Abstract
Cement-based stabilization/solidification (S/S) is a practical treatment approach for hazardous waste with anthropogenic As sources; however, its applicability for geogenic As-containing soil and the long-term leaching potential remain uncertain. In this study, semi-dynamic leaching test was performed to investigate the influence of S/S binders (cement blended with fuel ash (FA), furnace bottom ash (FBA), or ground granulated blast furnace slag (GGBS)) on the long-term leaching characteristics of geogenic As. The results showed that mineral admixtures with higher Ca content and pozzolanic activity were more effective in reducing the leached As concentrations. Thus, cement blended with FBA was inferior to other binders in suppressing the As leaching, while 20% replacement of ordinary Portland cement by GGBS was considered most feasible for the S/S treatment of As-containing soils. The leachability of geogenic As was suppressed by the encapsulation effect of solidified matrix and interlocking network of hydration products that were supported by scanning electron microscopy (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) results. The long-term leaching of geogenic As from the monolithic samples was diffusion-controlled. Increasing the Ca content in the samples led to a decrease in diffusion coefficient and an increase in feasibility for “controlled utilization” of the S/S-treated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is one of the most abundant naturally occurring elements in the earth’s crust and considered a human carcinogen (Stea et al. 2014; Chakraborti et al. 2016; Rahman et al. 2017). Environmental contamination of As due to anthropogenic and geogenic sources has been widely reported in soil/sediment and water around the world (Fendorf et al. 2010; Wang et al. 2015a; Chakraborty et al. 2017). Arsenic contamination resulting from human activities such as mining and pesticide application often receives more attention about its remediation, but natural sources of As have been increasingly recognized as the potential long-term threats to human health (Fendorf et al. 2010). The toxicity of As is dependent on the As speciation and the stability and chemical phase of the coexisting compounds (Kim et al. 2014; Ali et al. 2014; Zhang et al. 2016). The geochemistry of As in soils is complex with crosslinking interactions among Fe/Al minerals, organic carbon, microbes, pH, Eh conditions, etc., which presents challenge to practicing engineers and policy makers to design appropriate remediation methods at different sites (Bisone et al. 2016; Beiyuan et al. 2017a).
Among available techniques, cement-based solidification/stabilization (S/S) is one of the most effective methods to reduce the availability of metals in soils by transforming potentially hazardous phases into less hazardous ones (Leist et al. 2003; Phenrat et al. 2007; Zhang et al. 2013; Clancy et al. 2015) and prevent human exposure and metal transformation during the future land use. Over the past decade, S/S treatment has been used as a remediation option for soils contaminated by anthropogenic As (Leist et al. 2003). In the S/S process, an interlocking framework of minerals encapsulates and precipitates with the As present in the soil as cement hydration proceeds (Clancy et al. 2015; Wang et al. 2015b). The release of As is minimized as the solidified soil has a higher mechanical strength and lower surface area exposed to the environment with reduced permeability. Therefore, this study evaluates the efficacy of S/S technology for treating geogenic As-containing soils in Hong Kong, as the existing remediation literature primarily deal with anthropogenic As contamination of soils (Tsang and Yip 2014; Singh et al. 2015; Beiyuan et al. 2017b).
A number of standard leaching protocols are available for investigating the mobility of As in soils and S/S-treated samples. The Toxicity Characteristic Leaching Procedure (TCLP) is the most commonly employed batch leaching test as a performance indicator (Tsang et al. 2013a; Beiyuan et al. 2017b) and a remedial goal of contaminated site remediation (HK EPD 2011). However, the TCLP test is criticized for not reflecting the actual field conditions and As leaching may be underestimated because of the buffer capacity of the S/S-treated samples (Camacho et al. 2009; Voglar and Leštan 2013; Sima et al. 2015). Other batch leaching tests such as synthetic precipitation leaching procedure, simplified bioaccessibility extraction test, and rhizosphere-based phytoavailability test can be performed to simulate different exposure pathways of As (Karak et al. 2012; Tsang et al. 2013b; Bolan et al. 2015; Beiyuan et al. 2017c). The pH-based leaching method, sequential extraction procedure, and continuous column leaching procedure are also applicable for assessing the effectiveness of contaminated soils (Wenzel et al. 2001; Voglar and Leštan 2013; Tsang et al. 2014; Li et al. 2016). However, these single-point tests or granular leaching tests are less accurate for estimating contaminants’ release from the monolithic samples after S/S treatment.
