Abstract
Considering the possible effects of polycyclic aromatic hydrocarbons (PAHs) on thyroid function, the current study aims to investigate the association of PAH urinary metabolites with the level of thyroid hormones in a sample of Iranian children and adolescents. This cross-sectional study was conducted from September 2015 to July 2016 in Isfahan, Iran. Participants were 150 students, aged 6–18 years, who were selected by multistage cluster random sampling from schools of Isfahan province. Blood and urine samples of participants were obtained for measurement of thyroid hormone levels (measured by immunoradiometric assay) and PAH urinary metabolites, including 1-hydroxynaphthalene, 2-hydroxynaphthalene, 9-hydroxyphenanthrene, and 1-hydroxypyrene. The association of serum thyroid-stimulating hormone (TSH) and PAH urinary metabolites was determined by correlation and regression analyses. Multivariate regression analysis revealed significant association between serum TSH and PAH urinary metabolites; this association remained significant after adjustment for gender and age. The corresponding figures were r = 0.85 for 1-naphthol, r = 0.86 for 2-naphthol, r = 0.87 for 1-hydroxypyrene, and r = 0.42 for 9-phenantrol, respectively, all p values < 0.001. The mean levels of 1-hydroxypyrene and 9-phenanthrol were higher in boys than those in girls (p < 0.05). The findings of this study indicated significant positive association between urinary PAH biomarkers and the TSH level in children and adolescents. It can be suggested that long-term exposure to PAHs might result in thyroid function impairment. The clinical implication of the current findings should be confirmed by future longitudinal studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thyroid diseases, mainly hypothyroidism, are of the most prevalent endocrine disorders in the pediatric age group. Hypothyroidism could be developed as either overt or subclinical forms with an estimated prevalence rate of 5.3 and 4.3%, respectively (Duntas 2002; Hollowell et al. 2002; LaFranchi 2011).
There are emerging evidences that exposure to environmental toxicants and environmental endocrine disruptors (EDCs) could have a causative role in the development of chronic diseases (Kelishadi and Poursafa 2014) including thyroid disorders (Maqbool et al. 2016; Schmutzler et al. 2007). Several studies indicated that EDCs could alter thyroid function by interfering with the function of thyroid hormone in different stages of thyroid hormone synthesis, release, transport, metabolism, and clearance (Schmutzler et al. 2007; Skakkebaek et al. 2011).
Polycyclic aromatic hydrocarbons (PAHs) are a family of EDCs that are produced through incomplete combustion of oil, fossil fuel, gas, coal, and waste and are also produced naturally from volcanoes and forest fires (Kataria et al. 2015). Exposure to PAHs could be through inhalation of cigarette smoke, vehicle exhaust, and processed fossil fuels, or through ingestion of grilled and charred foods, contaminated flour and bread products, processed and pickled foods, and contaminated water and milk. Other sources of exposure are air, water, or soil in the area near hazardous waste sites (Ramesh et al. 2004; Buratti et al. 2007). A common way of exposure to PAHs is by inhalation. Secondhand smoking is another source of exposure, especially in children. Among non-smokers, ingestion of the abovementioned foods is another important source (Ramesh et al. 2004; Buratti et al. 2007; ASTDR 2005).
It is suggested that PAH may accumulate in the body over time; it is well documented that these pollutants have several adverse health effects, including cancer, cardiovascular disease, peripheral arterial disease, and fatal ischemic heart disease (Moorthy et al. 2015; Xu et al. 2010; Xu et al. 2013; Burstyn et al. 2005). Some other reports exist on the harming effects of PAHs on the reproductive system as well as on their carcinogenic and mutagenic effects (Izawa et al. 2007; Wu et al. 2004; Hua et al. 2007).
A growing body of evidence exists on the effects of PAHs on the endocrine system, including thyroid function, but the exact underlying mechanisms remain to be determined. Some previous studies indicated that PAHs might interfere with thyroid hormones receptor (THR)-mediated transcription, and in turn, they could inhibit thyroid function (Sun et al. 2008). Some experimental studies reported that specific PAH components could change the activity of thyroid peroxidase (TPO) but its effect on human thyroid function is not clearly determined yet (Fowles et al. 2016; Paul et al. 2013; Brar et al. 2010). It might be because the thyroid hormone turnover kinetics in humans are very different (Fowles et al. 2016). Song and colleagues indicated that some of the PAH metabolites, mainly pyrene, benzofluoranthene, and benzopyrene, could alter thyroid function by inhibition of TPO (Song et al. 2012).
