Abstract
A novel stereoselective removal behavior of isomeric endrin and dieldrin pesticides from sample solution is demonstrated using nanocomposite of graphene oxide (GO) and iron oxide (Fe3O4) magnetic nanoparticles (MNPs). The removal efficiency of endrin and dieldrin was found higher when GO-MNPs was used as a separating probe than the individual use of GO and MNPs. The removal efficiency of both the pesticides was found to be more favorable when the dosage amount of GO-MNPs was 30 mg for 30-min contact time with pH 4.0 at room temperature. The good correlation of determination (R 2) with 0.975 and 0.973 values obtained for endrin and dieldrin, respectively demonstrated a well fitting of Langmuir adsorption isotherm model. The higher removal percentage (86.0%) and higher slope value of Langmuir adsorption isotherm were estimated for endrin compared to dieldrin (74.0%). The reason for higher adsorption percentage of endrin is due to the endo-position of oxygen atom in molecule favors more interaction of molecules with GO-MNPs compared to the exo-position of oxygen present in dieldrin. In addition, the higher value of R 2 for endrin and dieldrin demonstrated better suitability of pseudo-first-order and pseudo-second-order kinetic models, respectively. The advantages of the present method are use of simple UV-vis spectrophotometry for monitoring and low-cost use of GO-MNPs nanomaterial for the removal of pesticides from sample solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, nanomaterials are gaining a lot of attention because of their varied structural forms, high surface area, optical properties, and biological and chemical stability (Navale et al. 2015). Graphene is a single layered, two-dimensional carbon nanostructure with sp2 hybridization. In graphene, the carbon atoms are honeycomb-like crystal lattice and numerous graphene sheets used in different fields such as storage of materials (Torrisi et al. 2012), drug delivery (Liu et al. 2013), sensors (Ang et al. 2008), polymer composites (Kuilla et al. 2010), and nano-electronics (Westervelt 2008). Graphene oxide (GO) contains different oxygen functional groups such as hydroxyl, carboxyl, and epoxide. The delocalization of π-electron in GO provides structural stability and property to adsorb the higher concentration of aromatic compounds on its surface. Graphene has limitation to be used as adsorbent in aqueous solutions because of incomplete solubility; whereas, GO contains free oxygen groups which are reactive for adsorption and then used for the removal of chemical substances from aqueous samples (Cao and Li 2014; Zhao et al. 2011; Saleh et al. 2017a). The addition of Fe3O4 magnetic nanoparticles (MNPs) to GO provides an extra stability and avoids the aggregation of single graphene sheet. The use of GO-MNPs composite material would be helpful in rapid separation and preconcentration of chemical substances from aqueous sample solution by applying an external magnetic source (Li and Cao 2011; Yang et al. 2010, 2013; Altintıg et al. 2017; Saleh et al. 2017b).
The use of chlorinated pesticides (endrin and dieldrin) in agriculture will increase the productivity of the crops. However, the improper handlings of these pesticides pollute the underground water reservoirs and entering of these hazardous chemical substances in to the human system causes the health problem. Chlorinated pesticides are having an adverse effect on the human health due to their bioaccumulation and toxic action on the nervous systems. The chemical characteristics of both the pesticides are given in Table S1 and Table S2 (supplementary material). Furthermore, several side effects of these pesticides such as headache, nausea, vomiting, convulsions, and inhibition of neurotransmitter hormone have been also reported. Therefore, a new material is needed to remove the contamination of these pesticides from polluted water bodies in order to prevent the risks on the human health (Shrivas et al. 2016; Shrivas and Wu 2008; Gao et al. 2011).
There are several analytical techniques such gas chromatography (GC) (Krechniak and Foss 1982), high performance liquid chromatography (HPLC) (Grice et al. 1999), GC-mass spectrometry (MS) (Rodrigues et al. 2007), and HPLC-MS (Sinha 2011) have been reported for monitoring the removal of endrin and dieldrin pesticides from variety of samples. However, the removal studies through GC, HPLC, GC-MS, and HPLC-MS involve tedious, time-consuming sample preparation steps and require the use of expensive chemicals and accessories to run these instruments. UV-vis spectrophotometry is found to be simple, rapid, and economic and could be applied at the sample source for monitoring the removal of dieldrin and endrin pesticides.