Semi-dynamic leaching test is an experimental test designed specifically for the S/S-treated samples considering their low permeability and monolithic nature, which can simulate in a more realistic way the actual leaching conditions of contaminants encapsulated in the solidified matrix (Li and Poon 2017; Xue et al. 2017). The semi-dynamic leaching test allows the determination of effective diffusion coefficient (D e) of the contaminants in the S/S-treated soils to assess the long-term leaching potential of the contaminants (Song et al. 2013; Du et al. 2014; Li et al. 2017). However, all of the reported studies applied the semi-dynamic leaching test to investigate the S/S-treated soils that had been contaminated by anthropogenic sources (Gwenzi and Mupatsi 2016; Li and Poon 2017). Uncertainties remain in the effectiveness of S/S-treated geogenic As-containing soils under semi-dynamic leaching and the adequacy of mineral admixtures as environmentally friendly cement substitutes.
In the present study, cement blended with mineral admixtures (fuel ash (FA), furnace bottom ash (FBA), and ground granulated blast furnace slag (GGBS)) was used as the binding agents for geogenic As-containing soils in Hong Kong in view of economic advantages and carbon emission reduction. The unconfined compressive strength (UCS) and the leaching characteristics (including cumulative fraction of As leached (CFL) and D e) of the different S/S-treated soils under semi-dynamic leaching tests were investigated. An effort was made to reveal the possible As mobilization mechanism through microscopic and spectroscopic analyses. These results could facilitate the remediation design of geogenic As-containing soils by using cement-based S/S technique.
Materials and methods
Geogenic As-containing soils and stabilization/solidification
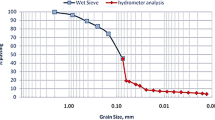
Four soil samples at varying depths from different boreholes that contained high As concentrations (> 400 mg kg−1) were collected from a geogenic As-containing site in Hong Kong, China. The soil columns were sealed to be air-tight at the site, frozen with ice packs in freezing boxes during transportation, and stored at −20 °C in the dark until freeze-drying for subsequent analysis. For S/S experiments, the soils were oven-dried at 60 °C and passed through a 2-mm sieve. The concentrations of total metals were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, SPECTROBLUE) after strong acid digestion (1:4 concentrated HNO3 and HClO4 (v/v)) according to ASTM D3974-81. Based on X-ray diffractometer analysis (XRD, D8 Advance, Bruker), the main minerals of the soils were quartz, kaolinite, muscovite, and iron oxides. The organic matter content was measured by loss on ignition at 550 °C; the cation exchange capacity (CEC) was measured after percolation of 1.0 mol L−1 ammonium acetate solution at pH 7. The grain-size distribution was analyzed by wet sieving and hydrometer tests. The physico-chemical properties of the four soils are shown in Table 1.
Four commercially available binders used in this study were ordinary Portland cement (OPC), FA (class F), FBA, and GGBS. The chemical compositions of the cementitious materials based on XRF results are shown in Table 2. The leaching characteristics of heavy metals in these four binders based on TCLP leaching process are presented in supplementary materials (Table S1 in Supplementary Materials). It can be concluded that all binders contain only trace amounts of heavy metals and the S/S process would not be affected. To keep the same workability of the mixtures, the S/S-treated samples were prepared with a water-to-solid ratio of 0.4 for soil 1, a ratio of 0.3 for soil 2, and a constant ratio of 0.5 for soils 3 and 4, because of their variation in clay content and water absorption. The binder content was 5% for low alkalinity and economic considerations. The FA, FBA, and GGBS were used to replace OPC progressively as indicated, and the replacement ratio was 20 and 50% for obtaining compressive strength above 0.35 MPa. The mix designs for As-containing soils are shown in Table 3. All the soils and cementitious powder materials were first mixed uniformly for about 1 min using a mechanical mixer before adding water. The blended paste was further mixed for another 2 min and cast into steel molds (2 × 2 × 2 cm) with vibration. The samples were cured at 23 °C and 75% relative humidity for 24 h, and then the hardened S/S materials were removed from the molds and cured in a steam cabinet at 60 ± 2 °C for 7 days.
