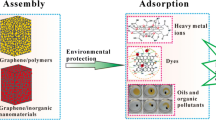
Abstract
Water pollution is a global environmental problem that affects the ecosystem severely. Treatment of oily wastewater and organic pollutants is a major challenge that waits to be solved as soon as possible. Adsorbing is one of the most effective strategies to deal with this problem. Three-dimensional (3D) porous adsorbents made of graphene or graphene-based nanomaterials skeletons had attracted more attention in wastewater treatment because of their large surface area, high porosity, low density, high chemical/thermal stability, and steady mechanical properties, which allow different pollutants to easily access and diffuse into 3D networks of adsorbents. This work presents an extensive summarization of recent progress in the synthesis methodologies and microstructures of 3D graphene foams and 3D graphene-based foams and highlights their adsorption performance for oils and organic solvents. Advantages and disadvantages of various preparation strategies are compared and the corresponded structures of these skeletons are studied in detail. Furthermore, the effects of the structures on oil-adsorption properties are analyzed and some data and parameters of the oil-adsorption properties are listed and studied for easier comparison. At last, the future research directions and technical challenges are prospected, which is hoped that the researchers will be inspired to develop the new graphene-based adsorbents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water contamination and related environmental concerns have become a hot issue in recent years. Water contamination is caused by dyes, oil spills, organic solvents, and heavy metals released by industry, as well as via changeable accidents. Among them, contamination caused by crude oil and toxic organic solvents (such as cyclohexane, benzene, dichloromethane, and toluene) poses a huge threat to marine ecosystems and economy(Schrope 2011). For example, the Gulf of Mexico oil spill in 2010 is a typical example, the largest ocean leak in history, which leaked 4.9 million pails of crude oil into the ocean, causing grave harm to fisheries, marine life, tourism, coastal wetlands, and so on (McNutt et al. 2012). The long-term environmental pollution caused by leakage of oils and organic solvents urgently requires the development of technologies and advanced materials to remove oil and water-insoluble organic pollutants from the surface of the water (Cheng et al. 2011; Li et al. 2013; Srinivasan and Viraraghavan 2010a; Toyoda and Inagaki 2000).
Currently, water pollution remediation methods are based on traditional artificial technologies such as in situ burning, mechanical method, adsorption, and bioremediation (Brakstad et al. 2018; Motta et al. 2018; Srinivasan and Viraraghavan 2010b; Wang et al. 2018a). Among these methods, in situ burning method can efficiently remove oil spills in a short time with low costs, but the gas released by combustion (CO, SO2) will cause threats to the environment. The mechanical method is less harmful to the environment. However, it relies heavily on specialized equipment and is more expensive. Bioremediation method uses microorganisms to decompose petroleum substances. Nevertheless, the speed of purifying water is slow and cannot meet emergency needs. The adsorption method removes and recovers oil by using porous adsorbent materials. It is considered to be one of the most promising methods because of its effectiveness, ease, and safety. Absorbents for oil removal involve inorganic mineral sorbents, natural organic sorbents, and synthetic polymers (Adebajo et al. 2003; Annunciado et al. 2005; Bandura et al. 2017; Bastani et al. 2006; Bayat et al. 2005; Doshi et al. 2018; Radetic et al. 2003). Inorganic mineral adsorbents, such as zeolites, perlite, graphite, clay minerals, and silica adsorbents, are widely investigated because of their low cost, easy availability, and non-flammability (Bandura et al. 2015). However, due to their lower buoyancy comparing with organic adsorbents and synthetic polymer adsorbents, the adsorption capacity is relatively low. Natural organic adsorbents such as straw, wood fiber, corn corb, and cotton fiber are easily available and environmentally friendly, but their poor hydrophobicity will cause these materials to absorb a large amount of water while absorbing oil. The synthetic polymers such as polyethylene, polypropylene, polyacrylate, polyurethane, and polystyrene have excellent adsorption capacity for spilled oil because of their excellent hydrophobicity and lipophilicity. The disadvantages are flame retardancy, non-biodegradability, and the possibility of returning the absorbed oil under external force. Therefore, developing new adsorbents that can reversibly and effectively remove oil spill pollutants and organic solvents is essential for future oil spill cleanup and water pollution remediation.
Three-dimensional (3D) spongy graphene has recently attracted enormous interests as a new type of sorbent with many excellent properties such as outstanding surface areas, highly porous structure, flexibility, highly hydrophobic/oleophilic, and remarkable chemical stability (Bi et al. 2012; Bolotin et al. 2008; Frank et al. 2007; Shen et al. 2015). The well-connected network and abundant pore structure of 3D graphene macrostructures provide sufficient transport channels which is beneficial to diffuse contaminants into the interior of the adsorbent. This also helps to promote the rate of diffusion, which leads to higher adsorption rates. In addition, the adsorption of contaminants on 3D graphene adsorbents has faster adsorption kinetics compared with the existing adsorbents (Chen et al. 2013; Wang et al. 2011; Xu et al. 2013).
The most primitive approach for preparing graphene is mechanical exfoliation, which is not applicable for industrial applications due to its low efficiency. Therefore, large-scale production of 3D graphene foams and its widespread distribution requires some alternative processes. In the past few years, various production methods had been exploited for the preparation of 3D graphene foam, including self-assembly and chemical vapor deposition (CVD) and other methods. Comparing with the mechanical exfoliation method, the production obtained by the CVD method exhibits a rich morphology and high conductivity. However, the growth of graphene is mainly concentrated on the outside of the catalyst, and the yield of graphene by this method is still not high. Self-assembly is a very promising technique for mass production of 3D graphene skeleton. During the use and recycling process, the release of pure graphene to the environment should be avoided, because of the lower toxicity of graphene to cells, animals, and plants. Modifying the structure of 3D graphene with functional materials is an effective way to solve the above problem and produce new types of 3D graphene-based foams. Although graphene-based adsorption materials are widely used in scientific research, there are still bottlenecks in large-scale industrial applications. Therefore, all of these techniques are calculated to find an optimum way for large-scale production. It is essential to contradistinguish these systems, analyze their advantages and disadvantages, and exploit a suitable method for industrial production of graphene materials with remarkable oil-adsorption properties and low costs.
Herein, this review summarized the synthesis methods of 3D graphene foams and 3D graphene-based foams. The relationship between the synthesis mechanism and adsorption properties of high-performance sponges is also elaborated. Finally, it briefly overviewed the current difficulties in assembly and application and looked forward to the future development prospects.
3D graphene foams obtaining methods
3D graphene foams with specific surface area and excellent porosity can generally be prepared by various procedures, including CVD, reduction self-assembly methods and others.
CVD
CVD is one of the most efficient techniques for synthesizing high quality 3D graphene sponges with controlled layer and size. In particular, the nanoporous graphene synthesized by the CVD method has the characteristics of high adsorption capacity, large specific surface area, large pore volume and small pore size as a nano adsorbent. In this way, three main components are required: carbon feedstocks, supporting template, and a heating source around 1000 °C within a controlled atmosphere. The supporting template types could be categorized into metal catalysts and non-metal catalysts.
