean oil, castor oil), and toxic organic solvents such as toluene and chloroform. The absorption efficiency was 20–86 times the weight of SG. In addition, the SG can be regenerated by heat to remove adsorbed materials and the regeneration of the material by heat treatment can also be performed 10 times. It was concluded that the method is low cost, safe, and possesses a high absorption capacity for various absorbates such as oils and organic solvents (Bi et al. 2012). Bi et al. (2014) reported the high performance for oil uptake on a spongy graphene sorbent. The absorption capacity of organic solvents was greatly enhanced as compared with previous studies. The absorption capacity of the material for chloroform reached almost 600 times its weight (Bi et al. 2014).
An independent graphene vessel has been developed for collection/suction and storage of the spilled oil in the vessel which works without the use of power. Here, reduced graphene oxide foam was coated by a self-assembly process, followed by annealing onto a copper mesh. The pores in the RGO foam suck oil by capillary action and then the suctioned oil flows into the vessel by gravity action. The material/vessel can confront a water head of 0.5 m and possessed excellent recyclability and chemical stability. The oil collection rate was about 20,000 l m2 h−1. It was suggested that this prototype of reduced graphene foam-coated vessel can be used to clean oil spills with speed and low cost (Kim et al. 2016).
The preparation of a polymer-based 3D material graphene foam was reported which was flexible with hydrophobic properties. The foam was prepared by the self-assembly of graphene sheets on a polymer skeleton. It was suggested that this material has potential to be used as an oil–water separator and as an elastic electric conductor (Wu et al. 2013b). A three-dimensional superwetting graphene mesh film on stainless-steel grids was synthesized. The material possessed unique adhesion force for liquids which can be used for the selective adsorption of oils or organic solvents from the water. Therefore, the prepared foam can be used to remove small quantities of oils and organic solvents for microanalysis of samples (Sun et al. 2013a). Superhydrophobic graphene foam was formed by thermal reduction of graphene oxide on melamine foam at the material possessed excellent absorption capacity for organic solvents and various oils. The preparation method can be readily scaled up because it uses low-cost materials and equipment. The prepared foam was robust and showed excellent recyclability (Zhu et al. 2015a).
Dye sorption studies
Dyes are of various types including acid or anionic water-soluble dyes (amaranth, orange G, eosin, India ink) and basic or cationic water-soluble dyes (rhodamine B). Acid dyes possess a negative charge while basic dyes possess a positive charge. Dyes are used in the textile dyeing, printing industry, hair dyeing, leather dyeing, and in staining of cells in microbiology and are produced at over 1 million tons per year. Adsorption is a preferred procedure for the removal of dyes from the wastewater because of the simplicity and the low cost of the operation and the relative ease of recycling of the adsorbent (Forgacs et al. 2004; Ip et al. 2010). Other techniques include coagulation by using iron and aluminum salts, biosorption by various agricultural wastes like rice husk, sugarcane bagasse, microbial degradation of dyes by fungi, etc. (Forgacs et al. 2004).
Ma et al. reported the formation of three-dimensional graphene oxide GO and reduced graphene oxide RGO gels, in which the GO sheets in the gel were strengthened by cross-linking with sodium alginate SA. The adsorption of methylene blue (MB) dye followed pseudo-second-order kinetics. The adsorption also followed the Langmuir and Freundlich isotherms, i.e., the adsorption of the dye gave a very good fit in both isotherms. The adsorption capacity for MB was almost 833 mg/g of GO-SA gel and 192 mg/g of reduced graphene oxide-SA gel. The adsorption of MB was a spontaneous process as indicated by thermodynamic parameters such as the negative value for ΔG 0 [ΔG 0 (kJ/mol) at 303 K, − 11.05 for GO/SA gel, and − 2.92 for RGO/SA gel]. It was suggested that the main interaction between cationic MB and negative (surface) oxygen-containing groups of graphene oxide was an electrostatic interaction (Ma et al. 2014). The sorption of various dyes by 3D graphene has been summarized in Table 3 and Fig. 6.