Unconfined compressive strength
The UCS of the S/S product was measured by using a universal testing machine (Testometric CXM 500-50KN) at a loading rate of 0.3 mm min−1 according to BSEN12390-3. The strength was compared with the criteria for reuse as fill materials for site formation in Hong Kong (i.e., ground leveling/stabilization to the design formation level prior to construction works) (HK EPD 2011). All the tests were duplicated (or triplicated where necessary) and the average results were reported.
Semi-dynamic leaching
Semi-dynamic leaching tests were applied to the long-term leaching potential of monolithic samples after S/S treatment (ASTM C1308). The ratio of volume of deionized water used to the surface area of the specimen was 10:1. The leaching test was conducted for 14 days at a constant temperature of 23 ± 2 °C, in which deionized water was replaced after 2, 5, and 17 h in the first day, then daily for the following 9 days and every other day for the last 4 days.
In the above tests, the final pH of leachate was measured and the solution sample was separated by centrifugation at 4000 rpm for 15 min followed by filtration through a 0.45-μm glass fiber filter. Afterwards, the samples were acidified by concentrated HNO3 to pH below 2 and stored at 4 °C in the dark prior to ICP-OES analysis. The cumulative fraction leached (CFL) of As in semi-dynamic leaching tests was calculated using Eq. (1), and its plot against time square root (t 1/2) enabled the computation of the diffusion coefficients (D e) of As using Eq. (2). All experiments were carried out in triplicate, and the average value with standard deviations less than 5% was reported.
where A t = the cumulative mass of the metal contaminant leached out of the medium surface (mg), A 0 = total mass of substance contained in the soil prior to the test (mg), V = volume of specimen (cm3), t = leaching time (s), S = surface of the sample used in the semi-dynamic leaching test (cm2), c i = measured concentration of the leached metal contaminant in the leachate at ith test interval during test (mg/L), and V L,i = volume of the leachant (L).
Microscopic/spectroscopic characteristics
Scanning electron microscopy (SEM) with elemental mapping (VEGA3, TESCAN) was conducted on freeze-dried, gold-coated soil samples to investigate the morphology and elemental distribution (As, Ca, Si, and Al) on the surface of the S/S-treated soils. The speciation of geogenic As in the S/S-treated soils was characterized using Kratos Axis Ultra Imaging X-ray photoelectron spectroscopy (XPS) with monochromatic Al Ka X-ray. A broadscan was obtained using 50 eV pass energy, while narrow high resolution scans of As 3d were obtained using 20 eV pass energy. The charge effect was corrected using the C 1s line at 285 eV. The component peaks were identified by comparison of their binding energies with values in the literatures. The crystalline-phase mineralogy of the ball-milled soil samples was evaluated by using a high resolution powdered X-ray diffractometer (Rigaku SmartLab, Japan) using CuKα (λ = 1.54059 Å) radiation obtained at 40 kV and 30 mA. Soil 2 had the highest As concentration and its microscopic and spectroscopic analysis was shown as the representative sample for illustrating the effects of different S/S treatments on As speciation.
