Abstract
Cocoa production is affected by the black pod disease caused by several Phytophthora species that bring, about each year, an estimated loss of 44% of world production. Chemical control remains expensive and poses an enormous risk of poisoning for the users and the environment. Biocontrol by using antagonistic microorganisms has become an alternative to the integrated control strategy against this disease. Trichoderma viride T7, T. harzanium T40, and T. asperellum T54, which showed in vivo and in vitro antagonistic activity against P. palmivora, were cultured and mycelia extracted. Inhibition activity of crude extracts was determined, and then organic compounds were isolated and characterized. The in vitro effect of each compound on the conidia germination and mycelia growth of four P. palmivora, two P. megakaria, and one P. capsici was evaluated. T. viride that displayed best activities produced two active metabolites, viridin and gliovirin, against P. palmivora and P. megakaria strains. However, no activity against P. capsici was observed. Besides being active separately, these two compounds have a synergistic effect for both inhibitions, mycelia growth and conidia germination. These results provide the basis for the development of a low-impact pesticide based on a mixture of viridin and gliovirine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The phytopathogens from the genus Phytophthora are capable of causing enormous economic losses on crops worldwide. These Oomycetes are responsible for rot affecting woody ornamentals, vegetables (Kamoun et al. 2015), and trees, of which is Theobroma cacao.
Chocolate is produced from the beans of cocoa, cultivated over 700,000 km2 in most tropical regions of the world. It is a major cash crop worldwide, especially in some African countries where 71% of world production of cocoa beans is produced, where one third of world production is from Ivory Coast (ICCO 2014). Cocoa production is threatened by several diseases that entail severe yield losses in many areas. Black pod causing Oomycetes are, among others, Phytophthora palmivora (Africa and Cuba), P. megakarya (Africa), and P. capsici (Brazil and Mexico). Cocoa pods mature within 5 to 6 months following pollination. All the stages of pod development are susceptible to black pod disease.
Copper and metalaxyl-based fungicide chemicals are traditionally used to treat the disease. However, these negatively impact both environment and farmers’ health (Acebo-Guerrero et al. 2011). In addition, the extensive use of chemicals could enhance resistance of some Phytophthora. Cultural methods such as the removal of diseased pods that showed relative efficiency when associated with other control methods (Ndoumbe-Nkeng et al. 2004) or the use of resistant cocoa cultivars (Nyassé et al. 2007) were reported.
The biopesticides and the related management products provide an alternative to the use of pesticides and fungicides (Seiber et al. 2014) and meet good public compliance. The biological control of some Phytophthora sp. was developed using fungi (Segarra et al. 2013) and bacteria (Zhou et al. 2008, Lee et al. 2013). Two mechanisms have been reported in the control of Oomycetes: (1) through production of hydrolytic enzymes especially glucanases which are able to hydrolyze the cellulose of their cell walls (Valois et al. 1996, Mishra 2010) and (2) through antibiosis mediated by few compounds (Lumsden et al. 1992, Trejo-Estrada et al. 1998, Haesler et al. 2008, El-Hasan et al. 2009, Weisshoff et al. 2014, Takeuchi et al. 2015). However, only few examples concern black pod disease (Tondje et al. 2007, Hanada et al. 2009). Endophytic fungi Geniculospotium sp. and Trichoderma martiale isolated from healthy leaves of Cocoa and from sapwood in trunks showed a potential effect against P. megakaria (Tondje et al. 2006) and P. palmivora (Hanada et al. 2008), respectively. Recently, it has been shown that Trichoderma virens, endophyte of leaves and roots of cocoa, reduced Phytophthora lesions on pods and seedlings (Sriwati et al. 2015). It was also showed that Pseudomonas from T. cacao rhizosphere has antagonistic activity against P. palmivora (Acebo-Guerrero et al. 2015 ).
Previous studies assessed the biological control using fungi. Thus, collections of microorganisms from cocoa plantations were created and their ability to inhibit the growth of P. megakaria (Tondje et al. 2007) and P. palmivora (Mpika et al. 2009) were analyzed. Antagonist microorganisms were then identified as Trichoderma strains.
Fungi of the genus Trichoderma, known to produce secondary metabolites with a key role in antagonistic activities (Verma et al. 2007, Vinale et al. 2014), was used as biocontrol agents (Leng et al. 2011). Despite the effectiveness of Trichoderma sp., there are few reported studies on the secondary metabolites involved in the inhibition of the growth of P. palmivora and P. megakarya. Furthermore, for the use of microorganisms as biocontrol agents, there is a need to identify the active metabolites, including those with non-targeted effect (Brimner and Boland 2003). The aim of this study was to test the antibiosis mechanism and to identify the secondary metabolites involved in the biocontrol of P. palmivora by T. virens T7, T. harzianum T40, and T. asperellum T54 previously described by Mpika (Mpika et al. 2009). The activities of metabolites on other Phytophthora species from various countries were also studied.