Sorption of various dyes by 3D graphene (a), methyl blue (b), methyl orange (c), orange G (d). Filtration of methyl blue through the 3D graphene and e kinetic sorption curves of methyl blue, methyl orange, and orange G reproduced from Zhang et al. (2015). Copyright Royal Society of Chemistry 2015
To remove organic dyes such as MB and rhodamine RhB from waste water, 3D reduced graphene oxide-based hydrogels were developed. The gels were prepared by the reduction of graphene oxide using sodium ascorbate. The toxicity tests were performed using bacterial cells such as Escherichia coli cells. The results showed that the quality of aqueous solution purified by these gels was comparable to distilled water. The dye capture or removal can be observed by the change in the color of the dye aqueous solution. The absorbance was noted at 664 nm for MB and at 554 nm for RhB. The adsorption isotherm plots (at 25 °C, pH 6.4, adsorption time 2 h) were obtained by plotting the amount of adsorbate adsorbed per unit weight of adsorbent (mg/g) and the concentration of adsorbate in the bulk solution (mg/l). The excellent removal abilities of dyes (about 100% for MB and about 97% for RhB) were observed and these were due to strong π–π interactions and anion–cation interactions. It was concluded that the prepared hydrogels have a mesoporous structure with a high surface area and a uniform pore size distribution. Further, it was found that the hydrogels can be recycled efficiently by the use of ethylene glycol (EG) solvent. It was suggested these gels can be used for the removal of organic dye pollutants (Tiwari et al. 2013).
Graphene oxide (GO)–polyethyleneimine (PEI) hydrogels (GEPMs) were prepared as dye adsorbents and gas (CO2) adsorbents. These materials were 3D and porous where PEI (with high amine density) was used as a cross-linking agent to interact with the GO sheets in water. GO possesses a high affinity to amines. The thermal stability of graphene oxide GO and GEPMs was studied by thermogravimetric analysis. These materials are lightweight (low density about 0.02 g cm−3) with a large pore volume and possess hierarchical morphology along with large specific surface area. The binding capacity of amaranth (acidic dye) was 0.8 g/g and of CO2 was about 11 wt.%. Therefore, it was suggested that these GEPMs can be used to remove waste dyes and CO2 greenhouse gas from the environment (Sui et al. 2013).
Liu et al. synthesized graphene oxide 3D sponge by centrifugation under vacuum from a graphene oxide aqueous colloidal suspension (10 mg/ml). The dye adsorption study on the prepared 3D GO sponge was conducted by measuring the absorbance at 630 nm for MB and at 584 nm for methyl violet (MV) at various time intervals based on Beer’s law to find the concentration of dyes. The adsorption capacities for MB and MV were 397 and 467 mg/g, respectively, and the adsorption process was completed in 2 min with a high efficiency of about 98% for the dyes. Furthermore, the adsorbed GO sponges can be quickly recovered by vacuum filtration. The mechanism of dye adsorption was investigated, and it was suggested that the strong adsorption between prepared graphene oxide and dyes was due to π–π and cation–anion interactions. The associated energy of activation for these interactions was about 50 and 71 kJ/mol, respectively, for MB and MV. The dyes have aromatic rings and cationic atoms (S+ in MB and N+ in MV) in their chemical structures and these (aromatic rings and cationic atoms) favor the adsorption of dyes on the surface of GO. It was concluded that the prepared 3D graphene oxide sponge possessed speed, capability, and efficiency to remove organic dyes (Liu et al. 2012).
Graphene oxide aqueous dispersion was reduced at 90 °C by the use of l-cysteine (weak reductant) to form graphene oxide hydrogel GH which on freeze drying gives graphene aerogel GA having a large specific area because of its highly porous structure and possessed high mechanical and thermal stability The adsorption capacity of GA was 660 mg/g for MB dye. Further, Pt nanoparticles were loaded in graphene hydrogel using l-cysteine as a reducing agent. The resulting product Pt NP-loaded GA was used as a catalyst in the reduction of p-aniline by NaBH4 (Zhang et al. 2015).