Results and discussion
UCS and leachate pH of the S/S-treated soils
The results of the compressive strength tests are shown in Fig. 1. The compressive strength of S/S soils varied significantly, and soils 3 and 4 with higher clay content generally showed higher strength. Cement content of 5% by mass of all soils fulfilled the acceptance criteria (1 MPa) for reuse as on-site fill materials in Hong Kong (HK EPD 2011), while 20–50% cement replacement by mineral admixtures (GGBS, FA, and FBA) provided UCS larger than 350 kPa for landfill disposal (US EPA 2013). The UCS of the S/S-treated soils decreased as the content of mineral admixtures increased, which was consistent with previous studies (Wang et al. 2015b). The reduction in UCS of the S/S-treated soils followed the order of FBA > FA > GGBS replacements. For example, addition of 2.5% FBA, FA, and GGBS resulted in a decrease of 48, 39, and 33% in UCS, respectively. This may be attributed to the difference in CaO content and pozzolanic activity of mineral admixtures (Wang et al. 2015c). The pozzolanic activity of GGBS, FA, and FBA can be activated in the presence of cement and water to form cementitious gels, the amount of which corresponded to the pozzolanic nature of supplementary cementitious materials.
Under semi-dynamic leaching, the S/S-treated soils showed variations in the leachate pH with respect to leaching time (Fig. 2). The pH of the leachate increased sharply in the first few hours and gradually during the initial 2 days, then remained steady and unchanged in the following days, but decreased slightly with time after approximately 8 days. The average values of the leachate pH ranged from 7 to 11. The variations of leachate pH were attributed to gradual dissolution of portlandite (Ca(OH)2) from the S/S-treated soils, which resulted in hydroxyl and Ca leaching (Du et al. 2014; Clancy et al. 2015). The pH values of the leachate of OPC-treated soils were higher than those of OPC-admixtures treatment due to the higher amount of Ca(OH)2 formed by OPC hydration. Increasing the incorporation of mineral admixtures in the binders decreased the leachate pHs, where the reduction of leachate pH followed the order of FBA > FA > GGBS, which was negatively correlated to their CaO content (Table 2).
Leached concentrations of As and Ca with respect to leaching time
The S/S-treated soils under semi-dynamic leaching showed different variations in the leached concentrations of As (Fig. 3) and Ca (Fig. 4) with respect to leaching time. The leached Ca concentrations were about 100 times greater than the leached As concentrations. In all the leachate, the As concentrations mainly ranged from 0.02 to 0.1 mg L−1, indicating a very low concentration even subject to prolonged and repetitive leaching in the long run. The leached As and Ca in the leachate increased during the initial stage (0–2 days) and then decreased slightly afterwards regardless of the difference in S/S treatments. The patterns of temporal variations may reflect the physical durability of solidified matrix of the S/S-treated soils as monolithic materials under the prolonged leaching condition.
There was a slight difference in the leached As and Ca concentrations between S/S treatments using different mineral admixtures. Supplementary binding materials with higher Ca content (GGBS > FA > FBA, Table 2) were more effective in reducing the leached As concentrations (Fig. 3), yet associated with an increase in the leached Ca concentrations (Fig. 4). As a result, FBA was inferior to other binders in suppressing the As leaching, while GGBS was considered as a suitable and environmentally friendly substitute of OPC (20–50%) for the S/S treatment of geogenic As-containing soils.
To further investigate the effect of the leached Ca on the leached As, Fig. 5 illustrated a negative but weakly linear relationship between them. The decrease in the leached As may be attributed to the sorption/co-precipitation of As with calcite in the leaching solution from the S/S-treated soils (Singh and Pant 2006). Arsenic can be also fixed within particular cementitious species through sorption onto calcium silicate hydrates (C-S-H) and substitution in the crystalline lattice of ettringite (Halim et al. 2004). It has been well recognized that precipitates of calcium arsenates could form in the reaction of As with Ca from the hydraulic binders (Vandecasteele et al. 2002). Specific Ca-As precipitates have been reported as the stable compounds responsible for As immobilization in the S/S processes, such as Ca3(AsO4)2, CaHAsO3 (Dutré and Vandecasteele 1998), Ca-As-O, Ca4(OH)2(AsO4)2·4H2O, and NaCaAsO4·7.5H2O (Moon et al. 2008).