Growth of graphene foam from metal catalysts
The metal catalysts are constituted by either Ni or Cu, deposited in a thin layer by electron beam evaporation (Chen et al. 2011b; Ito et al. 2014; Niu et al. 2012a; Singh et al. 2013; Wang et al. 2017c). The carburizing mechanism of preparing 3D graphene foam by CVD is mainly in accordance with that in growing graphene on Ni foils (Chen et al. 2011b). The oxide layer on the surface of Ni foam was removed by heating annealing at such short notice with the gas of hydrogen diluted with argon. During synthesis, a small quantity of methane was added into the reaction chamber at a constant rate, dissolving into Ni at 1000 °C. Subsequently, graphene foam was formed by a rapid convective cooling step of 100 °C min-1. Ni foam could be easily etched away via a hot HCl solution. Before removing Ni, polymer scaffolds are often needed to avoid the collapse of the graphene network (Chen et al. 2011b). The polymer scaffolds could be removed by acetone (Krueger et al. 2016). Poly (methyl methacrylate) (PMMA) is a typical template-support-sacrificial layer. The morphology of classical CVD-grown graphene foam was shown in Fig. 1. Such graphene foam had improved flexibility and mechanical strength due to the compact Ni foam scaffolds. The influencing factors of this process include carburization speed and cooling rate, which are relatively difficult to control. Therefore, some researchers had proposed using Cu as a template to grow graphene (Xia et al. 2017). Cu templates had a lower carbon saturation level and brilliant catalytic activity for graphene sponge growth. For example, Kim’s team used the CVD method to grow graphene on the 3D Cu foam and then spin-coated the PMMA layer on the 3D graphene/Cu sponge to preserve the 3D skeleton framework. The Cu foam was removed by chemical etching in an amount of 1 wt.% (NH4)2S2O8 solution and 3D graphene sponge was obtained (Kim et al. 2013). The growth mechanism of Cu is different from that of Ni foam. During the catalytic growth of graphene on Cu foam, carbon atoms adsorb on the Cu surface and crystallize to form graphene sponges. Therefore, when the Cu catalyst is used, the collapse of the graphene sponge could not be prevented. Additionally, metal oxide nanoparticles such as magnesium oxide, aluminum oxide, gallium oxide, and other oxides can be used as templates for individual 3D graphene foam formation (Bachmatiuk et al. 2013; Ning et al. 2017). Ning’s group reported 3D graphene capsules produced using the MgO-templated CVD process. They produced flexible 3D graphene capsules with surprising pore volume and good flexibility, which still exhibited high oil capacity (156 g/g) (Ning et al. 2017). There are some inherent disadvantages to using metal templates, including time-consuming template removal procedure and inevitable metal residue. In practical applications, cost issues should also be considered. Therefore, we should turn our attention to the development of low-cost and easily removable CVD growth templates for industrial production of the 3D graphene skeleton.
Characterization of graphene foam. a Image of graphene with scanning electron microscopy (SEM). b Raman spectroscopy indicates the structural composition of the foam (Krueger et al. 2016)
Growth of graphene foam from non-metal catalysts
In addition to Ni and Cu foams, graphene sponge can also be prepared on non-metal templates, such as silica and seashells. For example, Liu’s group found that annealing treated seashells exhibited a porous structure that provided a feasible template for the formation of 3D graphene foam. In this procedure, CVD can result in the preparation of graphene macroporous structure on a CaO porous framework which comes from CaCO3 calcination. Finally, etching CaO in dilute HCl and obtaining self-supporting 3D graphene foam after freeze-drying treatment (Shi et al. 2016). The seashell-derived 3D graphene foam had excellent electrical conductivity and mechanical flexibility. Figure 2 shown the fabrication process of 3D graphene sponge using a seashell template and the oil adsorption performance of 3D graphene sponges. Chen’s group used silicon dioxide as a template to grow high-quality graphene sponge by the CVD method after activating the growth substrates in the air at high temperatures (Chen et al. 2011a). 3D graphene foam prepared by this method has higher surface roughness, structural stability, and rich porosity, which make it the prominent candidate for oil-water separation. Unlike metal-catalyzed CVD growth, the method presented here is suitable for industrial production because of the low-cost and abundant nature of non-metallic templates and the high scalability.
Fabrication process of 3D graphene sponge using seashells template. a Diagram of the fabrication process of the graphene foam. b SEM image of the original seashells microstructure. c SEM image of calcined seashells at 1050 °C for 30 min in air. d Graphene-coated calcined seashells after CVD growth at 1020 °C for 30 min. e 3D graphene foam after removal of CaO skeleton. f Demonstration of methylene chloride adsorption underwater of graphene foam; g Adsorption kinetics of ethanol and water by graphene foam. h Adsorption capacities of different carbon-based materials for oil. i Adsorption capacity of various organic solvents and oils by graphene foams (Shi et al. 2016)
Reduction self-assembly methods
The most important precursor for the fabrication of 3D graphene foams is graphene oxide (GO). However, due to the presence of polar groups such as hydroxyl and carboxyl groups, the hydrophilic feature of GO limits its oil-adsorption capability seriously. It seems to be essential to reduce GO to superoleophilic- and superhydrophobic-reduced graphene oxide (rGO). Reduction self-assembly is a one-step method to prepare 3D oil-adsorption graphene foams by reducing and assembling GO simultaneously into ultra-light rGO foams with the help of crosslinking and reducing agents. The synthetic strategies can be roughly split into three categories, which are hydrothermal reduction, chemical reduction, and others.
Self-assembly via hydrothermal method
Hydrothermal method is one of the most facile, attractive, efficient, and green techniques for synthesizing 3D porous graphene foams, but the process requires high temperature (~ 90–200 °C), high pressure, and long reaction times (Bi et al. 2012; Zhao et al. 2012). During the hydrothermal synthesis, oxygen groups such as carboxyl and hydroxyl groups are eliminated gradually at the high temperature and the reduction of GO was performed (Garcia-Bordeje et al. 2018; Wu et al. 2016; Xu et al. 2010). At the same time, rGO sheets are assembled via π-π stacking at the graphene basal planar. For the first time, Zhao’s group (Zhao et al. 2012) fabricated self-assembled graphene foam with strong mechanical properties, tunable pore structure, and excellent surface properties via hydrothermal treatment. In addition, Zhao’s group found the connection between the structure of graphene foam and adsorption performance. The results showed that the adsorption performance of graphene foam on dyes has a great relationship with the surface charge concentration and electrostatic interaction, the adsorption capacity of oil is mainly dependent on the van der Waals interactions and specific surface area of graphene foam. In further work, Bi’s group annealed GO aerogel to synthesize graphene sponge with improved low density and absorption capability for oil removal (Fig. 3) (Bi et al. 2014). Bi’s group regulated the hydrophobicity using a flame soot on the outer surface of the graphene foam. The surface on graphene sponge produced a lotus leaf-like hierarchical carbon structure that significantly increased the absorption capacity of graphene sponge by 8 to 10 times that of previously reported graphene adsorbents. Figure 3a schematically shows the fabrication of graphene sponge. This process was improved to fabricate graphene sponge with high cavity content and lower density.
Images of the fabrication process of the graphene foam (Bi et al. 2014)
Self-assembly via chemical reduction
Chemical reduction is a facile, mild, and effective technique to prepare 3D graphene foams. The chemical reduction induced self-assembly of GO nanosheets usually occurs at atmospheric pressure below 100 °C without stirring, which is free from the harsh hydrothermal procedure (Ren et al. 2017; Tabish et al. 2018). For example, Liu’s group produced a highly compressed anisotropic graphene aerogel by reacting GO with ascorbic acid at 70 °C for 4 h, followed by directional freeze-drying (Liu et al. 2016). In this procedure, when the graphene foam was directional frozen, the ice crystals tend to grow in the elevation direction and remove the reduced GO flakes, which are sandwiched between adjacent ice crystals to constitute a 3D skeleton network. Graphene aerogel axial compression rebound tests demonstrated that directional freezing is beneficial to the mechanical properties of aerogels, which is closely related to the recovery performance of 3D oil-adsorption graphene foams. Due to its high porosity, brilliant flexibility, and good flame-resistance, the adsorbent had excellent recyclability for organic liquids and oils under adsorption combustion, adsorption distillation, and adsorption compression cycles.