The magnetic hybrids composed of graphene oxide GO and Fe3O4 nanoparticles were prepared and were found to be highly efficient for the removal of dyes and other organic pollutants. Physical affinities exist between sulfonated GO and Fe3O4 nanoparticles; therefore, GO was sulfonated to enhance the (surface) affinity for iron nanoparticles. Three dyes, namely, anionic dye (methyl blue) and two cationic dyes (methylene blue and rhodamine B), were used in this investigation. The removal rate for methylene blue was about 100%, and it was suggested that the fabrication method can be extended to an industrial scale (Jiao et al. 2015).
Miscellaneous sorbent studies to remove environmental pollutants
Three-dimensional graphene (3DG) was obtained by ethylene diamine (EDTA)-induced self-assembly of graphene oxide at 95 °C. The prepared material possessed a better adsorption capacity (119 mg/g) for paraquat (a toxic herbicide) than activated carbon which is a conventional adsorbent. A nylon teabag was used to protect the soft hydrogel against the repeated adsorption–desorption processes (Huang et al. 2014).
The radioactive 137Cs levels increased after the Fukushima Daiichi Nuclear Power Plant accident in 2011 in Japan. There is a need to find a method which can be used to remove radioactive cesium from water. The radioactive isotope has a half-life of 30 years and emits beta particles and strong gamma rays which can cause an adverse effect to human beings. It was found that a porous 3D composite made of graphene foam and Prussian blue (blue-colored iron-containing pigment) can effectively remove 137Cs. The excellent adsorption efficiency probably was due to the large surface area of porous 3D composite material and ion exchange ability of the material for Cs+1 by K+1 ions. The adsorption capacity obtained was about 18 mg/g and the adsorption isotherm followed the Langmuir model (Jang et al. 2015).
The in situ co-precipitation of graphene oxide and Fe3+/Fe2+ results in the formation of magnetic graphene oxide (MGO). The material can be used as an adsorbent for disinfection by-product (DBP) precursors from water samples. DBPs such as trihalomethanes (chloroform, bromoform, bromochloromethane, dibromochloromethane) are formed when chlorine or other disinfectants are used to decrease microbial contamination in drinking water. DBPs also cause adverse health effects and their level should not exceed the maximum allowed annual average level in the drinking water. MGO can be regenerated by the use of ethanol and its removal efficiency is stable even after five cycles (Liu et al. 2011). In previous studies, magnetic graphene oxide composites have also been used to remove metal ions such as Cu2+ (Hu et al. 2013) and Co2+ (Liu et al. 2011), ionic dyes (Deng et al. 2013), and tetracyclines (Lin et al. 2013).
Yang et al. reported the formation of graphene-based coated silica particles (GCMs) for adsorption/removal of organic pollutants such as model organic pollutant (phenanthrene) from the water. Adsorption isotherms of phenanthrene on the graphene-coated materials with graphene oxide and reduced graphene oxide were made. The superior adsorption of GCMs was due to strong the strong π–π stacking interactions due to exposed graphene nanosheets (Yang et al. 2015a).
Multifunctional 3D graphene oil sorbents
The recent trend is to make multifunctional 3D oil sorbents by the surface modification/engineering of 3D graphene and use them in other applications such as photocatalysis along with sorption. Table 4 provides some examples for such oil sorbents.
Conclusion
In 2010, the first 3D graphene material was synthesized and its exceptional properties have resulted in tremendous research in the areas of electronics, photonics, and environmental remediation in the last 6 years. In this review article, it has been shown that 3D graphene is a promising sorbent material as it can overcome problems associated with the conventional sorbents such as insufficient sorption capacity and non-recyclability. Not only the works utilizing 3D graphene for sorption of oils and organic solvents have been reviewed but also the sorption of various dyes and miscellaneous pollutants has been covered to give the readers a broader perspective of this research and the significance of 3D graphene as next-generation sorbent material for environmental pollution remediation.
Despite the advantages, various challenges still exist in this field, which are highlighted here for future work. Although the sorption capacities of 3D graphene materials are higher than conventional sorbents, they are still lower than various other nanomaterials recently synthesized; therefore, further work is needed to enhance the capacity values of 3D graphene. Moreover, the rate of sorption is an important parameter which has been neglected in many studies so far; therefore, detailed kinetic studies using 3D graphene materials should be done for various pollutants separately to determine the removal rate and the kinetic parameters.