Spectroscopic and microscopic characteristics of the S/S-treated As-containing soils
The XRD patterns of soil 2 with different treatments in our study (Fig. 6), however, did not show any distinct peaks for the As-containing precipitates. It was probably because of the stability of geogenic As in soils (Beiyuan et al. 2017c), where marginal amount of As leaching was insufficient to form noticeable amounts of Ca-As precipitates to be detectable by XRD analysis. According to Fig. 6, iron oxides/hydroxides and calcium silicate hydrate (C-S-H) were identified as the major components, on which As in soils may be strongly bound as adsorbed and/or co-precipitated species (Drahota et al. 2012; Li et al. 2017). The XPS results (Fig. 7) further indicated that the S/S treatment with OPC and different mineral admixtures did not alter the oxidation states and chemical species of geogenic As in soils. The binding energies of As 3d in the soil samples were 45.9 eV (untreated), 44.02 eV (O5-treated), 44.1 eV (O2.5F2.5-treated), 44.08 eV (O2.5B2.5-treated), and 43.77 eV (O2.5G2.5-treated), respectively. These values resembled those of As(V)-O bonded (Kim et al. 2015; Beiyuan et al. 2017c), suggesting that geogenic As in soils remained its stable forms after different S/S treatments. The slight change in the binding energy of As(V)-O in the S/S soils might be attributed to the changes in pH or redox conditions due to the S/S treatments.
Therefore, the S/S-treated soils primarily reduced the potential mobility and exposure pathway of geogenic As by means of physical encapsulation in the solidified matrix, where an interlocking framework of hydration products would increase with cement content. When the soil samples were mixed with cementitious materials, the Ca(OH)2, CSH, calcium aluminate hydrate (CAH) formed in the soil matrix might gradually increase (Li et al. 2014; Hernandez-Bautista et al. 2016), coupled with the filling of pore space and blocking of As leaching flow paths. Therefore, the variation in Ca content for hydration remarkably influenced the monolithic structure and As immobilization. A more compact structure was observed with higher OPC content in the cement blended with mineral admixtures (Subalakshmi et al. 2012). Partial OPC replacement by FA, GGBS, and FBA showed the corresponding decline in the compressive strength (Fig. 1) and increase in the leached As concentration under semi-dynamic leaching (Fig. 3). The influence followed the order of FBA > FA > GGBS, in agreement with their Ca content and pozzolanic activity.
The microstructural changes and the As distribution in S/S soils were determined by SEM-EDS analysis with elemental mapping (Fig. 8). The data indicated that Ca was present as the dominant element along with Si and Al in the OPC-treated soil (Fig. 8a), where more flocculent aggregate-like morphologies with dense structure were observed. This may result from quick release of Ca from OPC and subsequent precipitation of hydrate compounds, which could form relatively impermeable layer on the surface of soil particles. Because elemental dot maps indicated that As was rather evenly distributed on the soil surfaces, immobilization of geogenic As in the S/S-treated soils may be primarily attributed to its encapsulation into the dense hydrate network that could prevent leaching out, which was in agreement with the results of Randall (2012). In comparison, Ca-containing precipitates were less prevalent in the OPC/FBA-treated soil (Fig. 8b) due to the low content of Ca and pozzolanic activity of FBA, corroborating the lower compressive strength and higher leached As concentration under semi-dynamic leaching.
Temporal variation of CFL of As
The change of CFL of As with t 1/2 showed that very low percentages of total As were leached out during the semi-dynamic leaching tests (Fig. 9). For all the S/S-treated samples, only below 0.5% of As could be leached out in the first day. The plots of CFL-t 1/2 for As, regardless of the difference in S/S treatments, were well represented by linear lines throughout the entire leaching time. This indicated that the leaching of As was mainly through diffusion from the monolithic samples. Table 4 summarizes the computed values of D e of As in this study, which varied in the range of 7.07 × 10−20–1.44 × 10−17 m2 s−1. In most cases, D e values of As from soils treated by O5 and O4G1 were lower than the other treatments. Increasing the OPC content or Ca content of mineral admixtures in the S/S-treated soils led to a decrease in CFL at a given leaching time and a reduction of D e values.