In addition to ascorbic acid, phenolic acids and L-phenylalanine have also shown environment-friendly for reducing GO sheets (Dong et al. 2018; Wang et al. 2017b). Wang’s group heated the mixed solution of GO suspension and phenolic acids at 95 °C for 8 h to obtain graphene hydrogel, which was freeze-dried to fabricate graphene foam (Wang et al. 2012). Natural phenolic acids and GO sheets self-assembled under π-π interaction to prepare 3D graphene foam, which exhibited high porosity, electrical conductivity, superhydrophobicity, and high mechanical strength. Xu’s group heated the GO dispersion in an oil bath at 95 °C for 48 h in the presence of the l-phenylalanine solution to prepare graphene aerogel (Xu et al. 2015). The graphene aerogel had ultra-low density (5.0 mg cm-3), remarkable mechanical properties, large specific surface area, complete water repellency (water contact angle of 151.1°), and excellent recyclability. In addition, some crosslinking agents such as thiourea and ethylenediamine (EDA) can also gently reduce GO sheets and lead to self-assembly under low temperature and pressure. Li’s group used EDA acted as a crosslinker to reduce and self-assemble GO sheets in one step to prepare the highly compressive graphene aerogels (Fig. 4) (Li et al. 2014a). Due to the combination of hydrophobicity (water contact angle of 155°) and high porosity (~ 99.6%), aerogels had excellent absorptivity to various organic solvents and oils. Additionally, the absorbed oil in aerogel can be removed by the adsorption-extrusion process. This method of oil removal is fast and less energy consumption, which is more attractive in practical application.
a Images of the production of the graphene aerogel. b The recovery of the height of the aerogel in butanol. c Compressibility of the aerogel. d Fire-resistance of the aerogel (Li et al. 2014a)
Leavening method
Leavening is an effective method to convert dense graphene structures into 3D porous structures. It is well known that GO is quite unstable and often accompanied by the generation of gaseous CO2 and H2O during the reduction process. Therefore, if the gas released during the chemical reduction of GO can be integrated into the dense GO sheets, a porous graphene foam-like structure similar to “fermented bread” can be formed. For example, Niu’s group used small quantities of hydrazine to control the tightly packed graphite oxide films. They prepared flexible 3D graphene foam with amazing mechanical strength, open porous, and excellent capillary action, which made the foam a brilliant candidate for selective absorbers (Fig. 5) (Niu et al. 2012b).
Schematic diagram of the leavening process for preparing rGO foams (Niu et al. 2012b)
Oxidation and thermal reduction method
The above methods are all carried out under liquid phase conditions, while the oxidation and thermal reduction method is a solid phase procedure. 3D graphene foam produced by thermal exfoliation of graphite oxide possesses a smaller number of layers and high surface area. For example, Wang’s group placed graphite oxide powder in an airtight container at 1000 °C under a nitrogen atmosphere. The resulting 3D graphene sponge had very low bulk density, superhydrophobic-superoleophilic properties (Wang et al. 2014). In addition, the adsorption process is a combination of chemical adsorption and physical adsorption. The hydrophobic interaction of the surface of the 3D graphene adsorbents, the π-π bond, and the van der Waals interaction allow the oil molecules to adhere firmly to the surface of the foam.
3D graphene-based composite foam
Though 3D graphene foam is one of the most ideal oil absorbents at present, it is still confronted with many bottleneck problems, such as the fragile mechanical performance which restrict its cyclic utilization, poor linkage between graphene sheets, and weak interaction between graphene and oil molecules. Compounding inorganic nanoparticles or polymer molecular with graphene sheets or 3D graphene foams to synthesis 3D graphene-based composite foams were proved to be effective strategies to overcome the drawbacks mentioned above (Hu et al. 2018; Rahmani et al. 2018). The synthesis can be broadly categorized into dip-coating method, in situ self-assembly method and sol-gel method.
Dip-coating method
Dip coating is a convenient and inexpensive method, which use hydrophobic materials as a coating to deposit onto the desired matrices. In the dip-coating process, the shape and morphology of 3D graphene-based composite foams can be controlled. And the synthesized 3D graphene-based composite foams had excellent structural integrity and long-term stable performance. Typically, polyurethane (PU) foam and melamine foam have usually been applied as 3D porous substrates for the synthesis of graphene-based adsorbents. In this procedure, graphene was used for coatings on 3D polymer foam or sponge surfaces, which generally exhibited prominent oil spill cleanup due to their superior physical performances (Duc Dung et al. 2012; Liu et al. 2015b; Liu et al. 2013; Lv et al. 2019; Nguyen et al. 2012; Song et al. 2016; Tjandra et al. 2015; Wu et al. 2016; Xia et al. 2018; Zhou et al. 2016; Zhu et al. 2015b; Zhu et al. 2016).
Liu’s group reported graphene sponge prepared by using PU foam as substrates (Liu et al. 2013). After the dip-coating procedure, GO was coated on the foam and the GO was reduced with hydrazine at 80 °C for 1 h to form a robust superhydrophobic-superoleophilic coating. The porous structure of the PU, GO/PU, and rGO/PU foams was observed by SEM and the photos are shown in Fig. 6 (a–f). The same porous structure shows that the GO and rGO coatings have no effect on the porous structure of the PU sponge. The wall-surface morphologies of the GO/PU (Fig. 6d) and rGO/PU sponges (Fig. 6f) were full of wrinkles, which different from the surface of the PU sponge (Fig. 6b). The results showed that GO and rGO coatings are deposited uniformly on the surface of the PU sponge. The data in Fig. 6 g indicated that the absorption capacities of GO/PU foam was lower than rGO/PU sponge. There are two reasons for the phenomenon. The graphene coating on the PU sponge had higher porosity and fewer polar functional groups, which resulted in oleophilic, so it had a stronger affinity for oils and organic solvents. The recyclability of rGO/PU sponge was remarkable which the weight of the dry rGO/PU sponge did not change after reused for 50 times.
a, b SEM images of the pure PU sponge. c, d SEM images of the GO/PU sponge. e, f SEM images of the rGO/PU sponge. g Absorption capacity of the GO/PU and rGO/PU sponges for various organic liquids. h Recyclability of the rGO/PU sponge (Liu et al. 2013)
Melamine as a polymer template also enables graphene to achieve efficient oil absorption (Du et al. 2016; Ge et al. 2017; Ji et al. 2017; Peng et al. 2018; Song et al. 2016; Sun et al. 2019; Zhao et al. 2016). For example, Ji’s group dipped the melamine sponge into the GO dispersion for 12 h, then placed it in an oven to dry it and reduced it to melamine/graphene sponge (M/GS) at 160 °C (Ji et al. 2017). The hydrophobic graphene coating on the surface of the melamine sponge skeleton facilitates the formation of nanoscale roughness on the surface. Similar to above steps, M/GS was immersed in a homogeneous dispersion of carbon black to prepare melamine/graphene/carbon black composite sponges(M/G/CS). Figure 7 (a–f) demonstrated M/G/CS possessing rougher surface than melamine sponge and M/GS, which is beneficial to increase hydrophobicity. The super-hydrophobicity and microscale roughness of M/G/CS sponge were due to the its chemical composition and micro/nano structure, which resulted in high oil capacity (up to 50–130 times its weight for oils and organic solvents) and oil-water separation efficiency. Further work was studied by Sun’s group (Sun et al. 2019), fabricating a silver-reduced graphene oxide melamine sponge (Ag/RGO-MS) which exhibited prominent oil-water separation efficiency and bactericidal effects. As shown in Fig. 8, the pretreated melamine sponge was immersed in an Ag/RGO nanocomposite suspension for 24 hours. Then Ag/RGO-MS was dried for 2 hours at 180 °C in a vacuum oven. Ag/RGO-MS prepared by simple dip-coating method had hydrophobicity with water contact angle of 145°, rough surface, and high porosity. The sponge had strong adsorption capacity for various oils and organic solvents (26–49.2 g g-1), which also exhibited remarkable separation of oil-water and bacteriostatic effect. Moreover, graphene as a coating material can improve the hydrophobicity, lipophilic, and wettability of the adsorbent. But due to the weak mutual effect between polymer and graphene, separation occurs during the oil treatment process and result in degradation in oil absorption performance. The development of high-performance 3D graphene-based composite adsorbents which have strong physical adhesion between foam and graphene materials is still a challenge.
a-f SEM images of melamine sponge, M/GS, and M/G/CS. g Compressive stress-strain curves of M/G/CS at 60% strain. f Absorption capacity of M/G/CS for oil/water mixtures after different times (Ji et al. 2017)
a Schematic diagram of the synthesis processes of the functionalized Ag/RGO-MS. b Photographs of the processes of separating diesel from water using Ag/RGO-MS. c, d, e SEM images MS, RGO-MS, and Ag/RGO-MS. f, g Photographs of bactericidal effects of Ag/RGO-MS (Sun et al. 2019)
In situ self-assembly
In situ self-assembly is one of the most powerful methods for preparing free-standing 3D graphene-based adsorbents due to its facile and cost-effective procedure. In the assembling procedure, the 3D graphene skeleton would be modified by functional groups, metal oxides, heteroatom nanoparticles, or polymers (Bu et al. 2017; Chen et al. 2018; Chi et al. 2015; Eom et al. 2019; Hou et al. 2019; Hu et al. 2018; Kabiri et al. 2014; Li et al. 2014b; Rahmani et al. 2018; Wang et al. 2017a; Wang et al. 2015; Wang et al. 2018b; Wei et al. 2013; Yang et al. 2014). GO sheets contain a large amount of oxygen-containing functional groups, and the carboxyl group at the edge causes the GO to be negatively charged, which makes GO water soluble and easily chemical modified. As well, GO is considered as the optimum precursor for designing 3D graphene-based adsorbents for oil-water separation, due to these distinctive properties. During the self-assembly procedure, the removal of functional groups reduces the electrostatic repulsion between GO films. If the concentration of the starting GO can reach a certain critical value, these GO sheets could overlap each other to form a stable 3D skeleton frame. And appropriate alteration of graphene skeleton makes it exhibit water solubility and suitable surface properties, which can improve its adsorption capacity. Based on covered works, there are two primary ways to achieve the graphene-based composite foams with an ordered 3D network: (1) hydrothermal or solvothermal reduction and (2) cross-linking synthesis.