Materials and methods
General
Semi-preparative HPLC was performed using Agilent Technologies 1260 Infinity system coupled to a diode array detector with Agilent XDB-C18 column (150 × 21.2 mm). Mass spectra were recorded on an API Q-STAR PULSAR i of Applied Biosystem. NMR experiments were recorded on Bruker Avance III HD 400 MHz spectrometer (Wissembourg, France) equipped with a BBFO Plus Smartprobe.
Microorganisms and culture medium
P. palmivora BL7/11-2 and Trichoderma species were provided by the National Center for Agricultural Research (CNRA) in Ivory Coast. Other Phytophthora strains were kindly provided by the French Agricultural Research Organization for Development (CIRAD, Montpellier, France) (Table 1). The strains were maintained on PDA at 4 °C (Trichoderma) or room temperature (Phytophthora). V8 agar medium was prepared from V8 vegetable juice concentrate (100 ml) in distilled water (1 l) containing CaCO3 (3 g) and agar (20 g). Pod glucose agar (PGA) was prepared from fresh pod (30 g l−1 mixed with blender), glucose (20 g l−1), agar (20 g l−1), and distilled water (qsp) 1 l.
Antagonist activity
All antagonist-pathogen combinations were examined on V8 agar medium in 10-cm Petri dishes. For dual cultures, a 5-mm agar plug of 5-day-old cultures of Phytophthora (on V8 agar) and Trichoderma isolates (PDA) was placed at a distance of 3 cm of each other. Phytophthora and Trichoderma isolates were inoculated the same day. For monocultures as controls, agar plugs of Phytophthora strains were inoculated in the center of Petri dishes. Petri dishes were incubated at 25 ± 2 °C. The growth of Trichoderma and Phytophthora spp. was surveyed daily for 7 days.
Production, purification, and characterization of secondary metabolites
Trichoderma strains were grown in Roux culture flasks containing 250 ml potato dextrose agar (PDA). Agar plugs cut from 7-day-old culture (PDA) were placed into 50 ml Falcon tube with sterile distilled water (25 ml). After stirring (Vortex) and filtration on sterile gaze, either sterile distilled water or additional agar plugs were added to obtain a concentration of 107 spores ml−1. Roux culture flasks were inoculated with 1 ml of these suspensions and incubated at 27 °C in dark for 5 to 15 days.
After growth, biomasses and agar were transferred in 2 l Erlenmeyer, and ethyl acetate (500 ml) was added and mechanically stirred for extraction of metabolites. After 1 h, suspensions were filtered and this was repeated twice. Organic phases were pooled, concentrated, dried (MgS04), and evaporated in vacuo at 35 °C. The red-brown residue (crude extract) was recovered and underwent various chromatographic separation techniques.
T. virens T7 metabolites
The crude extract (4.79 g) from cultures in 20 Roux flasks of T. virens T7 was dissolved in methanol and chromatographied on Sephadex LH20 using methanol as eluant to give a fraction 3 (2.6 g) with an inhibitory activity against P. palmivora. This fraction was subjected to column chromatography on silica gel by eluting with gradient of CH2Cl2/MeOH (98/2 to 50/50). Fractions showing similar thin-layer chromatography (TLC) profiles were combined. Five fractions were then collected. Fraction 3–1 was further separated using silica gel column chromatography, C6H12/EtOAc (60/40 to 40/60), to yield 130 mg of mixture of α-viridin 1a and β-viridin 1b. Fraction 3-2 was further purified by silica gel column chromatography, CH2Cl2/MeOH (98/2 to 50/50) to give heptelidic acid 3a (20 mg), gliovirin 2a (100 mg), pretrichodermamide A 2b (13 mg), trichodermamide A 2c (11 mg), and viridiol 1c (7 mg). Fraction F3-3 was fractionated on a Sephadex LH-20 column eluting with MeOH to produce two sub-fractions (F3-3-1 and F3-3-2). F3-3-1 was subsequently separated on semi-preparative HPLC (colomn Agilent XDB-C18, 150 × 21.2 mm; gradient H2O-TFA (0.1%) 100% to H2O-TFA (0.1%)/AcN 50/50) to obtain hydroheptelic acid A 3b (6 mg) and gliocladic acid 4 (3 mg). F3-3-2 was a mixture (11 mg) of trichoderonic acid A 5a and a new metabolite which was named trichoderonic acid C 5b (see Fig. S2 and S3).