The D e values below 3 × 10−13 m2 s−1 suggested low mobility of As, while D e values lied in the range of 3 × 10−13–10−11 m2 s−1 indicated average mobility (Malviya and Chaudhary 2006). Because the S/S-treated soils showed D e values four to six orders of magnitude below 10−13 m2 s−1 in this study, they were considered appropriate for “controlled utilization” in road bases and quarry rehabilitation, etc. (Song et al. 2013; Xue et al. 2017). Therefore, the use of 5% binders with/without 20% replacement of OPC by GGBS was considered most feasible for the S/S treatment and reuse of physically encapsulated geogenic As-containing soils.
Conclusions
This study reveals that the S/S treatment using cement content of 5% by mass of geogenic As-containing soils fulfilled the acceptance criteria in Hong Kong for reuse as on-site fill materials. Partial replacement (20–50%) of cement by mineral admixtures compromised the compressive strength following the order of FBA > FA > GGBS, which inversely followed the order of CaO content and pozzolanic nature of mineral admixtures. Semi-dynamic leaching tests show that very low concentrations and percentages of As leached out from the S/S-treated soils in the long term. The leaching of As from the monolithic samples was diffusion-controlled, and there was a negative and linear relationship between leached Ca and As content in the leachate. The As oxidation state and species in the S/S process were not changed according to the XRD and XPS results, and the encapsulation of As into the dense net structures formed by hydration products suppressed the As leaching that was supported by the XRD results and SEM images and elemental dot maps. The S/S-treated soils with higher CaO content exhibited a higher leachate pH and higher amount of Ca leached, but a lower cumulative leached As fraction and decreased effective diffusion coefficient of As. This enhanced the feasibility for “controlled utilization” of the S/S-treated geogenic As-containing soils, for which 20% replacement of OPC by GGBS was considered the most feasible.
References
Ali I, Al-Othman ZA, Alwarthan A, Asim M, Khan TA (2014) Removal of arsenic species from water by batch and column operations on bagasse fly ash. Environ Sci Pollut Res 21:3218–3229
Beiyuan J, Awad YM, Beckers F, Tsang DCW, Ok YS, Rinklebe J (2017a) Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 178:110–118
Beiyuan J, Li JS, Tsang DCW, Wang L, Poon CS, Li XD, Fendorf S (2017b) Fate of residual arsenic after chemical-enhanced washing of an arsenic-containing soil in Hong Kong. Sci Total Environ 599-600:679–688
Beiyuan J, Tsang DCW, Valix M, Zhang W, Yang X, Ok YS, Li XD (2017c) Selective dissolution followed by EDDS washing of an e-waste contaminated soil: extraction efficiency, fate of residual metals, and impact on soil environment. Chemosphere 166:489–496
Bisone S, Chatain V, Blanc D, Gautier M, Bayard R, Sanchez F, Gourdon R (2016) Geochemical characterization and modeling of arsenic behavior in a highly contaminated mining soil. Environ Earth Sci 75:1–9
Bolan N, Mahimairaja S, Kunhikrishnan A, Seshadri B, Thangarajan R (2015) Bioavailability and ecotoxicity of arsenic species in solution culture and soil system: implications to remediation. Environ Sci Pollut Res 22:8866–8875
Camacho J, Wee HY, Kramer TA, Autenrieth R (2009) Arsenic stabilization on water treatment residuals by calcium addition. J Hazard Mater 165:599–603
Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, Mukherjee SC (2016) Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 152:520–529
Chakraborty S, Weindorf DC, Deb S, Li B, Paul S, Choudhury A, Ray DP (2017) Rapid assessment of regional soil arsenic pollution risk via diffuse reflectance spectroscopy. Geoderma 289:72–81