Hydrothermal or solvothermal method induced self-assembly
The 3D graphene skeleton can be decorated with functional materials by hydrothermal methods to improve performance, such as specific surface area, elasticity and oil absorption capacity (Li et al. 2013; Wan et al. 2016; Wu et al. 2019). For example, Hu’s group used 1H,1H,2H,2H-perfluorodecanethiol (PF) as additive to fabricate 3D amphiphobic graphene aerogel (Hu et al. 2018). Fluorographene precursor (F/rGO) was obtained by mixing the PF and GO dispersion and heat-treating them in a low temperature solvent at 50 °C for 24 h. Then the precursor was subjected to solution heat treatment at 90 °C for 3 h. The L-ascorbic acid (LA) was added to the solution and the reduction was continued for 96 h. Finally, the LA/F/rGO hydrogel production was prepared by freeze drying. The prepared 3D aerogel possessed excellent adsorption capacity (up to 20 times its original weight), excellent thermal stability and low density of 19 mg/ml. Due to the amphiphobic property of PF chains, the as-prepared LA/F/rGO hydrogel possessed amphiphobic surface properties, which made the hydrogel can selective separation/filtration of oil or water from their mixtures depending on the different pre-soak conditions. The illustration image is shown in Fig. 9. In another study, Li’s group used an improved hydrothermal reduction process to prepare 3D graphene-based aerogel decorated with powdered peanut hull (Li et al. 2019). Through this procedure, graphene could be assembled into a 3D skeleton structure with biocompatible molecules. Lignin, hemicellulose, and cellulose in peanut hull can improve the hydrophobicity and mechanical strength of 3D graphene-based aerogels. The addition of peanut shells made the graphene foams show excellent mechanical property (up to 2000 times its original weight), thermal stability, low density, remarkable adsorption capacity (32–79 g g-1), hydrophobicity, and lipophilicity.
a Diagram of the formation process for LA/F/rGO hydrogel. b Oil-water mixture separation process using different pre-soaking method (Hu et al. 2018)
Solvothermal method is an improved hydrothermal method to prepare 3D graphene-based skeleton. The solvents for this procedure could be N,N-dimethyl formamide (DMF), dopamine hydrochloride, ethanol and so on (Jayaramulu et al. 2017; Li et al. 2014b; Li et al. 2016; Liu et al. 2017; Pruna et al. 2019; Tran et al. 2015; Zhou et al. 2015). For example, Li’s group prepared a unique 3D graphene-based adsorbent by solvothermal reaction of GO sheets in DMF solution (Li et al. 2014b). The 3D graphene skeleton was functionalized by PVDF to prepare the superhydrophobic aerogels by a simple and low-cost technique. Meanwhile, the as-prepared foams exhibited high absorption capacity (20–70 g g-1) for oils and organic solvents, large specific surface area, outstanding water repellency (water contact angle is 153.6°) and superior absorption recyclability. In another study, Zhou’s group prepared polystyrene/Fe3O4/graphene hybrid sponge composites (CPFA) with high hydrophobicity (water contact angle above 140°), excellent compression ability, and strong magnetism (Zhou et al. 2015). Figure 10 illustrated the production process of CPFA systematically. In this work, polystyrene and porous Fe3O4 nanoparticles improved the compressibility of the graphene-based aerogel and enhanced the interconnection of graphene sheets, which made a huge oil adsorption capacity (40 g g-1). Beyond that, the addition of polystyrene microspheres and porous Fe3O4 nanoparticles allowed the graphene networks to form porous microporous substructures, which synergistically enhance hydrophobicity. Furthermore, the addition of porous Fe3O4 nanoparticles made magnetic possible, so that the oil-absorbing graphene aerogel can be easily gathered by the magnet and the oil can be easily extruded before the next cycle of operation.
Schematic illustration of the fabrication process of the CPFA (Zhou et al. 2015)
Chemical crosslinking induced self-assembly
Introducing chemical crosslinkers can enhance the interaction between graphene sheets and functionalize the 3D graphene surfaces (Alghunaimi et al. 2019; Cong et al. 2012; Eom et al. 2019; Kim 2016; Li et al. 2008; Stankovich et al. 2007; Zhan et al. 2018). In this way, even at very low concentrations, GO sheets can pass a series of supramolecular interaction. For example, Zhao’s group used thiourea as a crosslinking agent additive in the thermal reduction process to prepare 3D graphene foams with a maximum compressive stress of 14 kPa (Zhao et al. 2012). The addition of thiourea played an important role in the fabrication of porous and stable graphene foams. In the reduction process, thiourea decomposed into hydrogen sulfide, ammonia and other compounds, which could separate the GO sheets to create larger pores. In addition, the reaction of GO sheets with thiourea could form multiple new functional groups, such as amino (–NH2) and sulfonic acid (–SO3H), which could be combined with graphene surface operated to facilitate tougher inter-sheet cross-linking. And then, the GO sheets could be aggregated into 3D compact porous foams with brilliant structure stability. Due to the assistance of thiourea, the graphene foam exhibited adjustable surface properties and pore structure, which made high adsorption capacities for oils and organic solvents between 75 and 154 g g-1. Ha’s group used poly (acrylic acid) (PAA) as additive to produce the PAA/rGO networks with low density, excellent mechanical property (up to 10000 times its original weight), high porosity (> 99%), and prominent oil adsorption capacities (120 g g-1 for oils) (Ha et al. 2015). Figure 11 demonstrates the preparation process. Because of covalent interaction and hydrogen bonding, PAAs can shape into a filamentous dendritic structure on the surface of GO sheets, which is favorable to improve the overall robustness and stiffness of graphene-based foams.
a Illustration for process of preparing XPAA/rGO aerogels. b Scheme of proposed morphology of the XPAA/rGO aerogels. c Digital images showing the compressibility of 450 kDa/50 during the 10th compression/release cycle. d SEM images of PAA/GO (Ha et al. 2015)
Sol-gel method
The sol-gel method is an ideal synthesis approach for producing nano-modified graphene-based foams. This process is based on the hydroxylation and condensation of molecular precursors in solution to form nanoparticle sols. For example, Yang’s group used sol-gel method to combine silica nanoparticles with graphene sheets and subsequent modification using silane (Yang et al. 2017). In this work, graphene-based foam was synthesized by thermal treatment of GO sheets. The pH of the suspension of graphene and ethanol was adjusted to 11.5 with ammonium hydroxide. Then ethyl orthosilicate was slowly dropped into the above mixture and magnetic stirring was carried out after 12 h of reaction. Finally, it was washed with water and ethanol, then dried for 12 h to obtain graphene/SiO2 sample. Due to the combination of the super-oleophilic siloxane and the interconnected graphene skeleton, the adsorption capacity for organic solvents and oils were reinforced, ranging from 15 to 31 times of its own weight.