Porous properties can be more easily controlled in template-directed synthesis methods using templates of uniform pore sizes; however, the high cost of these CVD based processes makes them less favorable. Further work is needed in the solution-based synthesis of 3D graphene to understand all the parameters that affect the morphology of 3D graphene. An important challenge is to reduce the aggregation of graphene sheets as it reduces the surface area of the 3D graphene formed and lowers its sorption capacity. Various methods should be explored to enhance the surface area. For example, one such method is by the physical or chemical activation of graphene oxide sheets.
Another important scientific challenge in this research is the precise control of pore size distribution. Many other porous materials such as zeolites possess uniform pore size and shape and can act as molecular sieves that can exclusively separate various adsorbates based on their size; however, this is not the case for 3D graphene where its non-uniform morphology makes it impossible to selectively separate pollutant molecules based on their size.
Future work should also focus on properties of graphene oxide sheets used for the synthesis of 3D graphene and also the role of sheet size and geometric arrangement in the final structure need to be explored. Surface functionalities of graphene sheets usually make the graphene surface hydrophobic or hydrophilic; therefore, the effect due to nature of the graphene sheet surface in the final 3D structure should be quantified for the uptake of oils and non-polar organic solvents. Therefore, surface modification or functionalization of graphene sheets can pave the way to enhance capacity values for use in specific pollutants.
Most of the methods utilize harsh conditions of synthesis such as high temperature and pressure, suggesting the need to find safer, facile, and lower cost methods. Moreover, in the wet synthesis of 3D graphene, drying techniques such as freeze drying or supercritical drying and their specific operating conditions also affect its morphology, so further work in this direction can help to optimize these techniques for better sorption of pollutants. Future work should also investigate how to increase 3D graphene mechanical properties through making novel composite structures with other carbon nanomaterials or polymers so that these materials can withstand harsh environments.
References
Adebajo MO, Frost RL, Kloprogge JT et al (2003) Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J Porous Mater 10:159–170
Al-Majed AA, Adebayo AR, Hossain ME (2012) A sustainable approach to controlling oil spills. J Environ Manag 113:213–227. https://doi.org/10.1016/j.jenvman.2012.07.034
Al-Majed AA, Adebayo AR, Hossain ME (2014) A novel technology for sustainable oil spills control. Environ Eng Manag J 13:265–274
Ali Tahir A, Ullah H, Sudhagar P, et al (2016) The application of graphene and its derivatives to energy conversion, storage, and environmental and biosensing devices. Chem Rec 1591–1634. doi: https://doi.org/10.1002/tcr.201500279
Bai H, Li C, Wang X, Shi G (2011) On the gelation of graphene oxide. J Phys Chem C 115:5545–5551. https://doi.org/10.1021/jp1120299
Bartolomei M, Carmona-Novillo E, Hernández MI et al (2014) Penetration barrier of water through graphynes’ pores: first-principles predictions and force field optimization. J Phys Chem Lett 5:751–755. https://doi.org/10.1021/jz4026563
Bayat A, Aghamiri SF, Moheb A, Vakili-Nezhaad GR (2005) Oil spill cleanup from sea water by sorbent materials. Chem Eng Technol 28:1525–1528. https://doi.org/10.1002/ceat.200407083
Bhuyan MSA, Uddin MN, Islam MM et al (2016) Synthesis of graphene. Int Nano Lett 6:65–83. https://doi.org/10.1007/s40089-015-0176-1