There are few studies about the effects of PAH on different disorders in pediatric age group. Recently, some studies in children and adolescents have documented the association between PAHs and obesity, oxidative stress, inflammation, and renal function (Scinicariello and Buser 2014; Farzan et al. 2016; Li et al. 2015).
Though, both in vivo and in vitro studies have documented the association between PAHs and thyroid function, but to the best of our knowledge, no previous study has been conducted in the pediatric population. Thus, it is suggested that PAHs, as an EDC, might be a potential risk factor for pediatric thyroid disorders. The harmful effects of PAHs in children and adolescents are of special concern because of the important role of thyroid hormone in their growth and development. The current study aims to investigate the association of PAH urinary metabolites with the level of thyroid hormones in a sample of Iranian children and adolescents.
Materials and methods
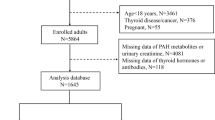
This cross-sectional study was conducted from September 2015 to July 2016 in Isfahan, the second largest and the most industrialized city in Iran. This study was conducted among school students, aged 6–18 years, who were selected by multistage cluster random sampling method from all schools of Isfahan province.
Students with a history of chronic disease, including thyroid disorders or using thyroid hormone medications, were not included.
Protocol of the study and details of the methods were described for all selected schoolchildren and their parents. Written informed consent was obtained from parents and oral assent from students. The study was approved by the regional Ethics committee of Isfahan University of Medical Sciences (Project No. 194274).
A pediatric endocrinologist examined all participants; a trained nurse recorded their demographic and anthropometric data. While one of the parents accompanied the students, blood and urine samples of all participants were obtained and examined in a referral laboratory for measurement of thyroid hormone levels and PAH urinary metabolites.
Laboratory measurements
Thyroid hormones
Venous blood samples were drawn from participants. The samples were centrifuged and stored at − 70 °C until analysis. Level of thyroxin (T4) and thyroid-stimulating hormone (TSH) were measured by radioimmunoassay (RIA) and immunoradiometric assay (IRMA), respectively, using Iran Kavoshyar diagnostic kits (Tehran, Iran). The normal range of T4 and TSH was considered as ≥ 10 μg/dL and ≤ 5 mU/L, respectively.
PAH urinary metabolites
Morning urine samples (10 mL) were collected for measuring PAH urinary metabolites. They were stored at − 80 °C until analysis in the laboratory of the Environment Research Center, Isfahan University of Medical Sciences.
We analyzed four hydroxylated urinary PAH metabolites, i.e., 1-hydroxynaphthalene (1-naphthol), 2-hydroxynaphthalene (2-naphthol), 9-hydroxyphenanthrene (9-phenanthrol), and 1-hydroxypyrene.
The indicators for quality assurance for measurement of the urinary PAH metabolites are presented in Table 3 of the Appendix section.
Sample preparation
We used methanol (Merck company, purity > 99.9%), dichloromethane (Merck company, purity > 99%), β-glucuronidase/arylsulfatase, and MSTFA (Sigma Aldrich company). Urine samples (3 mL) were aliquoted into test tubes. Sodium acetate buffer (0.1 M; pH 5.5; 5 mL) was added to each sample to adjust the pH for optimal deconjugation conditions for the enzyme. Samples were spiked with bisphenol A internal standard (10 μL, 200 pg/μL). Conjugates were hydrolyzed by adding β-glucuronidase/arylsulfatase (10 μL) to the samples followed by incubation (37 °C) for 17–18 h (overnight). Samples were mixed, allowed to equilibrate, then extracted on the RapidTrace® SPE workstation. Cartridges (focus 60 mg) were preconditioned with methanol (1 mL, 16 mL/min), followed by purified water (1 mL, 16 mL/min). Samples were added to the cartridge at 1 mL/min, rinsed using purified water (1 mL, 10 mL/min), and followed by methanol/sodium acetate buffer (3 mL, 4:6 by volume, pH 5.5, 10 mL/min). The sorbent was dried by applying a constant flow of nitrogen for 10 min to the cartridge and the final extract was eluted with dichloromethane (3 mL, 0.5 mL/min).