Trichoderonic acid C: 1H NMR (400.13 MHz, CD3OD): 7,24 (1H, d, 3,75, H-11), 5,05 (2H, d, 1,86, H-3),; 4,47 (2H, d, 12,2, H-15), 3,6 (1H, d, 12,6, H-5), 2,7 (1H, m, H-10), 2,15 (1H, m, H-7b), 2,1 (1H, m, H-12), 2,09 (3H, s, H-17), 1,7 (1H, m, H-8b), 1,5 (1H, m, H-9), 1,4 (1H, m, H-8a), 1,4 (1H, m, H-7a), 0,99 (3H, d, 6,8, H-14), 0,92 (3H, d, 6,8, H-13). 13C NMR (75,03 MHz, CD3OD) 174.4 (C-4), 172.9 (C-16), 168.8 (C-1), 146.5 (C-11), 131.0 (C-2), 73.6 (C-6), 66.0 (C-3), 65.9 (C-15), 54.2 (C-5), 49.5 (C-9), 41.0 (C-10), 35.9 (C-7), 28.6 (C-17), 22.2 (C-8), 21.6 (C-14), 20.8 (C-12), 15.5 (C-13), HRESI-TOf [M + H]+ 341.16.
T. harzianum T40 metabolites
The crude extract (1.2 g) from cultures of 15 days in 12 Roux flasks of T. harzianum T40 was chromatographied on silica gel using cyclohexane-ethyl acetate gradient. The active fraction (735 mg) was dissolved in distilled water, acidified (pH 3) with hydrochloric acid 0.1 N, and extracted with ethyl acetate. The extract was purified by chromatography on LH 20 (MeOH) and harzianic acid 6 was obtained (25 mg).
T. asperellum T54 metabolites
The crude extract (3.4 g) from cultures of 10 days in 18 Roux flasks of T. asperellum T54 underwent column chromatography on silica gel, by eluting with gradient of CH2Cl2/MeOH (98/2 to 50/50), to give mainly gliovirin 2a (300 mg).
Evaluation of the growth inhibiting activity against Phytophthora species
Agar plugs (5 mm diameter) from 7-day-old cultures were centrally transferred in the plates containing V8 agar medium. Five microliters of DMSO solution of the crude extracts, chromatographic fractions and purified compounds at concentrations ranging from 0.156 to 5 mg ml−1 were applied on antibiogram paper disks (6 mm diameter) placed 1.5 cm near the agar plugs. All experiments were done in triplicate. The controls were obtained by applying 5 μl of DMSO. The plates were incubated at 27 °C for P. palmivora and at 25 °C for P. megakaria strains. The pathogen radial growth was measured daily and percentages of inhibition were obtained after 3 days of incubation from the following equation:
Evaluation of the anti germination activity
Phytophthora strains were grown on V8 agar medium during 7-day cultures in culture plates (9 cm diameter). Suspensions of sporangia were prepared from these cultures with PDB (3 × 3 ml) and were refrigerated (5 °C) for 25 min and incubated in the dark at 25 °C for 30 min to induce zoospore release (Nyadanu et al. 2009). Zoospore suspensions were adjusted to 2 zoospores μl−1 with PDB, and 100 μl of suspensions were introduced in each well of a 96-well cell culture plate. Viridin, gliovirin, or mixture were added in DMSO solution (2 μl) to obtain final concentrations of 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, 100, and 200 μg ml−1. Controls were incubated with DMSO alone. All experiments were done in triplicate. The plates were incubated in orbital shaker (200 rpm) at 25 °C. The germination rate was recorded at 24 and 48 h under a phase contrast microscope (Olympus BX41). A minimal inhibitory concentration (MIC) was defined as concentration for which no germination was observed.
Confrontation zones analysis
Co-cultures of T. virens T7 with P. megakarya NS 269 on V8 agar or P. palmivora TRI 1 on pod agar and T. asperellum T54 with P. megakarya NS 269 on V8 agar were performed as previously described. Before recovery, the agar from the confrontation zones was excised with razor blade and extracted with ethyl acetate. After drying (Na2SO4) and evaporation, the crude extracts were dissolved in MeOH, filtered and analyzed in LC/MS.
Statistical analysis of data
The data collected on the growth inhibition of Phytophthora strains were subjected to an analysis of variance using Statistica software version 7.1. In case of significant difference, the averages were compared according to the Newman-Keuls test at 5% threshold.
Results
Bioassays in Petri dishes
The antagonisms between Trichorderma sp. and P. palmivora were essayed by plate confrontation using PDA and PGA. In both cases, the three strains of Trichoderma showed an effect on the growth of P. palmivora. This effect was not observed when assays were performed in compartmentalized Petri dishes. In contrary, P. palmivora was inhibited by crude ethyl acetate extracts of cultures of three Trichoderma on PDA. These results suggested that the antagonistic effects involved non-volatile organic compounds, which can be purified as usual natural products.
Purification and identification of metabolites
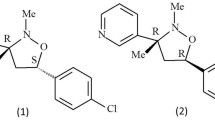
In preliminary study, T. virens T7 was cultured in PDB during 20 days and crude extract of culture medium showed an activity against P. palmivora. However, the chromatography failed in the purification of active compounds. The main isolated products were cyclo(L-Pro-L-Val) and cyclo(L-Pro-L-Tyr), for which toxic activity against P. infestans was reported (Puopolo et al. 2014). Then, in order to produce and purify the metabolites produced by the Trichoderma species, culture conditions were first investigated using T. virens T7. The most active crude extract was obtained on solid culture at 25 °C in the dark. After extraction of agar and mycelium, the metabolites (Fig. 1) were purified by flash chromatography, and their activity against P. palmivora BL7/11-2 was investigated.