Bi H, Xie X, Yin K et al (2014) Highly enhanced performance of spongy graphene as an oil sorbent. J Mater Chem A 2:1652–1656. https://doi.org/10.1039/C3TA14112H
Bi H, Xie X, Yin K et al (2012) Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv Funct Mater 22:4421–4425. https://doi.org/10.1002/adfm.201200888
Bonaccorso F, Lombardo A, Hasan T et al (2012) Production and processing of graphene and 2D crystals. Mater Today 15:564–589
Broje V, Keller AA (2006) Improved mechanical oil spill recovery using an optimized geometry for the skimmer surface. Environ Sci Technol 40:7914–7918. https://doi.org/10.1021/es061842m
Buist I, Potter S, Nedwed T, Mullin J (2011) Herding surfactants to contract and thicken oil spills in pack ice for in situ burning. Cold Reg Sci Technol 67:3–23. https://doi.org/10.1016/j.coldregions.2011.02.004
Calcagnile P, Fragouli D, Bayer IS et al (2012) Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 6:5413–5419. https://doi.org/10.1021/nn3012948
Cao N, Lyu Q, Li J et al (2017) Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem Eng J 326:17–28. https://doi.org/10.1016/j.cej.2017.05.117
Cao X, Zeng Z, Shi W et al (2013) Three-dimensional graphene network composites for detection of hydrogen peroxide. Small 9:1703–1707. https://doi.org/10.1002/smll.201200683
Carmalin Sophia A, Lima EC, Allaudeen N, Rajan S (2016) Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—a review. Desalin Water Treat 3994:1–14. https://doi.org/10.1080/19443994.2016.1172989
Chen Z, Ren W, Liu B et al (2010) Bulk growth of mono- to few-layer graphene on nickel particles by chemical vapor deposition from methane. Carbon 48:3543–3550. https://doi.org/10.1016/j.carbon.2010.05.052
Chen Z, Ren W, Gao L et al (2011) Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater 10:424–428. https://doi.org/10.1038/nmat3001
Chu Y, Pan Q (2012) Three-dimensionally macroporous Fe/C nanocomposites as highly selective oil-absorption materials. ACS Appl Mater Interfaces 4(5):2420. https://doi.org/10.1021/am3000825
Deng J, Zhang X, Zeng G et al (2013) Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem Eng J 226:189–200. https://doi.org/10.1016/j.cej.2013.04.045
Falcao EHL, Wudl F (2007) Carbon allotropes: beyond graphite and diamond. J Chem Technol Biotechnol 82:524–531. https://doi.org/10.1002/jctb
Fan X, Chen X, Dai L (2015a) 3D graphene based materials for energy storage. Curr Opin Colloid Interface Sci 20:429–438. https://doi.org/10.1016/j.cocis.2015.11.005
Fan Y, Ma W, Han D et al (2015b) Convenient recycling of 3D AgX/graphene aerogels (X = Br, Cl) for efficient photocatalytic degradation of water pollutants. Adv Mater 27:3767–3773. https://doi.org/10.1002/adma.201500391
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971. https://doi.org/10.1016/j.envint.2004.02.001
Gao X, Jang J, Nagase S (2010) Hydrazine and thermal reduction of graphene oxide: reaction mechanisms and design. J Phys Chem C 114:832–842
Georgakilas V, Perman JA, Tucek J, Zboril R (2015) Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev 115:4744–4822. https://doi.org/10.1021/cr500304f
Gui X, Wei J, Wang K et al (2010) Carbon nanotube sponges. Adv Mater 22:617–621. https://doi.org/10.1002/adma.200902986
Gupta S, Tai N-H (2016) Carbon materials as oil sorbents: a review on the synthesis and performance. J Mater Chem A 4:1550–1565. https://doi.org/10.1039/C5TA08321D
He Y, Liu Y, Wu T et al (2013) An environmentally friendly method for the fabrication of reduced graphene oxide foam with a super oil absorption capacity. J Hazard Mater 260:796–805. https://doi.org/10.1016/j.jhazmat.2013.06.042
Hou C, Zhang Q, Li Y, Wang H (2012) P25-graphene hydrogels: room-temperature synthesis and application for removal of methylene blue from aqueous solution. J Hazard Mater 205–206:229–235. https://doi.org/10.1016/j.jhazmat.2011.12.071