The sample extracts were evaporated using a gentle stream of nitrogen (5–10 psi, gradually increasing during evaporation) and a water bath (40 °C), then reconstituted with toluene (40 μL). Samples were derivatized to their trimethylsilylated derivative, prior to GC/MS measurement, by adding MSTFA (10 μL) and incubating (60 °C) for 30 min.
The extracts were analyzed by gas chromatography/mass spectrophotometry using a quadruple Agilent GC-MSD (Agilent Technologies, Palo Alto, CA, USA) model 7890A coupled to a mass selective detector model 5975C inert, operated in the electron-impact mode at 70 eV. Data recording and instrument control were performed by the MSD ChemStation software (G1701CA; version C.00.00; Agilent Technologies). Helium (99.999%) was employed as a carrier gas at the flow rate of 1 mL/min. The analytes were separated using a capillary column (HP-5, 30 m, 0.25-mm id., 0.25-μm coating thickness). The gas chromatographic conditions were as follows: injection volume, 2 μL; split ratio, 1:10; and injector temperature, 280 °C. The oven temperature was programmed from 100 °C (holding for 2 min) to 210 °C at 10 °C/min then to 250 °C at 5 °C/min and finally to 280 °C at 30 °C/min, keeping the final temperature for 4 min. The MS transfer line and ion source were kept at 280 and 230 °C, respectively. The MS was tuned to selective ion monitoring (SIM) mode with m/z 69, 219, and 502 for the electron impact (EI) corresponding to perfluorotetra butyl amine (PFTBA). Data acquisition was conducted in the selected ion monitoring (SIM) mode and results were qualified by comparison with the NIST and Wiley’s library spectral data bank (G1035B; Rev D.02.00; Agilent Technologies).
All samples were subsequently transferred to amber GC vials with 300-μL-fused inserts (Romanoff et al. 2006).
Statistical analysis
Quantitative variables are presented as mean (SD). Normality of data distribution was assessed by Kolmogorov-Smirnov test. Non-normal positive skewed data were subjected to logarithmic transformation. Independent sample t test was used for comparing measurements between groups.
Association between studying variables (PAHs metabolites and TSH) was evaluated using smoothing spline regression with linear trend. Data were analyzed using SPSS software (version 20.0, IBM, USA). p value of < 0.05 was considered as statistically significant.
Results
The study participants consisted of 150 students (46% boys) with mean (SD) age of 13.51 (3.11) years. Mean (SD) of age of boys and girls was 13.04 (2.75) and 13.9 (3.35) years, respectively (p = 0.08). Mean (SD) of BMI of boys and girls was 18.7 (1.23) and 18.9 (2.2) kg/m2, respectively (p = 0.08).
Mean (SD) of biochemical measurements including level of thyroid hormones and PAH urinary metabolites is presented in Table 1. Mean (SD) level of thyroid hormones was not different significantly among boys and girls (p > 0.05).
From urinary metabolites of PAH, mean level of 1-hydroxypyrene and 9-hydroxyphenanthrene was significantly higher in boys than that in girls (p = 0.01 and p = 0.03, respectively).
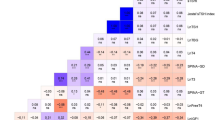
For evaluating the association of urinary metabolites of PAH with TSH levels, smoothing spline regression with linear trend was used to track the complex relationships; the regression coefficients after adjustment for the confounding impacts of sex and age showed significant positive association (Table 2) (p < 0.05). Figure 1 depicts the patterns of variation in THS levels in terms of metabolites of PAH levels. Among the examined different splines on study data, a dose-response linear relationship between continuous exposure (i.e., metabolites of PAH) and TSH levels only was statistically significant. Each presented regression coefficient in Table 2, indicating TSH increased per 1 unit increase in metabolites of PAH.
Discussion
In this study, the association between PAH urinary metabolites and thyroid hormone levels was determined in a sample of children and adolescents. Our findings revealed significant positive correlation between level of PAH metabolites and TSH in this group of population.