Adsorption behavior
The adsorption for various organic solvents and oils of 3D graphene-based adsorbents is a physicochemical process. In this procedure, pollutants such as oil molecules could be transferred from the liquid phase to the surface of the adsorbents. Moreover, the mutual effect between the adsorbents and the contaminants is essential to improve the adsorption performance (Dong et al. 2012; Hong et al. 2015; Zhu et al. 2015a). It is generally believed that 3D graphene adsorbents have interconnected 3D porous networks, extensive specific surface area and hydrophobicity, which promote the diffusion of oil droplet molecules into the inner of adsorbents and provide abundant adsorption sites [5]. For example, Liu’s group produced the 3D magnetic polymer-based graphene aerogel using dip-coating method (Liu et al. 2015a). The prepared graphene aerogel used PU sponge as the substrate and possessed large specific surface area and interconnect pore structure. The as-obtained aerogel exhibited superhydrophobicity and lipophilicity with the water contact angle of 158°. The graphene composites displayed high and rapid adsorption for lubricating oil, n-hexadecane, paraffin oil, peanut oil and hydraulic oil, and also had excellent adsorption capacities (the range of 9–27 times of its own weight). In addition, the strong hydrophobic π-π electron coupling/stacking interaction also played a considerable part in the adsorption process. For example, Li’s group reported a facile technique for the preparation of 3D graphene-polypyrrole adsorbent (Li et al. 2013). Due to the sp2 hybridized carbon atoms, there is a highly delocalized π electron-conjugated system on the external of graphene, which inclined to coupling/stacking with other σ or π electrons. As for aromatic hybrids such as benzene and toluene, π-π electron coupling/stacking interaction would be formed. As regards the hydrocarbons such as diesel and kerosene, the π electron on the 3D graphene-polypyrrole foam could couple with the σ electron on the absorbed molecules. Furthermore, the marked adsorption properties could be ascribed to the abundant adsorption active sites, porous structure, and specific hydrophobic surface area of rGO. Cao’s group fabricated 3D graphene/polydopamine aerogels, which modified by 1H,1H,2H,2H-perfluorodecanethiol and enhanced by chitosan, as the adsorbing material by a facile and environmentally friendly method (Fig. 12) (Cao et al. 2017). In this work, dopamine can self-polymerize into polydopamine on the graphene skeleton, and the abundant catechol and amino groups of polydopamine can provide active sites for adsorption. Rough surface and hydrophilic groups (–OH and –NH2) on chitosan made the aerogel superoleophobic underwater and superhydrophilic in air. In addition, layered porous structure also made the aerogel display highly adsorption capacity for various oils and organic pollutants (up to 12–21 times higher than its weight). The adsorption capacity of 3D graphene-based adsorbents is also affected by the C/O ratio. For example, Iqbal’s group prepared thermally reduced graphene (TRG) using oxidation and thermal reduction method. The results show that the C/O ratio can be used as an indicator of the hydrophobicity of TRG and has a great influence on the energy absorption (Iqbal and Abdala 2013).
a Schematic illustration of the preparation of chitosan/rGO composite aerogel (CGA) and polydopamine/chitosan/rGO composite aerogel. b The anti-oil-fouling and self-cleaning properties of CGA. c Underwater contact angle of CGA for various organic liquids and Oil adsorption capacity of fCGA aerogel (Cao et al. 2017)
The adsorption behavior of 3D graphene-based adsorbents on oil molecules often follows the intra-particle diffusion process. For example, Rahmani’s group prepared n-doped graphene foam through a hydrothermal method (Rahmani et al. 2018). In the beginning, the adsorption occurred in external surface, and at this stage the oil removal rate is higher. The second stage is the gradual adsorption process, the adsorption rate was intra-particle diffusion. At this stage, the adsorption sites decreased, and the adsorption rate increased slightly to a constant value (saturation point) which caused a low removal rate. Noteworthy, the synthesized n-doped graphene aerogel presented high adsorption capacity (210 times of its own weight) and maintained 95% of its original capacity after 10 pursuant cycles.
The performance of the 3D adsorbent can be evaluated by several criteria. Among them, the adsorption capacity is a key indicator for measuring the adsorbate uptake by unit mass of the adsorbent. The method of determining the adsorption capacity of graphene-based adsorbents generally follows: the initial mass of adsorbents are weighed and recorded as mbefore; then placed the adsorbents into the pollutant until saturated and weighed again which are recorded as mafter; the adsorption capacity is mafter minus mbefore and then divided by mbefore. Meanwhile, density, surface area and recycling generally provide benchmark to study the adsorption performance of 3D adsorbents. The adsorption performance of oils and various organic solvents onto 3D graphene foam and 3D graphene-based foam are shown in Table 1. Here, it can be seen that both 3D graphene foams and 3D graphene-based foams are effective adsorbents for removal organic pollutants and oils, exhibiting comparatively remarkable adsorption performance. However, pure 3D graphene foam is generally fragile due to the weak interaction between graphene sheets, which limilit its performance during the absorption and desorption process. The introduction of functional additive provides the 3D graphene-based foams with an ability to maintain the adsorption capability under improving mechanical strength and structural integrity.
Conclusion and outlook
A comprehensive review of the recent progresses in the characterization performance, synthesis, and basic research of 3D oil-adsorption graphene foams and 3D graphene-based composite foams were provided. Various strategies, such as CVD growth method, self-assembly method, oxidation and thermal reduction method, and dip-coating method, have been successfully developed to produce high-performance graphene foam adsorbents. Among these methods, CVD growth method can produce well-designed and large pore size network structures, and the quality of the adsorbents can be mainly controlled by process variables. Nevertheless, high temperature, sacrificial template, multi-step process, and high cost limit the scalability. For the dip-coating method, the combination of graphene sheets and polymer skeletons can achieve superhydrophobic surfaces. As the basic physical properties of the material, wettability will affect the adsorption performance of the oil-adsorbent. As for self-assembly method, the properties and performance of 3D graphene foams and 3D graphene-based composite foams are closely related to the size and quality of the GO precursor, the type of crosslinkers, doping agents, reducing agents, and the pH value of the synthetic mixture. Generally, pure 3D oil-adsorption graphene foam is fragile, while the mechanical properties of 3D graphene-based composite foam with functional additives prepared by self-assembly technology have been greatly improved. Referring to adsorption capacity, adsorbents prepared by the dip-coating method show the lowest adsorption capacity with the value ranges from several to several tens of times the weight of adsorbents. But for the self-assembled adsorbents, their oil absorption capacity can reach tens to hundreds of times the weight of adsorbents. CVD graphene skeletons have the largest oil adsorption capacity.
Regarding large scale production and practical applications, further efforts should be made to address the following challenges. Firstly, most 3D graphene-based foams discussed in this review are on a laboratory-scale. The quality of the production is largely dependent on the concentration of precursor and size of reaction vessels, which conduct the formation of ice crystal and the size of the foams. This problem must continue to be solved by future research which focuses on optimizing the cross-linking conditions and improving inter-sheet bonding. Secondly, most 3D graphene oil absorbents have high performance in absorbing low viscosity oils or organic solvents. Nevertheless, leaked crude-oils often have a high viscosity and enter the networks of the oil absorbents at a very slow velocity. In order to overcome the issue, increasing the pore channel and surface area of the adsorbents is worth further development. Finally, recycle for subsequent use is another major challenge in the application of 3D porous graphene skeleton, and despite reported elasticity and flexibility, most graphene foams are still brittle and easily broken if not handled carefully. Depending on the application, the introduction of highly elastic polymers and surface modification with functional materials should be an essential way to improve their recycle properties. In a word, there are many challenging tasks in front of us in preparing the next-generation of 3D graphene-based adsorbents.
Data availability statement
Some or all data, models, or code generated or used during the study are available in a repository or online in accordance with funder data retention policies.