In the present work, we used GO-MNPs as a separating probe for removal of endrin and dieldrin from aqueous sample solution using UV-vis. The factors such as pH of sample solution, contact time, and dosage amount of GO-MNPs that affected the adsorption followed by the removal of pesticides were investigated. Langmuir adsorption, pseudo-first-order kinetic, and pseudo-second-order kinetic models were studied in order to know the selective adsorption of both the pesticides on the surface of GO-MNPs.
Experimental section
Chemicals and solutions preparations
All chemicals used were of analytical grade. Graphite (99.5%), sodium nitrate (NaNO3, 99.5%), sulfuric acid (H2SO4, 98%), potassium permanganate (KMnO4, 99.5%), ferric chloride (FeCl3.6H2O), and ferrous sulfate (FeSO4.7H2O, 99.5%) were purchased from Hi Media Pvt. Ltd. (Mumbai, India). Hydrogen peroxide (H2O2, 30%) and ammonia (NH3) (30%) were obtained from S. D. Fine Chemical (Mumbai, India). Dieldrin (97.07%) and endrin (99.0%) were purchased from Sigma-Aldrich (MA, USA) and the structure is given in Fig. S1. The stock standard solution (100 mg/L) of endrin and dieldrin was prepared by dissolving an appropriate amount of the substances in 10 mL of acetone. The working standard solutions of endrin and dieldrin (5 mg L−1) were separately prepared in a 10 mL of deionized water (DW) by appropriate dilution of the stock standard solution.
Synthesis of GO-MNPs
The nanocomposite of GO-MNPs was prepared by reported wet chemical method (Li et al. 2012). Graphene oxide (GO) was prepared by Hummer’s method with slight modification. Briefly, 1.0 g of graphite, 1.0 g NaNO3, and 40 mL of H2SO4 were taken in a two-necked round bottom flask and the solution mixture was stirred in an ice bath for 30 min. Then, 6.0 g of KMnO4 was added and the reaction mixture was allowed to stir for 1 h. After stirring, 80 mL of DW was gradually added while maintaining the temperature of the solution mixture at 90 °C under constant stirring for 30 min. Afterwards, 150 mL of DW and 6 mL of H2O2 were added slowly, until the color of the solution changed from black to light brown. The solution mixture was filtered and the obtained filtrate was repeatedly washed with DW until the last traces of KMnO4 were eliminated. The residue was dried in an oven at 60 °C and dark brown powdered GO was obtained.
GO-MNP was prepared by taking a 0.2 g of GO in round bottom flask containing 250 mL DW and FeCl3.6H2O (1.86 g) and FeSO4·7H2O (0.96 g) were introduced into the round bottom flask. The temperature of solution mixture was maintained to 80 °C and then 0.5 mL of 30% NH3 solution was added to the solution mixture under vigorous stirring. The solution mixture was cooled and the suspended material was filtered and repeatedly washed until pH of the washout solution was found to be neutral. The obtained GO-MNPs were finally washed with acetone (2–3 times) and put in an oven at 60 °C to evaporate the traces of moisture (Zhang et al. 2015).
Apparatus
The removal of endrin and dieldrin from sample solution was monitored using UV-vis spectrophotometer (Shimadzu, Tokyo, Japan) in the range of 200–800 nm. Transmission electron microscopy (TEM) was used to determine size and morphology of GO and GO-MNPs. The TEM images of nanomaterials were taken (200 kV accelerating voltage) by placing a few drops of colloidal solution of GO and GO-MNPs on a carbon grid. Energy-dispersive X-ray spectroscopy (EDX) connected with scanning electron microscope (SEM) was employed for determination of composition of MNPs. The functional groups present on the surface of GO and GO-MNPs were confirmed by measurement with Fourier transform infrared spectroscopy (FTIR), Type-IRA affinity (Shimadzu, Japan).