PAHs are considered as one of the most prevalent global environmental contaminants (Kataria et al. 2015). Available reports indicated that determination of PAH metabolites in different populations could reflect the degree of environmental exposure of this contaminant in various communities. PAH urinary metabolites are the best marker for evaluation of PAH exposure. The advantages of PAH urine metabolites are their high excretion quantities and ease of sample collection (Li et al. 2010; De Craemer et al. 2016; Chetiyanukornkul et al. 2006). The concentration of urinary PAH metabolites of children has significant correlation with levels of ambient PAH (Poursafa et al. 2017) (Table 3 of the Appendix section). Previous studies showed that a single urine sample could predict an individual’s chronic exposure (CDC 2005). In this study, we used a morning urine sample for PAH metabolite measurement.
The exact mechanism of action of PAHs on the thyroid gland is not well understood. It is suggested that they could impair thyroid function by interfering with thyroid gland function in its different stages of synthesis, transporting, and biological function (Izawa et al. 2007; Wu et al. 2004; Hua et al. 2007).
Limited evidences proposed that PAHs might be involved in a number of mechanisms. Some human and experimental studies showed that PAH metabolites, including pyrene, benzofluoranthene, and benzopyrene, could affect thyroid function by inhibition of TPO (Song et al. 2012; Fowles et al. 2016; Paul et al. 2013; Brar et al. 2010). Another experimental study showed that rats exposed to benzopyrene enhance biliary excretion of thyroxine glucuronide (Goldstein and Taurog 1968). An in vitro study showed that PAH metabolites interfered with THR-mediated transcription, and in turn, inhibition of thyroid function (Sun et al. 2008). 3-Methylcholanthrene, a carcinogenic PAH, was found to also affect thyroid function (Newman and Moon 1967).
All of the available human studies in this field were conducted in adult population.
A study in China investigated the relationship between PAH urinary metabolites and thyroid hormone levels among 480 Chinese adult men with non-occupational exposure. It showed significant association between 2-hydroxyfluorene and elevated levels of TSH; the corresponding figure was not statistically significant for other metabolites (Zhu et al. 2009).
Findings of the National Health and Nutrition Examination Survey in the USA documented significant association between nine PAH metabolites and thyroid function among the adult population. The feature of association was not similar in terms of gender. In females, levels of 1-hydroxynapthalene, 2-hydroxyphenanthrene, and 1-hydroxypyrene were associated with elevated levels of TT3. In males, levels of 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, and 9-hydroxypyrene were associated with decreased levels of free T4 (Jain 2016). They reported significant association between PAH metabolites and TT3 in women. They indicated significant association between the PAH metabolites and FT4 in men (Jain 2016).
To our knowledge, our study is the first to examine the association between PAH and thyroid hormones in children and adolescents. The results of this study indicated that from various urinary PAH biomarkers, the mean level of 1-hydroxypyrene and 9-hydroxyphenanthrene (9-P) was higher in boys than that of girls. The mean age and BMI of boys and girls were not different significantly, therefore the higher level of these metabolites is suggested to be because boys have more outdoor activities, thus their exposure to ambient pollutants would be higher.
In this study, multivariate regression analysis indicated positive significant correlation between mentioned biomarkers with level of TSH after adjustment for age and gender.
Our results were not in line with those of a study in China, which did not find any association between PAH metabolites and thyroid hormones in adult men. They showed significant association between 2-hydroxyfluorene (2-F) and high reference TSH levels (Zhu et al. 2009).
Considering the positive association between PAH metabolites and level of TSH, it seems that long-term and continuous exposure to PAH could impair thyroid function mainly hypothyroidism. It is suggested that similar to other EDCs, long-term stimulation of the thyroid gland by the PAHs could impair the thyroid function. Thus, it is recommended to plan longitudinal studies to obtain more precise results on the long-term effects of exposure to high levels of PAHs.
As all studied schoolchildren were evaluated during the school time and almost all of them did not change their lifestyles and environments over several months before sample collection, we suggest that their PAH exposure may not have fluctuated to great extent.
The limitations of the current study are the cross-sectional design of the study and the relatively small sample size of the studied population. In addition, due to lack of enough laboratory facilities, we could not measure one of the PAH urine metabolites, like 2-hydroxyfluorene, which might affect the thyroid function (Zhu et al. 2009). Further, considering the possible association of PAH and autoimmunity, it is also recommended to evaluate the association between PAHs and thyroid auto-antibodies.
The strength of our study was its novelty as the first study in this field among the pediatric population.