Clancy TM, Snyder KV, Reddy R, Lanzirotti A, Amrose SE, Raskin L, Hayes KF (2015) Evaluating the cement stabilization of arsenic-bearing iron wastes from drinking water treatment. J Hazard Mater 300:522–529
Drahota P, Filippi M, Ettler V, Rohovec J, Mihaljevič M, Šebek O (2012) Natural attenuation of arsenic in soils near a highly contaminated historical mine waste dump. Sci Total Environ 414:546–555
Du YJ, Wei ML, Reddy KR, Liu ZP, Jin F (2014) Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J Hazard Mater 271:131–140
Dutré V, Vandecasteele C (1998) Immobilization mechanism of arsenic in waste solidified using cement and lime. Environ Sci Technol 32:2782–2787
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328:1123–1127
Gwenzi W, Mupatsi NM (2016) Evaluation of heavy metal leaching from coal ash-versus conventional concrete monoliths and debris. Waste Manag 49:114–123
Halim CE, Amal R, Beydoun D, Scott JA, Low G (2004) Implications of the structure of cementitious wastes containing Pb(II), Cd(II), As(V), and Cr(VI) on the leaching of metals. Cem Concr Res 34:1093–1102
Hernandez-Bautista E, Bentz DP, Sandoval-Torres S, Cano-Barrita PDJ (2016) Numerical simulation of heat and mass transport during hydration of Portland cement mortar in semi-adiabatic and steam curing conditions. Cem Concr Compos 69:38–48
HK EPD (2011) Practice guide for investigation and remediation of contaminated land. Environmental Protection Department, Hong Kong
Karak T, Abollino O, Bhattacharyya P, Das KK, Paul RK (2012) Fractionation and speciation of arsenic in three tea gardens soil profiles and distribution of As in different parts of tea plant (Camellia sinensis L.) Chemosphere 85:948–960
Kim EJ, Yoo JC, Baek K (2014) Arsenic speciation and bioaccessibility in arsenic-contaminated soils: sequential extraction and mineralogical investigation. Environ Pollut 186:29–35
Kim EJ, Lee JC, Baek K (2015) Abiotic reductive extraction of arsenic from contaminated soils enhanced by complexation: arsenic extraction by reducing agents and combination of reducing and chelating agents. J Hazard Mater 283:454–461
Leist M, Casey RJ, Caridi D (2003) The fixation and leaching of cement stabilized arsenic. Waste Manag 23:353–359
Li JS, Poon CS (2017) Innovative solidification/stabilization of lead contaminated soil using incineration sewage sludge ash. Chemosphere 173:143–152
Li JS, Xue Q, Wang P, Li ZZ, Liu L (2014) Effect of drying-wetting cycles on leaching behavior of cement solidified lead-contaminated soil. Chemosphere 117:10–13
Li JS, Xue Q, Wang P, Wang HQ, Zhang TT (2016) Evaluation of leaching characteristics of heavy metals from municipal solid waste incineration fly ash by up-flow percolation column tests. Environ Earth Sci 75:1–10
Li JS, Beiyuan J, Tsang DCW, Wang L, Poon CS, Li XD, Fendorf S (2017) Arsenic-containing soil from geogenic source in Hong Kong: leaching characteristics and stabilization/solidification. Chemosphere 182:31–39
Malviya R, Chaudhary R (2006) Leaching behavior and immobilization of heavy metals in solidified/stabilized products. J Hazard Mater 37:207–217
Moon DH, Wazne M, Yoon IH, Grubb DG (2008) Assessment of cement kiln dust (CKD) for stabilization/solidification (S/S) of arsenic contaminated soils. J Hazard Mater 159:512–518
Phenrat T, Marhaba TF, Rachakornkij M (2007) XRD and unconfined compressive strength study for a qualitative examination of calcium–arsenic compounds retardation of cement hydration in solidified/stabilized arsenic-iron hydroxide sludge. J Environ Eng 133:595–607
Rahman MS, Reichelt-Brushet AJ, Clark MW, Farzana T, Yee LH (2017) Arsenic bio-accessibility and bioaccumulation in aged pesticide contaminated soils: a multiline investigation to understand environmental risk. Sci Total Environ 581-582:782–793
Randall PM (2012) Arsenic encapsulation using Portland cement with ferrous sulfate/lime and Terra-Bond™ technologies—microcharacterization and leaching studies. Sci Total Environ 420:300–312