Procedure for removal of endrin and dieldrin using GO-MNPs
Figure 1 displays the schematic procure for separation and monitoring of endrin or dieldrin pesticides from aqueous sample solution using GO-MNPs as a separating probe. Endrin and dieldrin were separately taken into a 5-mL glass vial followed by the addition of nanomaterials (MNPs or GO or GO-MNPs) and total volume of the sample solution was maintained to 2 mL with DW. The pH of solution mixture was maintained to 4.0. After, the solution mixture was shaken for 30-min contact time on the vortex shaker at room temperature. After, the adsorbed pesticides on the surface of nanomaterials were separated using an external magnet. The obtained supernatant sample solution was taken into a sample cell for monitoring the removal of pesticide using UV-vis spectrophotometer.
Schematic diagram showing the removal of pesticides using GO-MNPs as a separating probe. a Glass vial containing aqueous solution of pesticide and GO-NMPs and agitated for 30 min at pH 4.0, b glass vial containing the GO-MNPs adsorbed with pesticide, c adsorbed pesticide on surface of GO-MNPs was separated with external magnet followed by the supernatant solution was transferred to sample cell, and d UV-vis used for monitoring the removal pesticide from aqueous solution
Results and discussion
Characterization of GO and GO-MNPs
The morphology and size of GO and GO-MNPs were determined with TEM. Figure 2a displays the TEM image of GO nanosheets showing the presence of number of stacked layers with wrinkles and folding. Figure 2b shows the TEM image of GO-MNPs where the two-dimensional GO was decorated with Fe3O4 MNPs. The magnetic property of synthesized GO-MNPs was important in present work because it would be helpful in fast separation of target analyte from aqueous solution by placing a magnet near the glass bottle. Figure 2c shows a glass vial containing the homogenous dispersed GO-MNPs in aqueous solution, with the precipitates settling down when the external magnet was placed near the bottle. Figure 2d presents the FTIR spectra of GO and GO-MNPs. The broad intense peak obtained around 3200 cm−1 attributed to the stretching mode of O─H bond, peak at 1747 cm−1 assigned to C═O stretching vibration mode in carboxyl group, peak at 1631 cm−1 corresponded to presence of C═C bond, and peak at 1078 cm−1 attributed to C─O stretching vibration from the epoxy group. The strong absorption band at 580 cm−1 was related to the presence of Fe─O bond from Fe3O4 MNPs. Therefore, the presence of these functional groups on the surface of GO and GO-MNPs would be responsible for better adsorption followed by the removal of pesticide from aqueous sample. The composition of MNPs was determined with EDX technique and the result is shown in Fig. 2e. The intense peaks of iron and oxygen elements were obtained in the spectrum confirmed the MNPs (Fe3O4) in the prepared composite nanomaterial.
Selection of GO-MNPs for the removal of endrin and dieldrin pesticides
The removal of endrin and dieldrin pesticides from aqueous sample by GO, MNPs, and GO-MNPs was monitored with UV-vis spectrophotometer. The absorbance value obtained at 265 nm in UV region was used to monitor the removal of endrin (Fig. 3a) and dieldrin (Fig. 3b). The decrease in signal intensity was observed when MNPs, GO, and GO-MNPs were used as separating probes for removal of both pesticides at 4.0 pH for 30 min of contact time. The maximum removal of both pesticides was determined when the GO-MNPs was used as a separating probe compared to the individual use of GO and MNPs. The use of GO and MNPs also showed the separation of both the pesticides through the adsorption process. Therefore, GO-MNPs were employed for further removal of endrin and dieldrin pesticides from sample solution.
Optimization for removal of endrin and dieldrin using GO-MNPs
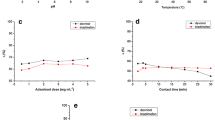
The parameters such as pH of sample solution, contact time, and dosage amount of GO-MNPs that affected the removal efficiency of endrin and dieldrin from sample solution were studied by measuring the absorbance value at 265 nm in UV-vis.