Hu XJ, Liu YG, Wang H et al (2013) Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite. Sep Purif Technol 108:189–195. https://doi.org/10.1016/j.seppur.2013.02.011
Huang S, Shi J (2014) Monolithic macroporous carbon materials as high-performance and ultralow-cost sorbents for efficiently solving organic pollution. Ind Eng Chem Res 53:4888–4893
Huang Y, Li C, Lin Z (2014) EDTA-induced self-assembly of 3D graphene and its superior adsorption ability for paraquat using a teabag. ACS Appl Mater {&} interfaces 6:19766–19773. https://doi.org/10.1021/am504922v
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Ip AWM, Barford JP, McKay G (2010) Biodegradation of reactive black 5 and bioregeneration in upflow fixed bed bioreactors packed with different adsorbents. J Chem Technol Biotechnol 85:658–667. https://doi.org/10.1002/jctb.2349
Jang S, Haldorai Y, Lee G, et al (2015) Porous three-dimensional graphene foam/Prussian blue composite for efficient removal of radioactive Cs. Nat Publ Gr 1–10. https://doi.org/10.1038/srep17510
Jiao T, Liu Y, Wu Y, et al (2015) Facile and scalable preparation of graphene oxide-based magnetic hybrids for fast and highly efficient removal of organic dyes. Sci Rep 1–10. https://doi.org/10.1038/srep12451
Juuso TK, Kettunen M, Ras RHA, Olli I (2011) Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl Mater Interfaces 3:1813–1816
Kim T, Lee JS, Lee G et al (2016) Autonomous graphene vessel for suctioning and storing liquid body of spilled oil. Sci Rep 6:22339. https://doi.org/10.1038/srep22339
Kujawinski EB, Kido Soule MC, Valentine DL et al (2011) Fate of dispersants associated with the Deepwater Horizon oil spill. Environ Sci Technol 45:1298–1306. https://doi.org/10.1021/es103838p
Lei W, Portehault D, Liu D et al (2013) Porous boron nitride nanosheets for effective water cleaning. Nat Commun 4:1777. https://doi.org/10.1038/ncomms2818
Lessard R, DeMarco G (2000) The significance of oil spill dispersants. Spill Sci Technol Bull 6:59–68. https://doi.org/10.1016/S1353-2561(99)00061-4
Li N, Zhang Q, Gao S et al (2013) Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci Rep 3:1604. https://doi.org/10.1038/srep01604
Lin Y, Xu S, Li J (2013) Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem Eng J 225:679–685. https://doi.org/10.1016/j.cej.2013.03.104
Liu F, Chung S, Oh G, Seo TS (2012) A three-dimensional graphene oxide nanostructure for fast and efficient dye removal. ACS Appl Mater Interfaces 4:1–2
Liu M, Chen C, Hu J et al (2011) Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J Phys Chem C 115:25234–25240
Liu W, Cai J, Li Z (2015a) Self-assembly of semiconductor nanoparticles/reduced graphene oxide (RGO) composite aerogels for enhanced photocatalytic performance and facile recycling in aqueous photocatalysis. ACS Sustain Chem Eng 3:277–282. https://doi.org/10.1021/sc5006473
Liu Y, Ma J, Wu T et al (2013) Cost effective reduced graphene oxide-coated polyurethane sponge as highly efficient and reusable oil absorbent. ACS Appl Mater Interfaces 5:10018–10026
Liu Z, Wang X, Luo Z, et al (2015b) Removing of disinfection by-product precursors from surface water by using magnetic graphene oxide. PLoS One 1–16. https://doi.org/10.1371/journal.pone.0143819
Loeblein M, Bolker A, Tsang SH et al (2015) 3D Graphene-infused polyimide with enhanced electrothermal performance for long-term flexible space applications. Small 11:6425–6434. https://doi.org/10.1002/smll.201502670
Luo J, Liu J, Zeng Z et al (2013) Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett 13:6136–6143. https://doi.org/10.1021/nl403461n
Ma T, Chang PR, Zheng P et al (2014) Fabrication of ultra-light graphene-based gels and their adsorption of methylene blue. Chem Eng J 240:595–600. https://doi.org/10.1016/j.cej.2013.10.077