References
Adebajo MO, Frost RL, Kloprogge JT, Carmody O, Kokot S (2003) Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J Porous Mater 10:159–170. https://doi.org/10.1023/a:1027484117065
Alghunaimi FI, Alsaeed DJ, Harith AM, Saleh TA (2019) Synthesis of 9-octadecenoic acid grafted graphene modified with polystyrene for efficient light oil removal from water. J Clean Prod 233:946–953. https://doi.org/10.1016/j.jclepro.2019.05.239
Annunciado TR, Sydenstricker THD, Amico SC (2005) Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar Pollut Bull 50:1340–1346. https://doi.org/10.1016/j.marpolbul.2005.04.043
Bachmatiuk A et al (2013) Few-layer graphene shells and nonmagnetic encapsulates: a versatile and nontoxic carbon nanomaterial. ACS Nano 7:10552–10562. https://doi.org/10.1021/nn4051562
Bandura L, Franus M, Jozefaciuk G, Franus W (2015) Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 147:100–107. https://doi.org/10.1016/j.fuel.2015.01.067
Bandura L, Woszuk A, Kolodynska D, Franus W (2017) Application of mineral sorbents for removal of petroleum substances: a review. Minerals 7. https://doi.org/10.3390/min7030037
Bastani D, Safekordi AA, Alihosseini A, Taghikhani V (2006) Study of oil sorption by expanded perlite at 298.15 K. Sep Purif Technol 52:295–300. https://doi.org/10.1016/j.seppur.2006.05.004
Bayat A, Aghamiri SF, Moheb A, Vakili-Nezhaad GR (2005) Oil spill cleanup from sea water by sorbent materials. Chem Eng Technol 28:1525–1528. https://doi.org/10.1002/ceat.200407083
Bi H et al (2012) Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv Funct Mater 22:4421–4425. https://doi.org/10.1002/adfm.201200888
Bi H, Xie X, Yin K, Zhou Y, Wan S, Ruoff RS, Sun L (2014) Highly enhanced performance of spongy graphene as an oil sorbent. J Mater Chem A 2:1652–1656. https://doi.org/10.1039/c3ta14112h
Bolotin KI et al (2008) Ultrahigh electron mobility in suspended graphene. Solid State Commun 146:351–355. https://doi.org/10.1016/j.ssc.2008.02.024
Brakstad OG, Lewis A, Beegle-Krause CJ (2018) A critical review of marine snow in the context of oil spills and oil spill dispersant treatment with focus on the Deepwater Horizon oil spill. Mar Pollut Bull 135:346–356. https://doi.org/10.1016/j.marpolbul.2018.07.028
Bu ZP, Zang LL, Zhang YH, Cao XJ, Sun LG, Qin CL, Wang C (2017) Magnetic porous graphene/multi-walled carbon nanotube beads from microfluidics: a flexible and robust oil/water separation material. RSC Adv 7:25334–25340. https://doi.org/10.1039/c7ra03910g
Cao N, Lyu Q, Li J, Wang Y, Yang B, Szunerits S, Boukherroub R (2017) Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem Eng J 326:17–28. https://doi.org/10.1016/j.cej.2017.05.117
Chen C, Li F, Zhang Y, Wang B, Fan Y, Wang X, Sun R (2018) Compressive, ultralight and fire-resistant lignin-modified graphene aerogels as recyclable absorbents for oil and organic solvents. Chem Eng J 350:173–180. https://doi.org/10.1016/j.cej.2018.05.189
Chen J et al (2011a) Oxygen-aided synthesis of polycrystalline graphene on silicon dioxide substrates. J Am Chem Soc 133:17548–17551. https://doi.org/10.1021/ja2063633
Chen Y, Zhang Q, Chen L, Bai H, Li L (2013) Basic aluminum sulfate@graphene hydrogel composites: preparation and application for removal of fluoride. J Mater Chem A 1:13101–13110. https://doi.org/10.1039/c3ta13285d
Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng H-M (2011b) Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater 10:424–428. https://doi.org/10.1038/nmat3001
Cheng M, Gao Y, Guo X, Shi Z, J-f C, Shi F (2011) A functionally integrated device for effective and facile oil spill cleanup. Langmuir 27:7371–7375. https://doi.org/10.1021/la201168j
Chi CX et al (2015) 3D hierarchical porous graphene aerogels for highly improved adsorption and recycled capacity. Mater Sci Eng B-Adv Funct Solid-State Mater 194:62–67. https://doi.org/10.1016/j.mseb.2014.12.026
Cong H-P, Ren X-C, Wang P, Yu S-H (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 6:2693–2703. https://doi.org/10.1021/nn300082k
Dong SY et al (2018) Controlled synthesis of flexible graphene aerogels macroscopic monolith as versatile agents for wastewater treatment. Appl Surf Sci 445:30–38. https://doi.org/10.1016/j.apsusc.2018.03.132
Dong X et al (2012) Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem Commun 48:10660–10662. https://doi.org/10.1039/c2cc35844a
Doshi B, Sillanpaa M, Kalliola S (2018) A review of bio-based materials for oil spill treatment. Water Res 135:262–277. https://doi.org/10.1016/j.watres.2018.02.034
Du QC et al (2016) A graphene-melamine-sponge for efficient and recyclable dye adsorption. RSC Adv 6:54589–54596. https://doi.org/10.1039/c6ra08412e
Duc Dung N, Tai N-H, Lee S-B, Kuo W-S (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci 5:7908–7912. https://doi.org/10.1039/c2ee21848h
Eom S, Kang DW, Kang M, Choe JH, Kim H, Kim DW, Hong CS (2019) Fine-tuning of wettability in a single metal-organic framework via postcoordination modification and its reduced graphene oxide aerogel for oil-water separation. Chem Sci 10:2663–2669. https://doi.org/10.1039/c8sc04581j
Frank IW, Tanenbaum DM, Van der Zande AM, McEuen PL (2007) Mechanical properties of suspended graphene sheets. J Vac Sci Technol B 25:2558–2561. https://doi.org/10.1116/1.2789446
Garcia-Bordeje E, Victor-Roman S, Sanahuja-Parejo O, Benito AM, Maser WK (2018) Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method. Nanoscale 10:3526–3539. https://doi.org/10.1039/c7nr08732b
Ge J et al (2017) Joule-heated graphene-wrapped sponge enables fast clean-up of viscous crude-oil spill Nature Nanotechnology 12:434-440. https://doi.org/10.1038/nnano.2017.33
Ha H, Shanmuganathan K, Ellison CJ (2015) Mechanically stable thermally cross linked poly (acrylic acid)/reduced graphene oxide aerogels. ACS Appl Mater Interfaces 7:6220–6229. https://doi.org/10.1021/acsami.5b00407
He Y et al (2013) An environmentally friendly method for the fabrication of reduced graphene oxide foam with a super oil absorption capacity. J Hazard Mater 260:796–805. https://doi.org/10.1016/j.jhazmat.2013.06.042
Hong J-Y, Sohn E-H, Park S, Park HS (2015) Highly-efficient and recyclable oil absorbing performance of functionalized graphene aerogel. Chem Eng J 269:229–235. https://doi.org/10.1016/j.cej.2015.01.066
Hou P et al (2019) Hollow carbon spheres/graphene hybrid aerogels as high-performance adsorbents for organic pollution. Sep Purif Technol 213:524–532. https://doi.org/10.1016/j.seppur.2018.12.032
Hu W et al (2018) An amphiphobic graphene-based hydrogel as oil-water separator and oil fence material. Chem Eng J 353:708–716. https://doi.org/10.1016/j.cej.2018.07.147
Iqbal MZ, Abdala AA (2013) Oil spill cleanup using graphene. Environ Sci Pollut Res 20:3271–3279. https://doi.org/10.1007/s11356-012-1257-6
Ito Y et al (2014) High-quality three-dimensional nanoporous graphene. Angew Chem-Int Edit 53:4822–4826. https://doi.org/10.1002/anie.201402662
Jayaramulu K, Geyer F, Petr M, Zboril R, Vollmer D, Fischer RA (2017) Shape controlled hierarchical porous hydrophobic/oleophilic metal-organic nanofibrous gel composites for oil adsorption. Adv Mater:29. https://doi.org/10.1002/adma.201605307
Ji CH et al (2017) High performance graphene-based foam fabricated by a facile approach for oil absorption. J Mater Chem A 5:11263–11270. https://doi.org/10.1039/c7ta02613g