The pH of sample solution plays an important role in determining the extent of adsorption of substances on the surface of NPs. For this, 5 mg L−1 of endrin and dieldrin were taken into different glass vials containing 30 mg of GO-MNPs while maintaining the pH of sample solution from 2.0 to 12.0 using 0.1 M NaOH and 0.1 M HCl. The solution mixture was agitated for 30 min of contact time at room temperature. The results are given in Fig. 4. The higher removal of efficiency of both the pesticides was obtained when the pH of sample solution was kept between 4.0 and 6.0. This is due to the hydrogen bonding between the oxygen atom of endrin and dieldrin molecules and carboxylic acid (COOH) and hydroxyl (OH) groups found on the surface of GO-MNPs (Liu et al. 2012; Mkhoyan et al. 2009; Shrivas et al. 2017; Liang et al. 2009). In acidic condition (< pH 4.0), decrease in removal efficiency of both the pesticide was observed possibly due to the dissolving of Fe3O4 NPs which resulted the decrease in adsorption of pesticides on surface of NPs (Dreyer et al. 2010). In basic conditions (> pH 8.0), the anionic functional groups on the GO-MNPs did not favor the interaction with electronegative atoms (Cl and O atoms). Figure 5 shows mechanism of adsorption process of endrin and dieldrin through the hydrogen bonding between hydrogen of carboxylic or hydroxyl groups of GO-MNPs and oxygen of pesticide as well as the π-π stacking between pesticide and GO. Therefore, 4.0 pH of sample solution was used for removal of both pesticides using GO-MNPs.
The contact time on the removal of pesticides was studied in the range of 0–30 min with pH of 4.0 at room temperature (Meral and Metin 2014). The removal efficiency of both the pesticides was increased (decrease in the absorbance value at 265 nm) with increasing contact time from 0 to 30 min and then remained constant. The results are given in Fig. 6a. The reason for increase in the removal efficiency of both the pesticides up to 30 min was due to the increase in the adsorption reaction kinetic and then after remained constant due to the attainment of equilibrium condition for adsorption of analytes on GO-MNPs. Therefore, the 30 min was selected for further studies to get the maximum enrichment of both the pesticides from aqueous sample.
The dosage amounts of nanomaterials also affect the adsorption and removal of target analyte form aqueous samples (Zhu et al. 2012). Thus, the different dosages of GO-MNPs (0, 5, 10, 15, 20, 25, and 30 mg) were studied for removal of both the pesticides when the pH of sample solution was 4.0 for 30-min contact time at room temperature. Fig. 6b and Tables S3 and S4 display the effect of dosage amount of GO-MNPs for removal percentage of endrin and dieldrin pesticides from sample solution (formula for removal percentage is given in supporting information). The removal percentage of endrin and dieldrin pesticides was found increased with the increasing the dosage amount up to 30 mg of GO-MNPs and after there was no further enhancement. The maximum removal efficiency of both the pesticide was found when the dosage amount was 30 mg and beyond there was no sites available for attachment of target analyte on GO-MNPs. The maximum removal efficiency of pesticides was obtained when 30 mg of GO-MNPs used as a separating probe for removal of pesticides.
Langmuir adsorption isotherm of endrin and dieldrin pesticides
Langmuir adsorption model (formula is given in supporting information) was studied at different temperatures (at 0 °C, room temperature (RT) and 80 °C) in order to understand the adsorption behavior of endrin and dieldrin on the surface of GO-MNPs at the optimized conditions (Mittal et al. 2007; Jabeen et al. 2011). The results are given in Fig. 7a, b and Tables S5 and S6. Different dosage amount of GO-MNPs (5, 10, 15, 20, 25, and 30 mg) were taken into the different glass vial containing endrin/dieldrin (5 mg L−1) with pH 4.0 and the sample solution was agitated for 30 min of contact time at different temperatures. The good Langmuir adsorption curve was obtained for endrin and dieldrin with correlation of determination (R 2) of 0.972 and 0.975, respectively at room temperature. The data obtained were well fitted with Langmuir adsorption model. In addition, we calculated the slope and intercept from the same adsorption curve to know the adsorption capacity of both the pesticides. The values of slope and intercept were used to find the values of a and b (given in supplementary material in the equation) indicating the adsorption capacity (a) and affinity of binding sites (b), respectively. The adsorption capacity for dieldrin was 1 mg g−1 with GO-MNPs and binding affinity value was 193.66 L g−1 with GO-MNPs. Similarly, the adsorption capacity and binding affinity for endrin were 99 mg g−1 of GO-MNPs and 0.47 L g−1, respectively. However, the value of ab for dieldrin was 28 mg L−1 and 2.153 g L−1 for endrin. Since, the value of ab for endrin was greater than 1 showing the adsorption isotherm was found to be more favorable and this also confirmed with the experimental results. The stereoselective adsorption behavior seemed to be more favorable for endrin compared to dieldrin. The similar results were also obtained when the experiments were performed at lower temperature (0 °C) and higher temperature (80 °C) revealing the change in temperature did not alter the results for adsorption behavior of both pesticides on the surface of GO-MNPs.