The crude extract of T. virens T7 solid culture was partitioned on LH 20, giving five chromatographic fractions. Among them, fractions 2 and 3 were active against P. palmivora. Further purification of fraction 2 yielded α-viridin 1a and a mixture of α-viridin and β-viridin 1b. α-Viridin 1a is a known steroidal antifungal metabolite produced by Gliocladium and Trichoderma strains, first described in 1945 (Brian and McGowan 1945) while β-viridin 1b is described as a result of its isomerization (Grove et al. 1965) which can occur during chromatography on silica (Avent et al. 1992). Fraction 3 gave gliovirin 2a which is a diketopiperazine previously isolated from Gliocladium virens culture media (Stipanovic and Howell 1982) with an activity against P. ultinum and other Phytophthora sp. (Howell and Stipanovic 1983).
Further purification of fraction 4 allowed identifying four known metabolites. One was a reduction product of α-viridin, viridiol 2c, which was previously described as a phytotoxin (Howell and Stipanovic 1984) and having necrotic activity (Andersson et al. 2010). The second, heptelidic acid 3a, was a sesquiterpene produced by different strains of fungi isolated from soil samples (Itoh et al. 1980) and by fungal endophytes (Calhoun et al. 1992). It has been described with antimicrobial, against anaerobic bacteria (Itoh et al. 1980), and antiplasmodial (Tanaka et al. 1998) activities. This compound was inactive against P. palmivora.
The two other products were derived from gliovirin and previously isolated from Trichoderma sp. (Seephonkai et al. 2006), pretrichodermamide A 2b and trichodermamide 2c. In addition, four products were purified from fraction 5 by HPLC. Three were known metabolites, hydroheptelidic acid 3b (Calhoun et al. 1992), gliocladic acid 4 (Itoh et al. 1982), and trichoderonic acid A 5a (Yamaguchi et al. 2010) which was obtained in mixture with the new compound 5b. The molecular formula of 5b was determined to be C17H24O7 (m/z 363.14 found [M + Na]+) using HR-ESIMS spectrum. Compared to that of compound 5a, the 13C NMR spectrum of compound 5b showed one new carbonyl signal at δ 172.9 and one new methyl group at δ 28.6. These carbonyl (C-16) and methyl (C-17) groups were localized near C-15 on the basis of HMBC correlations between C-16 (δ 172.9) and H-15 (δ 4.47, d, J = 12.2 Hz) and H-17 (δ 2.09, s). Accordingly the C-16 and C-17 signals formed an acetyl group in compound 5c. This compound was identified as a new derivative named trichoderonic acid C.
The separation of T. harzanium T40 extract gave one active fraction. Purification of this fraction yielded harzianic acid. It has been previously described as having an activity against Pythium irregulare and having a plant growth promoting effect (Vinale et al. 2009). Since it had no activity against P. palmivora, it was concluded that the active compound of the extract may be present in a very small amount, at least difficulty isolatable in hour hand.
Purification of the active compound from T. asperellum T54 extract afforded gliovirin in a good yield (300 mg, 9% of the crude extract) obtained from 18 Roux flasks.
Growth inhibiting activity
The antibiotic activities of purified compounds against seven Phytophthora strains (four P. palmivora, two P. megakaria, and one P. capsici) were determined as inhibition diameters using antibiogram paper disks soaked with solutions of the compounds (Table 2). Among them, only viridin and gliovirin were active against Phytophthora strains. Viridin inhibited the growth of four P. palmivora and P. megakaria NS 269 when the disk was soaked with 25 μg of product but was inactive against other P. capsici. Gliovirin was comparatively more active as 30% inhibition was observed with disks soaked with 6.25 μg of compound. It was also active on the mycelia growth of both P. megakaria but inactive against P. capsici.
While Mpika et al. selected T. virens T7 with the best antagonistic effect on P. palmivora, we showed that this strain produced both viridin and gliovirin. In order to study a possible synergistic effect, experiments with mixtures of viridin and gliovirin were also conducted. The same amounts of both compounds were deposited on the disks. A slight increase in inhibition was observed for P. palmivora and P. megakaria spp., especially for P. palmivora NS 487. However, no effect was observed for P. capsici which remained resistant to all of these conditions.
Anti-germination activity
The activities of purified compounds against spore germination of P. palmivora strains were determined. Bioassays were performed in 96-well plates by incubation of Phytophthora zoospores in PDB medium containing variable concentrations of the products (0.097 to 200 μg ml−1). After 24 h, we observed that only viridin and gliovirin remained active. The minimum inhibitory concentrations (MICs) are reported in Table 3.