Kabiri S, Tran DNH, Altalhi T, Losic D (2014) Outstanding adsorption performance of graphene-carbon nanotube aerogels for continuous oil removal. Carbon 80:523–533. https://doi.org/10.1016/j.carbon.2014.08.092
Kim B-J, Yang G, Park MJ, Kwak JS, Baik KH, Kim D, Kim J (2013) Three-dimensional graphene foam-based transparent conductive electrodes in GaN-based blue light-emitting diodes. Appl Phys Lett:102. https://doi.org/10.1063/1.4801763
Kim BS (2016) Characteristics of graphene production from graphite using plant extracts. Korean Soc Biotechnol Bioeng J 31:208–213
Krueger E et al (2016) Graphene Foam as a three-dimensional platform for myotube growth. ACS Biomater Sci Eng 2:1234–1241. https://doi.org/10.1021/acsbiomaterials.6b00139
Li D, Muller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3:101–105. https://doi.org/10.1038/nnano.2007.451
Li H, Liu L, Yang F (2013) Covalent assembly of 3D graphene/polypyrrole foams for oil spill cleanup. J Mater Chem A 1:3446–3453. https://doi.org/10.1039/c3ta00166k
Li J et al (2014a) Ultra-light, compressible and fire-resistant graphene aerogel as a highly efficient and recyclable absorbent for organic liquids. J Mater Chem A 2:2934–2941. https://doi.org/10.1039/c3ta14725h
Li N, Yue QY, Gao BY, Xu X, Su RD, Yu BJ (2019) One-step synthesis of peanut hull/graphene aerogel for highly efficient oil-water separation. J Clean Prod 207:764–771. https://doi.org/10.1016/j.jclepro.2018.10.038
Li R, Chen C, Li J, Xu L, Xiao G, Yan D (2014b) A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J Mater Chem A 2:3057–3064. https://doi.org/10.1039/c3ta14262k
Li Y, Zhang RF, Tian XK, Yang C, Zhou ZX (2016) Facile synthesis of Fe3O4 nanoparticles decorated on 3D graphene aerogels as broad-spectrum sorbents for water treatment. Appl Surf Sci 369:11–18. https://doi.org/10.1016/j.apsusc.2016.02.019
Liu C, Yang J, Tang YC, Yin LT, Tang H, Li CS (2015a) Versatile fabrication of the magnetic polymer-based graphene foam and applications for oil-water separation. Colloid Surf A-Physicochem Eng Asp 468:10–16. https://doi.org/10.1016/j.colsurfa.2014.12.005
Liu T, Huang M, Li X, Wang C, Gui C-X, Yu Z-Z (2016) Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids. Carbon 100:456–464. https://doi.org/10.1016/j.carbon.2016.01.038
Liu T, Zhao G, Zhang W, Chi H, Hou C, Sun Y (2015b) The preparation of superhydrophobic graphene/melamine composite sponge applied in treatment of oil pollution. J Porous Mater 22:1573–1580. https://doi.org/10.1007/s10934-015-0040-8
Liu Y et al (2013) Cost-Effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent. ACS Appl Mater Interfaces 5:10018–10026. https://doi.org/10.1021/am4024252
Liu Y, Xiang MH, Hong L (2017) Three-dimensional nitrogen and boron codoped graphene for carbon dioxide and oils adsorption. RSC Adv 7:6467–6473. https://doi.org/10.1039/c6ra22297h
Lv X, Tian D, Peng Y, Li J, Jiang G (2019) Superhydrophobic magnetic reduced graphene oxide-decorated foam for efficient and repeatable oil-water separation. Appl Surf Sci 466:937–945. https://doi.org/10.1016/j.apsusc.2018.10.110
McNutt MK et al (2012) Review of flow rate estimates of the Deepwater Horizon oil spill. Proc Natl Acad Sci U S A 109:20260–20267. https://doi.org/10.1073/pnas.1112139108
Motta FL, Stoyanov SR, Soares JBP (2018) Application of solidifiers for oil spill containment: a review. Chemosphere 194:837–846. https://doi.org/10.1016/j.chemosphere.2017.11.103
Nguyen DD, Tai NH, Lee SB, Kuo WS (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci 5:7908–7912. https://doi.org/10.1039/c2ee21848h
Ning G, Ma X, Wang M, Li Y (2017) High capacity oil adsorption by graphene capsules. Nanoscale 9:12647–12651. https://doi.org/10.1039/c7nr04383j
Niu Y, Zhao J, Zhang X, Wang X, Wu J, Li Y, Li Y (2012a) Large area orientation films based on graphene oxide self-assembly and low-temperature thermal reduction. Appl Phys Lett:101. https://doi.org/10.1063/1.4764549
Niu ZQ, Chen J, Hng HH, Ma J, Chen XD (2012b) A leavening strategy to prepare reduced graphene oxide foams. Adv Mater 24:4144–4150. https://doi.org/10.1002/adma.201200197
Peng M et al (2018) Superhydrophobic kaolinite modified graphene oxide-melamine sponge with excellent properties for oil-water separation. Appl Clay Sci 163:63–71. https://doi.org/10.1016/j.clay.2018.07.008
Pourmand S, Abdouss M, Rashidi A (2015) Fabrication of nanoporous graphene by chemical vapor deposition (CVD) and its application in oil spill removal as a recyclable nanosorbent. J Ind Eng Chem 22:8–18. https://doi.org/10.1016/j.jiec.2014.06.018
Pruna A, Carcel AC, Barjola A, Benedito A, Gimenez E (2019) Tailoring the performance of graphene aerogels for oil/organic solvent separation by 1-step solvothermal approach. Nanomaterials (Basel, Switzerland) 9. https://doi.org/10.3390/nano9081077
Radetic MM, Jocic DM, Jovancic PM, Petrovic ZL, Thomas HF (2003) Recycled wool-based nonwoven material as an oil sorbent. Environ Sci Technol 37:1008–1012. https://doi.org/10.1021/es0201303
Rahmani Z, Rashidi AM, Kazemi A, Samadi MT, Rahmani AR (2018) N-doped reduced graphene oxide aerogel for the selective adsorption of oil pollutants from water: isotherm and kinetic study. J Ind Eng Chem 61:416–426. https://doi.org/10.1016/j.jiec.2017.12.041
Ren RP, Li W, Lv YK (2017) A robust, superhydrophobic graphene aerogel as a recyclable sorbent for oils and organic solvents at various temperatures. J Colloid Interface Sci 500:63–68. https://doi.org/10.1016/j.jcis.2017.01.071
Schrope M (2011) Deep wounds. Nature 472:152
Shen Y, Fang QL, Chen BL (2015) Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection. Environ Sci Technol 49:67–84. https://doi.org/10.1021/es504421y
Shi L et al (2016) Scalable seashell-based chemical vapor deposition growth of three-dimensional graphene foams for oil-water separation. J Am Chem Soc 138:6360–6363. https://doi.org/10.1021/jacs.6b02262
Singh E, Chen Z, Houshmand F, Ren W, Peles Y, Cheng H-M, Koratkar N (2013) Superhydrophobic graphene foams. Small 9:75–80. https://doi.org/10.1002/smll.201201176
Song S, Yang H, Su C, Jiang Z, Lu Z (2016) Ultrasonic-microwave assisted synthesis of stable reduced graphene oxide modified melamine foam with superhydrophobicity and high oil adsorption capacities. Chem Eng J 306:504–511. https://doi.org/10.1016/j.cej.2016.07.086
Srinivasan A, Viraraghavan T (2010a) Oil removal from water by fungal biomass: a factorial design analysis. J Hazard Mater 175:695–702. https://doi.org/10.1016/j.jhazmat.2009.10.065
Srinivasan A, Viraraghavan T (2010b) Oil removal from water using biomaterials. Bioresour Technol 101:6594–6600. https://doi.org/10.1016/j.biortech.2010.03.079
Stankovich S et al (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565. https://doi.org/10.1016/j.carbon.2007.02.034
Sun SB et al (2019) A bifunctional melamine sponge decorated with silver-reduced graphene oxide nanocomposite for oil-water separation and antibacterial applications. Appl Surf Sci 473:1049–1061. https://doi.org/10.1016/j.apsusc.2018.12.215
Tabish TA, Memon FA, Gomez DE, Horsell DW, Zhang SW (2018) A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci Rep 8. https://doi.org/10.1038/s41598-018-19978-8
Tjandra R, Lui G, Veilleux A, Broughton J, Chiu G, Yu A (2015) Introduction of an enhanced binding of reduced graphene oxide to polyurethane sponge for oil absorption. Ind Eng Chem Res 54:3657–3663. https://doi.org/10.1021/acs.iecr.5b00748