Conclusion
The findings of this study indicated a significant positive association between urinary PAH biomarkers and level of TSH in children and adolescents. Our results propose that long-term exposure to PAHs could result in thyroid function impairment. However, for obtaining more accurate results in this field, evaluation of other urinary PAH biomarkers as well as thyroid auto-antibodies in a larger sample size of children and adolescents is recommended.
References
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxicological profile for naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. ATSDR, Atlanta
Brar NK, Waggoner C, Reyes JA, Fairey R, Kelley KM (2010) Evidence for thyroid endocrine disruption in wild fish in San Francisco Bay, California, USA. Relationships to contaminant exposures. Aquat Toxicol 96:203–215. https://doi.org/10.1016/j.aquatox.2009.10.023
Buratti M, Campo L, Fustinoni S, Cirla PE, Martinotti I, Cavallo D et al (2007) Urinary hydroxylated metabolites of polycyclic aromatic hydrocarbons as biomarkers of exposure in asphalt workers. Biomarkers 12:221–239
Burstyn I, Kromhout H, Partanen T, Svane O, Langard S, Ahrens W et al (2005) Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 16:744–750
CDC (2005) Third national report on human exposure to environmental chemicals. Centers for Disease Control and Prevention, Atlanta http://www.cdc.gov/exposurereport/3rd/pdf/thirdreport.pdf
Chetiyanukornkul T, Toriba A, Kameda T, Tang N, Hayakawa K (2006) Simultaneous determination of urinary hydroxylated metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene as multiple biomarkers of exposure to polycyclic aromatic hydrocarbons. Anal Bioanal Chem 386:712–718
De Craemer S, Croes K, van Larebeke N, Sioen I, Schoeters G, Loots I et al (2016) Investigating unmetabolized polycyclic aromatic hydrocarbons in adolescents’ urine as biomarkers of environmental exposure. Chemosphere 155:48–56. https://doi.org/10.1016/j.chemosphere.2016.04.017
Duntas LH (2002) Thyroid disease and lipids. Thyroid 12:287–293
Farzan SF, Chen Y, Trachtman H, Trasande L (2016) Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003-2008. Environ Res 144:149–157. https://doi.org/10.1016/j.envres.2015.11.012.
Fowles JR, Banton MI, Boogaard PJ, Ketelslegers HB, Rohde AM (2016) Assessment of petroleum streams for thyroid toxicity. Toxicol Lett 254:52–62. https://doi.org/10.1016/j.toxlet.2016.05.001
Goldstein JA, Taurog A (1968) Enhanced biliary excretion of thyroxineglucuronide in rats pretreated with benzpyrene. Biochem Pharmacol 17:1049–1065
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA et al (2002) Serum TSH, T (4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499
Hua G, Broderick J, Semple KT, Killham K, Singleton I (2007) Rapid quantification of polycyclic aromatic hydrocarbons in hydroxypropyl-beta-cyclodextrin (HPCD) soil extracts by synchronous fluorescence spectroscopy (SFS). Environ Pollut 148:176–181
Izawa H, Kohara M, Watanabe G, Taya K, Sagai M (2007) Effects of diesel exhaust particles on the male reproductive system in strains of mice with different aryl hydrocarbon receptor responsiveness. J Reprod Dev 53:1191–1197
Jain RB (2016) Association between polycyclic aromatic hydrocarbons and thyroid function among males and females: data from NHANES 2007-2008. Int J Environ Health Res 20:1–15. https://doi.org/10.1080/09603123.2015.1135311.