The results showed that activities were strongly dependent on the strains, and that viridin was more active than gliovirin. P. palmivora TRI 1 was the more sensitive to viridin with MIC ≤ 10 μg ml−1. Mixtures of viridin and gliovirin were also tested as anti-germinative. Both compounds were used at the same concentration. The results demonstrated that the activity of the mixture was much greater than each compound individually. Especially for P. palmivora NS 487, responding to the MIC of 50 μg ml−1 of viridin and 100 μg ml−1 of gliovirin, only 0.78 μg ml−1 of the mixture inhibited the germination. Moreover, the activity of the mixture was stable at 48 h, except in the case of P. palmivora BL7/11-2.
Since T. virens has been used for the biocontrol of plant pathogens, we also investigated the anti-germinative activity of these compounds against Botrytis cinerea and Fusarium oxyporum. Gliovirin exhibited no activity on these fungi while viridin showed a MIC of 25 and 12.5 μg ml−1 respectively, after 48-h incubation.
Detection of metabolites in the confrontation zones of fungal co-cultures
In order to verify the involvement of viridin and gliovirin in the antagonistic effect, Trichoderma and Phytophthora strains were cultured alone and in co-culture using Petri dishes containing V8 juice agar or cacao pod agar. In both media, the radial mycelial growth rates of fungi were fast and the colonies reached the edge of the plates after 5 days of inoculation. However, on V8 juice agar, colonies were compact and clearly visible (Fig. 2a) when on cacao pod agar, mycelia were thin and inconspicuous, although a significant sporulation of Trichoderma was observed (data no shown). In both culture media, T. virens T7 and T. asperellum T54 produced active metabolites. The growth of P. palmivora BL7/11-2 on cacao pod agar medium was inhibited by viridine and gliovirin (Fig. 2b). In the case of co-culture of T. virens T7 and P. palmivora TRI 1 on pod agar medium, LC/MS analysis showed the presence of gliovirin and viridin in the confrontation area before recovery.
Discussion
Trichoderma were selected because of their antagonistic effect on the growth of P. palmivora, a causative agent of cocoa black pod disease. The Trichoderma strains were isolated from soil samples of a cocoa plantation in Ivory Coast by trapping, by burying fragments of pods infected with P. palmivora (Mpika et al. 2009). Among the Trichoderma isolated by Mpika, the CNRA in Ivory Coast provided us with three species T. virens T7, T. harzanium T40, and T. asperellum T54, and with the P. palmivora strain used in the previous screening study. Additional Phytophthora strains were isolated in other countries by the CIRAD.
The purification of crude extracts produced 12 metabolites. Among them, one was a new compound and two were already known with antagonist effects against Phytophthora spp., except P. capsici, the causing agents of the cacao black-pod disease. Viridin was reported to prevent the germination of spores of several fungi such as B. allii, Colletotrichum lini, and F. caeruleum at low concentrations and other strains such as Penicillium expansum, Aspergillus niger, and Stachybotrys atra at higher concentrations (Reino et al. 2007). It was a better inhibitor of the growth of fungi than Pythium ultimum (Lumsden et al. 1992). Viridin was known for its antihelminthic activity (Bacikova et al. 1965) and its antiproliferative cytostatic effects (Smith et al. 2009). Our results showed that viridin was more active in inhibiting spore germination of P. palmivora strains in comparison to their mycelial growth.
Moreover, gliovirin, described to be selectively active against Oomycetes (Howell and Stipanovic 1983), presented a low antigerminative activity but strongly inhibited the growth of P. palmivora and P. megakarya. This may be due to the gliovirin mechanisms of action as previously evidenced to cause protoplasm coagulation in P. ultinum (Howell 1982). It was shown in the epidithiodiketopiperazines like gliotoxin that the presence of the disulfide bridge (Iwasa et al. 2011) could be one of the causes of their toxicity. Furthermore, pretrichodermamide A 2b, an hydrolyzed-epoxide form of gliovirin, was showed to be inactive against any Phytophthora species. Therefore, the activity of gliovirin on the Phytophthora species could be due to the presence of the epoxide rather than one disulfide bridge.
A synergistic effect of viridin and gliovirin, i.e., antigerminative and growth inhibiting activities, was underscored particularly in P. palmivora strains. We showed the production of both these compounds as well as the synergistic effect on microorganisms cultivated on pod glucose agar medium. Similar synergies were already mentioned by previous studies, involving either two products of biological control agent like secondary metabolites and a hydrolytic enzyme (Dipietro et al. 1993, Saravanakumar et al. 2016) or one antifungal chemical and a biological control agent (Howell 1991). Here, we showed for the first time a synergetic effect against Oomycetes between two metabolites produced by one microorganism. This effect of both gliovirin and viridin not only resulted in significantly higher activities but also in the stability of activities in a long run. This latter point was important for assessing the antigerminative activity.