Toyoda M, Inagaki M (2000) Heavy oil sorption using exfoliated graphite - new application of exfoliated graphite to protect heavy oil pollution. Carbon 38:199–210. https://doi.org/10.1016/s0008-6223(99)00174-8
Tran DNH, Kabiri S, Sim TR, Losic D (2015) Selective adsorption of oil-water mixtures using polydimethylsiloxane (PDMS)-graphene sponges. Environ Sci-Wat Res Technol 1:298–305. https://doi.org/10.1039/c5ew00035a
Wan WC, Zhang RY, Li W, Liu H, Lin YH, Li LN, Zhou Y (2016) Graphene-carbon nanotube aerogel as an ultra-light, compressible and recyclable highly efficient absorbent for oil and dyes. Environ-Sci Nano 3:107–113. https://doi.org/10.1039/c5en00125k
Wang C, Feng C, Gao Y, Ma X, Wu Q, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem Eng J 173:92–97. https://doi.org/10.1016/j.cej.2011.07.041
Wang CC, Yang SD, Ma Q, Jia X, Ma PC (2017a) Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon 118:765–771. https://doi.org/10.1016/j.carbon.2017.04.001
Wang F, Wang Y, Zhan WW, Yu SR, Zhong WH, Sui G, Yang XP (2017b) Facile synthesis of ultra-light graphene aerogels with super absorption crossManc capability for organic solvents and strain-sensitive electrical conductivity. Chem Eng J 320:539–548. https://doi.org/10.1016/j.cej.2017.03.082
Wang J, Shang L, Cheng Y, Ding H, Zhao Y, Gu Z (2015) Microfluidic generation of porous particles encapsulating spongy graphene for oil absorption. Small 11:3890–3895. https://doi.org/10.1002/smll.201500691
Wang J, Shi Z, Fan J, Ge Y, Yin J, Hu G (2012) Self-assembly of graphene into three-dimensional structures promoted by natural phenolic acids. J Mater Chem 22:22459–22466. https://doi.org/10.1039/c2jm35024f
Wang N, Zhang YC, Zhu FZ, Li JY, Liu SS, Na P (2014) Adsorption of soluble oil from water to graphene. Environ Sci Pollut Res 21:6495–6505. https://doi.org/10.1007/s11356-014-2504-9
Wang P, Wang W, Ci T, Li L, Han H (2018a) Pump-free oil droplet transfer by combining microfibre array and superoleophobic mesh. Appl Surf Sci 455:980–986. https://doi.org/10.1016/j.apsusc.2018.06.037
Wang SY et al (2017c) Novel flower-like graphene foam directly grown on a nickel template by chemical vapor deposition. Carbon 120:103–110. https://doi.org/10.1016/j.carbon.2017.04.010
Wang YK, Wang B, Wang JH, Ren YF, Xuan CY, Liu CT, Shen CY (2018b) Superhydrophobic and superoleophilic porous reduced graphene oxide/polycarbonate monoliths for high-efficiency oil/water separation. J Hazard Mater 344:849–856. https://doi.org/10.1016/j.jhazmat.2017.11.040
Wei G, Miao YE, Zhang C, Yang Z, Liu ZY, Tjiu WW, Liu TX (2013) Ni-doped graphene/carbon cryogels and their applications as versatile sorbents for water purification. ACS Appl Mater Interfaces 5:7584–7591. https://doi.org/10.1021/am401887g
Wu H, Wang Z-M, Kumagai A, Endo T (2019) Amphiphilic cellulose nanofiber-interwoven graphene aerogel monolith for dyes and silicon oil removal. Compos Sci Technol 171:190–198. https://doi.org/10.1016/j.compscitech.2018.12.017
Wu R et al (2016) One-pot hydrothermal preparation of graphene sponge for the removal of oils and organic solvents. Appl Surf Sci 362:56–62. https://doi.org/10.1016/j.apsusc.2015.11.215
Xia CB, Li YB, Fei T, Gong WL (2018) Facile one-pot synthesis of superhydrophobic reduced graphene oxidecoated polyurethane sponge at the presence of ethanol for oil-water separation. Chem Eng J 345:648–658. https://doi.org/10.1016/j.cej.2018.01.079
Xia DY et al (2017) Solvent-free process to produce three dimensional graphene network with high electrochemical stability. J Phys Chem C 121:3062–3069. https://doi.org/10.1021/acs.jpcc.6b10082
Xu J, Lv H, Yang S-T, Luo J (2013) Preparation of graphene adsorbents and their applications in water purification. Rev Inorg Chem 33:139–160. https://doi.org/10.1515/revic-2013-0007
Xu L, Xiao G, Chen C, Li R, Mai Y, Sun G, Yan D (2015) Superhydrophobic and superoleophilic graphene aerogel prepared by facile chemical reduction. J Mater Chem A 3:7498–7504. https://doi.org/10.1039/c5ta00383k
Xu Y, Sheng K, Li C, Shi G (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4:4324–4330. https://doi.org/10.1021/nn101187z
Yang S, Chen L, Mu L, Ma P-C (2014) Magnetic graphene foam for efficient adsorption of oil and organic solvents. J Colloid Interface Sci 430:337–344. https://doi.org/10.1016/j.jcis.2014.05.062
Yang S, Chen L, Wang C, Rana M, Ma P-C (2017) Surface roughness induced superhydrophobicity of graphene foam for oil-water separation. J Colloid Interface Sci 508:254–262. https://doi.org/10.1016/j.jcis.2017.08.061
Zhan W, Yu S, Gao L, Wang F, Fu X, Sui G, Yang X (2018) Bioinspired assembly of carbon nanotube into graphene aerogel with "cabbagelike" hierarchical porous structure for highly efficient organic pollutants cleanup. ACS Appl Mater Interfaces 10:1093–1103. https://doi.org/10.1021/acsami.7b15322
Zhang L, Li HQ, Lai XJ, Su XJ, Liang T, Zeng XR (2017) Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem Eng J 316:736–743. https://doi.org/10.1016/j.cej.2017.02.030
Zhao J, Guo QJ, Wang X, Xie HL, Chen YZ (2016) Recycle and reusable melamine sponge coated by graphene for highly efficient oil-absorption. Colloid Surf A-Physicochem Eng Asp 488:93–99. https://doi.org/10.1016/j.colsurfa.2015.09.048
Zhao J, Ren W, Cheng H-M (2012) Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J Mater Chem 22:20197–20202. https://doi.org/10.1039/c2jm34128j
Zhou S, Hao GZ, Zhou X, Jiang W, Wang TH, Zhang N, Yu LH (2016) One-pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil-water separation. Chem Eng J 302:155–162. https://doi.org/10.1016/j.cej.2016.05.051
Zhou S, Jiang W, Wang TH, Lu Y (2015) Highly hydrophobic, compressible, and magnetic polystyrene/fe3o4/graphene aerogel composite for oil-water separation. Ind Eng Chem Res 54:5460–5467. https://doi.org/10.1021/acs.iecr.5b00296
Zhu H, Chen D, Li N, Xu Q, Li H, He J, Lu J (2015a) Graphene foam with switchable oil wettability for oil and organic solvents recovery. Adv Funct Mater 25:597–605. https://doi.org/10.1002/adfm.201403864
Zhu HG et al (2015b) A robust and cost-effective superhydrophobic graphene foam for efficient oil and organic solvent recovery. Small 11:5222–5229. https://doi.org/10.1002/smll.201501004
Zhu HG et al (2016) A versatile and cost-effective reduced graphene oxide-crosslinked polyurethane sponge for highly effective wastewater treatment. RSC Adv 6:38350–38355. https://doi.org/10.1039/c6ra05450a
Funding
This work was supported by the China Postdoctoral Science Foundation (2018M640240 and 2019T120189).
Author information
Authors and Affiliations
Contributions
Dandan Weng: investigation, writing-original draft, conceptualization, and methodology. Leilei Song: conceptualization, supervision, writing-review, and editing. Wenxiao Li: Writing-original draft, investigation. Jun Yan: investigation, writing-review, and editing. Lei Chen: conceptualization, writing-original draft, writing-review, and editing, and supervision. Yong Liu: conceptualization, writing-original draft, writing-review and editing, and supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weng, D., Song, L., Li, W. et al. Review on synthesis of three-dimensional graphene skeletons and their absorption performance for oily wastewater. Environ Sci Pollut Res 28, 16–34 (2021). https://doi.org/10.1007/s11356-020-10971-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10971-1