Adsorption kinetics for endrin and dieldrin on the surface of GO-MNPs
We also studied the pseudo-first-order (Ho et al. 2000) and pseudo-second-order kinetic models (Ai et al. 2011) as according to formula given in supporting information for both the pesticides to understand the selective adsorption behavior of pesticides on GO-MNPs (Rahimi et al. 2011). For this, both the pesticides (5 mg L−1) were taken into a glass vial containing 30 mg of GO-MNPs with pH 4.0 and the contact time (t) was varied from 2 to 30 min. The suitability of the model was evaluated by calculating the correlation of determination (R 2) between the Qt (amount of pesticides adsorbed per unit weight of adsorbent at certain time) and t (contact time) for pseudo-first order, and t/Qt and t for pseudo-second-order. The results obtained are given in Figs. 8a, b and Tables S7 and S8. The higher value of R 2 (close or equal to 1) demonstrated the better suitability of the model to explain the adsorption kinetics of both the pesticides on the surface of GO-MNPs. The R 2 obtained for dieldrin (0.991) and endrin (0.998) showing pseudo-first order and pseudo-second order, respectively at same contact time and experimental conditions. Further, the value of rate constant for dieldrin was 3.89 mg g−1 min−1 (pseudo-second order) and for endrin was 1.55 mg g−1 min−1 (pseudo-first order). This showed that the rate of adsorption for dieldrin was found more than endrin. The difference in adsorption behavior of both the pesticides is probably due to the difference in their configurations of oxygen entities in their chemical structure resulted in the different interaction and adsorption behavior on the surface of GO-MNPs.
Comparison of adsorption capacity of pesticides using GO-MNPs with other reported method
Table 1 demonstrates the comparison for the adsorption capacity of pesticides using GO-MNPs with different nanomaterials (Li et al. 2013, Hoan et al. 2016; Chandra et al. 2010; Zhu et al. 2011; Yao et al. 2012; Fan et al. 2012). Higher adsorption capacity of dieldrin was obtained with GO-MNPs compared to other nanomaterials used for separation of different chemical species. However, the lower adsorption capacity was acquired when GO-MNPs were used as a separating probe for endrin compared to other chemical species. The reason for obtaining a higher adsorption capacity for dieldrin is due to the stereoselective adsorption behavior of pesticide on the surface of GO-MNPs compared to endrin.
Conclusions
In summary, GO-MNPs were successfully demonstrated for removal of endrin and dieldrin from sample solution dependent on the pH, contact time, and dosage amount of NPs. The Langmuir adsorption isotherm study showed the different adsorption of behavior of both pesticides on the surface of GO-MNPs due to the endo- and exo-position of oxygen atom in the structure of pesticides. The adsorption process seems to be directed by π-π stacking as well as hydrogen bonding by interaction with oxygen entities of pesticides to hydroxyl groups present on the surface of GO-MNPs. In addition, endrin showed a pseudo-first-order kinetic model whereas that of dieldrin followed the pseudo second order showing the different adsorption behavior of both the pesticides on the surface of GO-MNPs due to the endo- and exo- position of endrin and dieldrin, respectively. The results obtained with present investigation demonstrate the development of low-cost technology for stereoselective removal of pesticide from environmental water samples.