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Mullin JV, Champ M a (2003) Introduction/overview to in situ burning of oil spills. Spill Sci Technol Bull 8:323–330. https://doi.org/10.1016/S1353-2561(03)00076-8
Nguyen DD, Tai N-H, Lee S-B, Kuo W-S (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci 5:7908. https://doi.org/10.1039/c2ee21848h
Process S, Cong H, Ren X et al (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced. ACS Nano 6:2693–2703. https://doi.org/10.1021/nn300082k
Qiu B, Xing M, Zhang J et al (2014) Mesoporous TiO2 nanocrystals grown in-situ on graphene aerogels for high photocatalysis and lithium ion batteries. J Am Chem Soc 136:5852–5855. https://doi.org/10.1021/ja500873u
Ratna PBS (2012) Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int J Environ Sci 3:940–955. https://doi.org/10.6088/ijes.2012030133002
Ren RP, Li W, Lv YK (2017) A robust, superhydrophobic graphene aerogel as a recyclable sorbent for oils and organic solvents at various temperatures. J Colloid Interface Sci 500:63–68. https://doi.org/10.1016/j.jcis.2017.01.071
Riaz MA, Hadi P, Abidi IH et al (2017) Recyclable 3D graphene aerogel with bimodal pore structure for ultrafast and selective oil sorption from water. RSC Adv 7:29722–29731. https://doi.org/10.1039/C7RA02886E
Rogowska J, Namie J (2010) Reviews of environmental contamination and toxicology volume 206. Springer, New York
Saleem J, Adil Riaz M, Gordon M (2018) Oil sorbents from plastic wastes and polymers: a review. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2017.07.072
Saleem J, Bazargan A, Barford J, McKay G (2014) Super-fast oil uptake using porous ultra-high molecular weight polyethylene sheets. Polym Adv Technol 25:1181–1185. https://doi.org/10.1002/pat.3376
Saleem J, Ning C, Barford J, McKay G (2015) Combating oil spill problem using plastic waste. Waste Manag 44:34–38. https://doi.org/10.1016/j.wasman.2015.06.003
Schulze R (1998) Oil spill response performance review of skimmers, ASTM International Volume 34
Sha J, Gao C, Lee S-K et al (2016) Preparation of three-dimensional graphene foams using powder metallurgy templates. ACS Nano 10:1411–1416. https://doi.org/10.1021/acsnano.5b06857
Shen Y, Li L, Xiao K, Xi J (2016) Constructing three-dimensional hierarchical architectures by integrating carbon nanofibers into graphite felts for water purification. ACS Sustain Chem Eng 4:2351–2358. https://doi.org/10.1021/acssuschemeng.6b00030
Sheng K, Xu Y, Li C, Shi G (2011) High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide. New Carbon Mater 26:9–15. https://doi.org/10.1016/S1872-5805(11)60062-0
Sui Z-Y, Cui Y, Zhu J-H, Han B-H (2013) Preparation of three-dimensional graphene oxide-polyethylenimine porous materials as dye and gas adsorbents. ACS Appl Mater Interfaces 5:9172–9179. https://doi.org/10.1021/am402661t
Sun H, Li A, Qin X et al (2013a) Three-dimensional superwetting mesh film based on graphene assembly for liquid transportation and selective absorption. ChemSusChem 6:2377–2381. https://doi.org/10.1002/cssc.201300319
Sun H, Xu Z, Gao C (2013b) Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv Mater 25:2554–2560. https://doi.org/10.1002/adma.201204576
Tiwari JN, Mahesh K, Le NH et al (2013) Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon N Y 56:173–182. https://doi.org/10.1016/j.carbon.2013.01.001
Wan W b, Yu S, Dong F et al (2016) Efficient C3N4/graphene oxide macroscopic aerogel visible-light photocatalyst. J Mater Chem A 4:7823–7829. https://doi.org/10.1039/c6ta01804a
Wang C, Lin S (2013) Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from. Water 8861–8864
Wang C, Yang S, Ma Q et al (2017a) Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon N Y 118:765–771. https://doi.org/10.1016/j.carbon.2017.04.001