It is known that the biotransformation of toxic molecules is a strategy to overcome their toxicity. Furthermore, a loss of activity of gliovirin in a non-sterile soil was demonstrated, while it was preserved in a sterile soil, suggesting a biodegradation of the active molecule (Howell and Stipanovic 1983). Such biotransformations could involve the reduction of viridin into viridiol and the hydrolysis of the epoxide of gliovirine leading to inactive metabolites. We also pointed out that the growth of both tested P. megakarya was not inhibited in the same manner by gliovirin, but identical inhibitions (40%) were observed when treated by mixture of compounds. Further studies on the biotransformation of both compounds by phytopathogens may be necessary to explain these results.
T. virens strains are separated into two groups, “P” and “Q”, on the basis of their secondary metabolites (Howell et al. 1993). The P-strains produce gliovirin and heptelidic acid and are ineffective biocontrol agents of seedling disease in cotton (Howell and Puckhaber 2005). The Q-strains, which are effective biocontrol agents of cotton seedling disease, produce gliotoxin but not gliovirin and heptelidic acid. In summary, T. virens T7 isolated by Mpika et al. belongs to the P group. In accordance with the synergistic effect showed in this study, the production of both the gliovirin and viridin explains the high activities observed against P. palmivora. Furthermore, as genes involved in the biosynthesis of secondary metabolites are usually in cluster, their expression strongly depends on the environmental factors. Our study indicated that viridin and gliovirin were co-produced by T. virens T7 within near-natural conditions to reach higher activity against the phytopathogenic competitors such as Phytophthora species.
Finally, our analysis on Phytophthora species from various parts of the world highlights the precautions for the use of Trichoderma species in the control of black pod disease of cocoa. Since Trichoderma strains used in the present work are not effective against P. capsici, they cannot be used as biocontrol agents in Brazil where cocoa is mainly infested by this pathogen. However, promising results were recently obtained with other strains of Trichoderma (Bae et al. 2016) and with Streptomyces sp. (Chen et al. 2016) as biological control agents against P. capsici on pepper and tomato plants. Likewise, in Cameroon, P. megakarya is the most abundant and its control could be achieved with T. virens T7 that produces the two effective molecules. In Ivory Coast, the first world producer of cocoa, there is a fear because P. megakarya is replacing P. palmivora in terms of populations. Hopefully, our data show once more that T. virens T7 could be used as a biocontrol agent over there. In addition, since both of the pathogens are sensitive to viridin and gliovirin, each one of them could be used on any one of the pathogens.
References
Acebo-Guerrero Y, Hernandez-Rodriguez A, Heydrich-Perez M, El Jaziri M, Hernandes-Lauzardo AN (2011) Management of black pod rot in cacao (Theobroma cacao L.): a review. Fruits 67:41–48
Acebo-Guerrero Y, Hernandez-Rodriguez A, Vandeputte O, Miguelez-Sierra Y, Heydrich-Perez M, Ye L, Cornelis P, Bertin P, El Jaziri M (2015) Characterization of Pseudomonas chlororaphis from Theobroma cacao L. rhizosphere with antagonistic activity against Phytophthora palmivora (Butler). J Appl Microbiol 119:1112–1126
Andersson PF, Johansson SBK, Stenlid J, Broberg A (2010) Isolation, identification and necrotic activity of viridiol from Chalara fraxinea, the fungus responsible for dieback of ash. For Pathol 40:43–46
Avent AG, Hanson JR, Truneh A (1992) Metabolites of Gliocladium flavofuscum. Phytochemistry 32:197–198
Bacikova D, Betina V, Nemec P (1965) Antihelminthic activity of antibiotics. Nature 206:1371–1372
Bae SJ, Mohanta TK, Chung JY et al (2016) Trichoderma metabolites as biological control agents against Phytophthora pathogens. Biol Control 92:128–138
Brian PW, McGowan JG (1945) Viridin: a highly fungistatic substance produced by Trichoderma viride. Nature 156:144–145
Brimner TA, Boland GJ (2003) A review of the non-target effects of fungi used to biologically control plant diseases. Agric Ecosyst Environ 100:3–16
Calhoun LA, Findlay JA, David MJ, Whitney NJ (1992) Metabolites toxic to spruce budworm from balsam fir needle endophytes. Mycol Res 96:281–286
Chen Y-Y, Chen P-C, Tsay T-T (2016) The biocontrol efficacy and antibiotic activity of Streptomyces plicatus on the oomycete Phytophthora capsici. Biol Control 98:34–42