References
Ai L, Zhang C, Liao F, Wang Y, Li M, Meng L, Jiang J (2011) Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater 198:282–290
Altintıg E, Altundag H, Tuzen M (2017) Ahmet Sarı, effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent. Chem Eng Res Des 122:151–163
Ang PK, Chen W, Wee ATS, Loh KP (2008) Solution-gated epitaxial graphene as pH sensor. J Am Chem Soc 130:14392–14393
Cao Y, Li X (2014) Adsorption of graphene for the removal of inorganic pollutants in water purification: a review. Adsorption 20:713–727
Chandra V, Park J, Chun Y, Lee JW, Hwang IC, Kim KS (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4:3979–3986
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Fan L, Luo C, Li X, Lu F, Qiu H, Sun M (2012) Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J Hazard Mater 215–216:272–279
Gao Z, Li W, Liu B, Liang F, He H, Yang S, Sun C (2011) Nano-structured polyaniline-ionic liquid composite film coated steel wire for headspace solid-phase microextraction of organochlorine pesticides in water. J Chromatogr A 1218:6285–6291
Grice ID, Salzmann M, Stiff I, Griffiths LR (1999) Simultaneous determination of aldrin, dieldrin, heptachlor and p,p’-DDT in medicinal extracts using a novel high performance liquid chromatography method. J Liq Chromatogr Relat Technol 22:2337–2344
Ho YS, McKay G, Wase DAJ, Forster CF (2000) Study of the sorption of divalent metal ions on to peat. Adsorpt Sci Technol 18:639–650
Hoan NTV, Thu NTA, Duc HV, Cuong ND, Khieu DQ, Vo F (2016) Fe3O4/reduced graphene oxide nanocomposite: synthesis and its application for toxic metal ion removal. J Chem 2418172:1–10
Jabeen H, Chandra V, Jung S, Lee JW, Kim KS, Kim SB (2011) Enhanced Cr(VI) removal using iron nanoparticle decorated grapheme. Nano 3:3583–3585
Krechniak J, Foss W (1982) Determination of aldrin, dieldrin and endrin in the air by gas chromatography. Med Pr 33:211–214
Kuilla T, Bhandra S, Lee JH (2010) Recent advances in graphene based polymer nanocomposites. Prog Polym Sci 35:1350–1375
Li B, Cao H (2011) ZnO@graphene composite with enhanced performance for the removal of dye from water. J Mater Chem 21:3346–3349
Li J, Zhang S, Chen C, Zhao G, Yang X, Li J, Wang X (2012) Removal of Cu(II) and fulvic acid by graphene xxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl Mater Interfaces 4:4991–5000
Li L, Fan L, Sun M, Qiu H, Li X, Duan H, Luo C (2013) Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin–chitosan. Colloids Surf B 107:76–83
Liang J, Huang Y, Zhang L, Wang Y, Ma Y, Guo T, Chen Y (2009) Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv Funct Mater 19:2297–2302
Liu F, Chung S, Oh G, Seo TS (2012) Three-dimensional graphene oxide nanostructure for fast and efficient water- soluble dye removal. ACS Appl Mater Interfaces 4:922–927
Liu J, Cui L, Losic D (2013) Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater 9:9243–9257
Meral K, Metin O (2014) Graphene oxide magnetite nanocomposite as an efficient and magnetically separable adsorbent for methylene blue removal from aqueous solution. Turk J Chem 38:775–782
Mittal A, Kurup L, Mittal J (2007) Freundlich and Langmuir adsorption isotherms and kinetics for the removal of tartrazine from aqueous solutions using hen feathers. J Hazard Mater 146:243–248
Mkhoyan KA, Contryman AW, Silcox J, Stewart DA, Eda G, Mattevi C, Miller S, Chhowalla M (2009) Atomic and electronic structure of graphene-oxide. Nano Lett 9:1058–1063
Navale GR, Rout CS, Gohil KN, Dharne MS, Late DJ, Shinde SS (2015) Oxidative and membrane stress-mediated antibacterial activity of WS2 and rGO-WS2 nanosheets. RSC Adv 5:74726–74733