Wang F, Wang Y, Zhan W et al (2017b) Facile synthesis of ultra-light graphene aerogels with super absorption capability for organic solvents and strain-sensitive electrical conductivity. Chem Eng J 320:539–548. https://doi.org/10.1016/j.cej.2017.03.082
Wu C, Huang X, Wu X et al (2013a) Mechanically flexible and multifunctional polymer-based graphene foams for elastic conductors and oil–water separators. Adv Mater 25:5658–5662. https://doi.org/10.1002/adma.201302406
Wu SY, An SSA, Hulme J (2015) Current applications of graphene oxide in nanomedicine. Int J Nanomedicine 10:9–24. https://doi.org/10.2147/IJN.S88285
Wu T, Chen M, Zhang L et al (2013b) Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J Mater Chem A 1:7612. https://doi.org/10.1039/c3ta10989e
Xia X, Chao D, Fan Z et al (2014) A new type of porous graphite foams and their integrated composites with oxide/polymer core/shell nanowires for supercapacitors: structural design, fabrication, and full supercapacitor demonstrations. Nano Lett 14:1651–1658. https://doi.org/10.1021/nl5001778
Xu X, Li H, Zhang Q et al (2015) 3D graphene/iron oxide aerogel elastomer deformable in a magnetic field. ACS Nano 9:3969–3977. https://doi.org/10.1021/nn507426u
Xu Y, Wu Q, Sun Y et al (2010) Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 4:7358–7362. https://doi.org/10.1021/nn1027104
Yang K, Chen B, Zhu L (2015a) Graphene-coated materials using silica particles as a framework for highly efficient removal of aromatic pollutants in water. Nat Publ Gr 5:1–12. https://doi.org/10.1038/srep11641
Yang Z, Chabi S, Xia Y, Zhu Y (2015b) Preparation of 3D graphene-based architectures and their applications in supercapacitors. Prog Nat Sci Mater Int 25:554–562. https://doi.org/10.1016/j.pnsc.2015.11.010
Ye S, Liu Y, Feng J (2017) Low-density, mechanical compressible, water-induced self-recoverable graphene aerogels for water treatment. ACS Appl Mater Interfaces 9:22456–22464. https://doi.org/10.1021/acsami.7b04536
Yuan J, Liu X, Akbulut O et al (2008) Superwetting nanowire membranes for selective absorption. Nat Nanotechnol 3(6):332. https://doi.org/10.1038/nnano.2008.136
Zahed MA, Aziz HA, Isa MH et al (2010) Optimal conditions for bioremediation of oily seawater. Bioresour Technol 101:9455–9460. https://doi.org/10.1016/j.biortech.2010.07.077
Żelechowska K, Kondratowicz I, Sadowski W (2014) 3D porous graphene-based structures—synthesis and applications. Carbon Nanotechnol 4438–4457. https://doi.org/10.1039/c3nr06858g
Zhang X, Liu D, Yang L et al (2015) Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J Mater Chem A 3:10031–10037. https://doi.org/10.1039/C5TA00355E
Zhao J, Ren W, Cheng H-M (2012a) Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J Mater Chem 22:20197. https://doi.org/10.1039/c2jm34128j
Zhao Y, Hu C, Hu Y et al (2012b) A versatile, ultralight, nitrogen-doped graphene framework. Angew Chem Int Ed Engl 51:11371–11375. https://doi.org/10.1002/anie.201206554
Zhu H, Chen D, An W et al (2015a) A robust and cost-effective superhydrophobic graphene foam for efficient oil and organic solvent recovery. Small 11:5222–5229. https://doi.org/10.1002/smll.201501004
Zhu H, Chen D, Li N et al (2015b) Graphene foam with switchable oil wettability for oil and organic solvents recovery. Adv Funct Mater 25:597–605. https://doi.org/10.1002/adfm.201403864
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Riaz, M.A., McKay, G. & Saleem, J. 3D graphene-based nanostructured materials as sorbents for cleaning oil spills and for the removal of dyes and miscellaneous pollutants present in water. Environ Sci Pollut Res 24, 27731–27745 (2017). https://doi.org/10.1007/s11356-017-0606-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0606-x