Dipietro A, Lorito M, Hayes CK, Broadway RM, Harman GE (1993) Endochitinase from Gliocladium virens—isolation, characterization, and synergistic antifungal activity in combination with gliotoxin. Phytopathology 83:308–313
El-Hasan A, Walker F, Schone J, Buchenauer H (2009) Detection of viridiofungin A and other antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur J Plant Pathol 124:457–470
Grove J.F., Moffatt J.S. & Vischer E.B. (1965) Viridin. Part I. Isolation and characterisation. J Chem Soc 3803–3811. https://doi.org/10.1039/JR9650003803
Haesler F, Hagn A, Frommberger M, Hertkorn N, Schmitt-Kopplin P, Munch JC, Schloter M (2008) In vitro antagonism of an actinobacterial Kitasatospora isolate against the plant pathogen Phytophthora citricola as elucidated with ultrahigh resolution mass spectrometry. J Microbiol Methods 75:188–195
Hanada RE, Souza TD, Pomella AWV, Hebbar KP, Pereira JO, Ismaiel A, Samuels GJ (2008) Trichoderma martiale sp nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. Mycol Res 112:1335–1343
Hanada RE, Pomella AWV, Soberanis W, Loguercio LL, Pereira JO (2009) Biocontrol potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora) of cacao. Biol Control 50:143–149
Howell CR (1982) Effect of Gliocladium virens on Pythium ultimum, Rhizoctonia solani, and Damping-Off of Cotton Seedlings. Phytopathology 72:496–498
Howell CR (1991) Biological-control of pythium damping-off of cotton with seed-coating preparations of Gliocladium virens. Phytopathology 81:738–741
Howell CR, Puckhaber LS (2005) Study of the characteristics of “P” and “Q” strains of Trichoderma virens to account for differences in biological control efficacy against cotton seedling diseases. Biol Control 33:217–222
Howell CR, Stipanovic RD (1983) Gliovirin, a new antibiotic from Gliocladium virens, and its role in the biological-control of Pythium ultimum. Can J Microbiol 29:321–324
Howell CR, Stipanovic RD (1984) Mycoherbicidal activity of Gliocladium virens by means of viridiol production. Phytopathology 74:836–836
Howell CR, Stipanovic RD, Lumsden RD (1993) Antibiotic production by strains of Gliocladium virens and its relation to the biocontrol of cotton seedling diseases. Biocontrol Sci Tech 3:435–441
ICCO (2014) Quarterly Bulletin of Cocoa Statistics, Vol XL, No 2, Cocoa year 2013/14 accessed online at. http://www.icco.org/statistics/production-and-grindings/production.html
Itoh Y, Kodama K, Furuya K, Takahashi S, Haneishi T, Takiguchi Y, Arai M (1980) A new sesquiterpene antibiotic, heptelidic acid producing organisms, fermentation, isolation and characterization. J Antibiot 33:468–473
Itoh Y, Takahashi S, Arai M (1982) Structure of gliocladic acid. J Antibiot 35:541–542
Iwasa E, Hamashima Y, Sodeoka M (2011) Epipolythiodiketopiperazine alkaloids: total syntheses and biological activities. Israel Journal of Chemistry 51:420–433
Kamoun S, Furzer O, Jones JDG et al (2015) The Top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol 16:413–434
Lee SH, Cho YE, Park SH, Balaraju K, Park JW, Lee SW, Park K (2013) An antibiotic fusaricidin: a cyclic depsipeptide from Paenibacillus polymyxa E681 induces systemic resistance against Phytophthora blight of red-pepper. Phytoparasitica 41:49–58
Leng PF, Zhang ZM, Pan GT, Zhao MJ (2011) Applications and development trends in biopesticides. Afr J Biotechnol 10:19864–19873
Lumsden RD, Ridout CJ, Vendemia ME, Harrison DJ, Waters RM, Walter JF (1992) Characterization of major secondary metabolites produced in soilless mix by a formulated strain of the biocontrol fungus Gliocladium virens. Can J Microbiol 38:1274–1280
Mishra V (2010) In vitro antagonism of Trichoderma species against Pythium aphanidermatum. J Phytol 2:28–35
Mpika J, Kebe IB, Issali AE, N'Guessan FK, Druzhinina S, Komon-Zelazowska M, Kubicek CP, Ake S (2009) Antagonist potential of Trichoderma indigenous isolates for biological control of Phytophthora palmivora the causative agent of black pod disease on cocoa (Theobroma cacao L.) in Cote d'Ivoire. Afr J Biotechnol 8:5280–5293
Ndoumbe-Nkeng M, Cilas C, Nyemb E, Nyasse S, Bieysse D, Flori A, Sache I (2004) Impact of removing diseased pods on cocoa black pod caused by Phytophthora megakarya and on cocoa production in Cameroon. Crop Prot 23:415–424
Nyadanu D, Assuah MK, Adomako B, Asiama YO, Opoku IY, Adu-Ampomah Y (2009) Efficacy of screening methods used in breeding for black pod disease resistance varieties in cocoa. Afri Crop Sci J 17:175–186
Nyassé S, Efombagn MIB, Kébé BI, Tahi M, Despréaux D, Cilas C (2007) Integrated management of Phytophthora diseases on cocoa (Theobroma cacao L): impact of plant breeding on pod rot incidence. Crop Prot 26:40–45