Rahimi R, Kerdari H, Rabbani M, Shafiee M (2011) Synthesis, characterization and adsorbing properties of hollow Zn-Fe2O4 nanospheres on removal of Congo red from aqueous solution. Desalination 280:412–418
Rodrigues MVN, Reyes FGR, Magalhaes PM, Rath S (2007) GC-MS determination of organochlorine pesticides in medicinal plants harvested in Brazil. J Braz Chem Soc 18:135–142
Saleh TA, Sari A, Tuzen M (2017a) Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem Eng J 307:230–238
Saleh TA, Tuzen M, Sari A (2017b) Magnetic activated carbon loaded with tungsten oxide nanoparticles for aluminum removal from waters. J Environ Chem Eng 5:2853–2860
Shrivas K, Wu HF (2008) Ultrasonication followed by single-drop microextraction combined with GC/MS for rapid determination of organochlorine pesticides from fish. J Sep Sci 31:380–386
Shrivas K, Nirmalkar N, Ghosale A, Thakur SS (2016) Application of silver nanoparticles for a highly selective colorimetric assay of endrin in water and food samples based on stereoselective endo-recognition. RSC Adv 6:29855–29862
Shrivas K, Ghosale A; Maji P (2017) Advanced nanomaterials for the removal of chemical substances and microbes from contaminated and waste water (chapter-4), Advanced nanomaterials for water engineering, Treatment, and Hydraulics, IGI Global publication USA, 127–161
Sinha SN (2011) Liquid chromatography mass spectrometer (LC-MS/MS) study of distribution patterns of base peak ions and reaction mechanism with quantification of pesticides in drinking water using a lyophilization technique. Am J Anal Chem 2:511–521
Torrisi F, Hasan T, Wu W, Sun Z, Lombardo A, Kulmala TS, Hsieh GW, Jung S, Bonaccorso F, Paul PJ, Chu D, Ferrari AC (2012) Inkjet-printed graphene electronics. ACS Nano 6:2992–3006
Westervelt RM (2008) Graphene nanoelectronics. Science 320:324–325
Yang ST, Chang Y, Wang H, Liu G, Chen S, Wang Y, Liu Y, Cao A (2010) Folding/aggregation of graphene oxide and its application in Cu2+ removal. J Colloid Interface Sci 351:122–127
Yang Z, Ji S, Gao W, Zhang C, Ren L, Tjiu WW, Zhang Z, Pan J, Liu T (2013) Magnetic nanomaterial derived from graphene oxide/layered double hydroxide hybrid for efficient removal of methyl orange from aqueous solution. J Colloid Interface Sci 408:25–32
Yao Y, Miao S, Liu S, Ma LP, Sun H, Wang S (2012) Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem Eng J 184:326–332
Zhang S, Zhang H, Chen D (2015) Preparation and application of Fe3O4 magnetic nanoparticles graphene sheet in the magnetic solid-phase extraction of organochiorine pesticides from water. J Chem Pharm Res 7:1378–1383
Zhao G, Ren X, Gao X, Tan X, Li J, Chen C, Huan Y, Wang X (2011) Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans 40:10945–10952
Zhu HY, Fu YQ, Jiang R, Jiang JH, Xiao L, Zeng GM, Zhao SL, Wang Y (2011) Adsorption removal of Congo red onto magnetic cellulose/Fe3O4/activated carbon composite: equilibrium, kinetic and thermodynamic studies. Chem Eng J 173:494–502
Zhu J, Wei S, Gu H, Rapole SB, Wang Q, Luo Z, Haldolaarachchige N, Young DP, Guo Z (2012) One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ Sci Technol 46:977–985
Acknowledgements
We would like to thank the Department of Science Technology, New Delhi, for awarding Kamlesh Shrivas a fast track project (NO.SB/FT/CS-128/2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 33 kb).
Rights and permissions
About this article
Cite this article
Shrivas, K., Ghosale, A., Nirmalkar, N. et al. Removal of endrin and dieldrin isomeric pesticides through stereoselective adsorption behavior on the graphene oxide-magnetic nanoparticles. Environ Sci Pollut Res 24, 24980–24988 (2017). https://doi.org/10.1007/s11356-017-0159-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0159-z