Sima J, Cao X, Zhao L, Luo Q (2015) Toxicity characteristic leaching procedure over-or under-estimates leachability of lead in phosphate-amended contaminated soils. Chemosphere 138:744–750
Singh TS, Pant KK (2006) Solidification/stabilization of arsenic containing solid wastes using Portland cement, fly ash and polymericmaterials. J Hazard Mater 131:29–36
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotox Environ Safe 112:247–270
Song F, Gu L, Zhu N, Yuan H (2013) Leaching behavior of heavy metals from sewage sludge solidified by cement-based binders. Chemosphere 92:344–350
Stea F, Bianchi F, Cori L, Sicari R (2014) Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res 21:244–251
Subalakshmi P, Saraswathy V, Sivasankaran SK (2012) Hydraulicity of mineral admixtures in cement blends. Constr Build Mater 36:648–653
Tsang DCW, Yip ACK (2014) Comparing chemical-enhanced washing and waste-based stabilisation approach for soil remediation. J Soils Sediments 14:936–947
Tsang DCW, Olds WE, Weber PA (2013a) Residual leachability of CCA-contaminated soil after treatment with biodegradable chelating agents and lignite-derived humic substances. J Soils Sediments 13:895–905
Tsang DCW, Olds WE, Weber PA, Yip ACK (2013b) Soil stabilisation using AMD sludge, compost and lignite: TCLP leachability and continuous acid leaching. Chemosphere 93:2839–2847
Tsang DCW, Yip ACK, Olds WE, Weber PA (2014) Arsenic and copper stabilisation in a contaminated soil by coal fly ash and green waste compost. Environ Sci Pollut Res 21:10194–10204
US EPA (2013) Prohibition on the disposal of bulk liquid hazardous waste in landfills-statutory interpretive guidance. Washington DC
Vandecasteele C, Dutré V, Geysen D, Wauters G (2002) Solidification/stabilization of arsenic bearing fly ash from the metallurgical industry. Immobilisation mechanism of arsenic. Waste Manag 22:143–146
Voglar GE, Leštan D (2013) Equilibrium leaching of toxic elements from cement stabilized soil. J Hazard Mater 246:18–25
Wang X, Peng B, Tan C, Ma L, Rathinasabapathi B (2015a) Recent advances in arsenic bioavailability, transport, and speciation in rice. Environ Sci Pollut Res 22:5742–5750
Wang L, Tsang DCW, Poon CS (2015b) Green remediation and recycling of contaminated sediment by waste-incorporated stabilization/solidification. Chemosphere 122:257–264
Wang F, Wang H, Al-Tabbaa A (2015c) Time-dependent performance of soil mix technology stabilized/solidified contaminated site soils. J Hazard Mater 286:503–508
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Xue Q, Wang P, Li JS, Zhang TT, Wang SY (2017) Investigation of the leaching behavior of lead in stabilized/solidified waste using a two-year semi-dynamic leaching test. Chemosphere 166:1–7
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20:8472–8483
Zhang J, Kim H, Dubey B, Townsend T (2016) Arsenic leaching and speciation in C&D debris landfills and the relationship with gypsum drywall content. Waste Manag 59:324–329
Acknowledgements
The authors appreciate the financial support from the Hong Kong Research Grants Council (PolyU 15222115 and 152132/14E), the Civil Engineering and Development Department of HKSAR Government, the Hong Kong Scholars Program (XJ2016035), and the National Natural Science Foundation of China (41602315) for this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Zhihong Xu
Electronic supplementary material
Table S1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Li, JS., Wang, L., Tsang, D.C. et al. Dynamic leaching behavior of geogenic As in soils after cement-based stabilization/solidification. Environ Sci Pollut Res 24, 27822–27832 (2017). https://doi.org/10.1007/s11356-017-0266-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0266-x