Kataria A, Trasande L, Trachtman H (2015) The effects of environmental chemicals on renal function. Nat Rev Nephrol 11:610–625. https://doi.org/10.1038/nrneph.2015.94
Kelishadi R, Poursafa P (2014) A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care 44(3):54–72. https://doi.org/10.1016/j.cppeds.2013.12.005
LaFranchi S (2011) Disorders of the thyroid gland. In: Kliegman R, Behrman R, Jenson H, Stanton B (eds) Nelson textbook of pediatrics, 19th edn. Elsevier, Philadelphia, pp 557–561
Li Z, Mulholland JA, Romanoff LC, Pittman EN, Trinidad DA, Lewin MD et al (2010) Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. J Environ Monit 12:1110–1118
Li J, Lu S, Liu G, Zhou Y, Lv Y, She J et al (2015) Co-exposure to polycyclic aromatic hydrocarbons, benzene and toluene and their dose-effects on oxidative stress damage in kindergarten-aged children in Guangzhou, China. Sci Total Environ 524-525:74–80. https://doi.org/10.1016/j.scitotenv.2015.04.020
Maqbool F, Mostafalou S, Bahadar H, Abdollahi M (2016) Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci 145:265–273. https://doi.org/10.1016/j.lfs.2015.10.022
Moorthy B, Chu C, Carlin DJ (2015) Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci 145:5–15. https://doi.org/10.1093/toxsci/kfv040
Newman WC, Moon RC (1967) Altered thyroxine metabolism resulting from the chemical carcinogen 3-methylcholanthrene. Endocrinology 80:896–900
Paul KB, Hedge JM, Macherla C, Filer DL, Burgess E, Simmons SO et al (2013) Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology 312:97–107. https://doi.org/10.1016/j.tox.2013.08.006
Poursafa P, Amin MM, Hajizadeh Y, Mansourian M, Pourzamani H, Ebrahim K et al (2017) Association of atmospheric concentrations of polycyclic aromatic hydrocarbons with their urinary metabolites in children and adolescents. Environ Sci Pollut Res Int 24(20):17136–17144. https://doi.org/10.1007/s11356-017-9315-8
Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH (2004) Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol 23:301–333
Romanoff LC, Li Z, Young KJ, Blakely NC, Patterson DG Jr, Sandau CD (2006) Automated solid-phase extraction method for measuring urinary polycyclic aromatic hydrocarbon metabolites in human biomonitoring using isotope-dilution gas chromatography high-resolution mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 835:47–54
Schmutzler C, Gotthardt I, Hofmann PJ, Radovic B, Kovacs G, Stemmler L (2007) Endocrine disruptors and the thyroid gland–a combined in vitro and in vivo analysis of potential new biomarkers. Environ Health Perspect 115:77–83. https://doi.org/10.1289/ehp.9369
Scinicariello F, Buser MC (2014) Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001-2006). Environ Health Perspect 122:299–303. https://doi.org/10.1289/ehp.1307234.
Skakkebaek NE, Toppari J, Söder O, Gordon CM, Divall S, Draznin M (2011) The exposure of fetuses and children to endocrine disrupting chemicals: a European Society for Paediatric Endocrinology (ESPE) and Pediatric Endocrine Society (PES) call to action statement. J Clin Endocrinol Metab 96:3056–3058. https://doi.org/10.1210/jc.2011-1269
Song M, Kim YJ, Park YK, Ryu JC (2012) Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit 14:2121–2126. https://doi.org/10.1039/c2em30106g
Sun H, Shen OX, Xu XL, Song L, Wang XR (2008) Carbaryl, 1-naphthol and 2-naphthol inhibit the beta-1 thyroid hormone receptor-mediated transcription in vitro. Toxicology 249:238–242. https://doi.org/10.1016/j.tox.2008.05.008
Wu MT, Lee LH, Ho CK, SC W, Lin LY, Cheng BH et al (2004) Environmental exposure to cooking oil fumes and cervical intraepithelial neoplasm. Environ Res 94:25–32
Xu X, Cook RL, Ilacqua VA, Kan H, Talbott EO, Kearney G (2010) Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci Total Environ 408:4943–4948. https://doi.org/10.1016/j.scitotenv.2010.07.034.
Xu X, Hu H, Kearney GD, Kan H, Sheps DS (2013) Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci Total Environ 461–462:341–347. https://doi.org/10.1016/j.scitotenv.2013.04.089
Zhu P, Bian Z, Xia Y, Han Y, Qiao S, Zhao R et al (2009) Relationship between urinary metabolites of polycyclic aromatic hydrocarbons and thyroid hormone levels in Chinese non-occupational exposure adult males. Chemosphere 77:883–888. https://doi.org/10.1016/j.chemosphere.2009.08.054
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from parents and oral assent from students. The study was approved by the regional Ethics committee of Isfahan University of Medical Sciences (Project No. 194274).
Additional information
Responsible editor: Philippe Garrigues
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Kelishadi, R., Sobhani, P., Poursafa, P. et al. Is there any association between urinary metabolites of polycyclic aromatic hydrocarbons and thyroid hormone levels in children and adolescents?. Environ Sci Pollut Res 25, 1962–1968 (2018). https://doi.org/10.1007/s11356-017-0577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0577-y