Puopolo G, Cimmino A, Palmieri MC, Giovannini O, Evidente A, Pertot I (2014) Lysobacter capsici AZ78 produces cyclo(L-Pro-L-Tyr), a 2,5-diketopiperazine with toxic activity against sporangia of Phytophthora infestans and Plasmopara viticola. J Appl Microbiol 117:1168–1180
Reino JL, Guerrero RF, Hernandez-Galan R, Collado IG (2007) Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem Rev 7:89–123
Saravanakumar K, Yu CJ, Dou K, Wang M, Li YQ, Chen J (2016) Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp cucumerinum. Biol Control 94:37–46
Seephonkai P, Kongsaeree P, Prabpai S, Isaka M, Thebtaranonth Y (2006) Transformation of an irregularly bridged epidithiodiketopiperazine to trichodermamide A. Org Lett 8:3073–3075
Segarra G, Aviles M, Casanova E, Borrero C, Trillas I (2013) Effectiveness of biological control of Phytophthora capsici in pepper by Trichoderma asperellum strain T34. Phytopathol Mediterr 52:77–83
Seiber JN, Coats J, Duke SO, Gross AD (2014) Biopesticides: state of the art and future opportunities. J Agric Food Chem 62:11613–11619
Smith A, Blois J, Yuan H et al (2009) The antiproliferative cytostatic effects of a self-activating viridin prodrug. Mol Cancer Ther 8:1666–1675
Sriwati R, Melnick RL, Muarif R, Strem MD, Samuels GJ, Bailey BA (2015) Trichoderma from Aceh Sumatra reduce Phytophthora lesions on pods and cacao seedlings. Biol Control 89:33–41
Stipanovic RD, Howell CR (1982) The structure of gliovirin, a new antibiotic from Gliocladium virens. J Antibiot 35:1326–1330
Takeuchi K, Noda N, Katayose Y, Mukai Y, Numa H, Yamada K, Someya N (2015) Rhizoxin analogs contribute to the biocontrol activity of a newly isolated Pseudomonas strain. Mol Plant-Microbe Interact 28:333–342
Tanaka Y, Shiomi K, Kamei K et al (1998) Antimalarial activity of radicicol, heptelidic acid and other fungal metabolites. J Antibiot 51:153–160
Tondje PR, Hebbar KP, Samuels G, Bowers JH, Weise S, Nyemb E, Begoude D, Foko J, Fontem D (2006) Bioassay of Genicolosporium species for Phytophthora megakarya biological control on cacao pod husk pieces. Afr J Biotechnol 5:648–652
Tondje PR, Roberts DP, Bon MC et al (2007) Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol Control 43:202–212
Trejo-Estrada SR, Paszczynski A, Crawford DL (1998) Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger YCED-9. J Ind Microbiol Biotechnol 21:81–90
Valois D, Fayad K, Barasubiye T, Garon M, Dery C, Brzezinski R, Beaulieu C (1996) Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var rubi, the causal agent of raspberry root rot. Appl Environ Microbiol 62:1630–1635
Verma M, Brar SK, Tyagi RD, Surampalli RY, Valero JR (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J 37:1–20
Vinale F, Flematti G, Sivasithamparam K, Lorito M, Marra R, Skelton BW, Ghisalberti EL (2009) Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J Nat Prod 72:2032–2035
Vinale F, Sivasithamparam K, Ghisalberti EL et al (2014) Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol J 8:127–139
Weisshoff H, Hentschel S, Zaspel I, Jarling R, Krause E, Pham TLH (2014) PPZPMs—a novel group of cyclic lipodepsipeptides produced by the Phytophthora alni associated strain Pseudomonas sp JX090307—the missing link between the viscosin and amphisin group. Nat Prod Commun 9:989–996
Yamaguchi Y, Manita D, Takeuchi T, Kuramochi K, Kuriyama I, Sugawara F, Yoshida H, Mizushina Y (2010) Novel terpenoids, trichoderonic acids A and B isolated from Trichoderma virens, are selective inhibitors of family X DNA polymerases. Biosci Biotechnol Biochem 74:793–801
Zhou Y, Choi YL, Sun M, Yu ZN (2008) Novel roles of Bacillus thuringiensis to control plant diseases. Appl Microbiol Biotechnol 80:563–572
Acknowledgements
The authors wish to thank C. Bance for technical assistance, L. Dubost for mass spectra, A. Deville for NMR spectra, and J. Mpika for providing the Trichoderma strains used in this study. The Government of Ivory Coast is acknowledged for the PhD fellowship to G.-A. P.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
The preliminary results of this study were presented (oral communication) at the Congress “Natural Product & Biocontrol 2014.”
Electronic supplementary material
ESM 1
(PPTX 938 kb).
Rights and permissions
About this article
Cite this article
Pakora, GA., Mpika, J., Kone, D. et al. Inhibition of Phytophthora species, agents of cocoa black pod disease, by secondary metabolites of Trichoderma species. Environ Sci Pollut Res 25, 29901–29909 (2018). https://doi.org/10.1007/s11356-017-0283-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0283-9