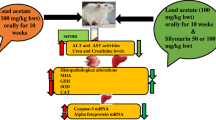
Abstract
Exposure to either lead (Pb) or γ-irradiation (IR) results in oxidative stress in biological systems. Herein, we explored the potential anti-apoptotic effect of spermine (Spm) against lead and/or γ-irradiation-induced hepatotoxicity in male albino rats. Rats were divided into eight experimental groups of ten rats each: groups including negative control, whole body γ-irradiated (6 Gray (Gy)), lead acetate (PbAct) trihydrate orally administered (75 mg/kg bw ≡ 40 mg/kg bw Pb for 14 consecutive days), and Spm intraperitoneally dosed (10 mg/kg bw for 14 consecutive days) rats and groups subjected to combinations of Pb + IR, Spm + IR, Spm + Pb, and Spm + Pb followed by IR on day 14 (Spm + Pb + IR). A significant decrease in arginase activity as well as mRNA and protein levels of Bcl-2 and p21 was observed in rats intoxicated with Pb and/or γ-irradiation compared to controls, whereas Bax mRNA and protein levels were significantly increased. Also, an increased level of nitric oxide (NO) with a reduced arginase activity was observed in liver tissues of intoxicated rats. Spm co-treatment with lead and/or γ-irradiation attenuated the increase in Bax mRNA and protein expression, while it restored those of Bcl-2 and p21 together with NO levels and arginase activity to control values. Altogether, we suggest that Spm may be useful in combating free radical-induced apoptosis in Pb-intoxicated and/or γ-irradiated rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a widely distributed environmental and industrial pollutant having no known physiological benefits (Carocci et al. 2016). Increased lead exposure consequent to industrial and environmental pollution leads to well-recognized hazardous health problems. It has been found to cause a wide range of biochemical and physiological dysfunctions that range from subclinical and subtle features to severe complications (Carocci et al. 2016). Acute toxicity of Pb is closely related to excessive occupational exposure over a short period of time and is quite uncommon. On the other hand, chronic toxicity is manifested as conditions that develop over extended periods from chronic exposure to relatively low concentrations. It is much more common and occurs in occupationally exposed populations at blood lead levels (BLLs) of about 40–60 μg/dL. If left untreated, it can be much more severe and is characterized by persistent vomiting, encephalopathy, lethargy, delirium, convulsions, and coma (Flora et al. 2007; Pearce 2007). It has been proposed that acute or chronic Pb toxicity occurs possibly due to the disturbance of prooxidant and antioxidant balance by generation of reactive oxygen species (Aykin-Burns et al. 2003; Flora 2002; Gurer and Ercal 2000). Also, it has been reported that lead exposure inhibits the activities of notable antioxidant enzymes including superoxide dismutase (SOD, EC 1.15.1.1) and catalase (CAT, EC 1.11.1.6) (Flora et al. 2007). In addition, lead inactivates other enzymes, such as δ-amino levulinic acid dehydratase (ALAD, EC 4.2.1.24), glutathione reductase (GR, EC 1.8.1.7), glutathione peroxidase (GPX, EC 1.11.1.9), and glutathione S-transferase (EC 2.5.1.18), that subsequently leads to reduction in glutathione (GSH) levels (Ahamed and Siddiqui 2007). Among the organs affected by Pb toxicity, the liver is considered the most common depository of Pb, followed by the kidney cortex and medulla (Mudipalli 2007). Acute high-dose exposure to lead has been reported to be associated with hepatotoxicity (Omotoso et al. 2015; Shalan et al. 2005), kidney dysfunction (Vargas et al. 2003; Witzmann et al. 1999), and might be an environmental risk factor for cardiovascular diseases (CVDs) (Fioresi et al. 2013; Simões et al. 2011).
Ionizing radiation (IR) is considered to be a powerful physical genotoxic agent as it affects three related systems including redox homeostasis, cell cycle regulation, and DNA repair (Islam 2017; Maier et al. 2016; Wang 2014). Exposure of mammals to IR results in the development of a complex dose-dependent series of changes, including injury to different organs, which causes changes in the structure and function of cellular components and of DNA (DNA double-strand breaks; DSBs), resulting in tissue damage and cell death via apoptosis (Abdelhalim and Moussa 2013; Ashry et al. 2017). Ionizing radiations induce the generation of free oxygen radicals, known to have adverse effects on cells and tissues that may or may not be a constituent of the target area (Luckey 2008). Free oxygen radicals interact with body tissues and cause lipid peroxidation, DNA lesions, and enzyme inactivation, all of which are mediators of radiation damage. In addition, IR induces nitrogen-containing species, indicated as reactive nitrogen species (RNS), which include nitric oxide (NO·) (Mikkelsen and Wardman 2003). Nitric oxide contributes significantly to the sensitivity of mammalian cells to ionizing radiation both in vivo and in vitro (Konopliannikov et al. 2007).
Combined action of IR and other toxic agents, including heavy metals, is of potentially great importance, because there are many incidents when interactions might occur in our environment. The observed increase of radioactive background which greatly exceeds the natural dose and also technogenic contamination of main sources of fresh water with chemical pollutants including heavy metals leads to increased radiation-chemical loading upon residents of natural and/or occupational environments. Possibly, the combined exposure to IR and lead can be extremely toxic to tissues due to elevated oxidative stress. Hence, the biological effects in organisms inhabiting such environments that are contaminated by multiple toxicants should be studied considering the multiple stressor exposures. Indeed, various in vitro and in vivo studies have addressed the combination effect of heavy metals, including lead (Pb) and cadmium (Cd), and IR (Bao et al. 2012; Iagunov et al. 2006; Mothersill et al. 2014; Olsvik et al. 2010; Osman 2013; Qiu et al. 2012; Zaichkina et al. 2001).
Apoptosis, or programmed cell death, is a process that is regulated via a number of pro- and anti-apoptotic genes encoding proteins of the B cell lymphoma-2 (Bcl-2) family, including Bcl-2-associated X (Bax) and Bcl-2, known to play a main role in determining whether or not a cell undergoes apoptosis (Danial and Korsmeyer 2004). The mitochondrial apoptotic pathway which results in mitochondrial membrane permeabilization (MMP) has been a subject of intense study. Concomitant to the increase in matrix calcium levels, a non-specific pore, known as mitochondrial permeability transition pore (MPTP), is opened which consequently leads to MMP (Hurst et al. 2017). Oxidative stress (OS), ATP depletion, and mitochondrial depolarization are among the most powerful and related factors that considerably boost the sensitivity of MPTP to calcium (Halestrap and Pasdois 2009). Consequent to MMP, a number of soluble proteins confined to the matrix and the intermembrane space are translocated to the cytosol, where they enhance cell death by numerous mechanisms (Cosentino and García-Sáez 2014).
The polyamine spermine (Spm) is widely distributed in many living organisms including animals, plants, some fungi and bacteria, and protozoa (Pegg and Michael 2010). It plays essential roles in myriad processes including proliferation, differentiation, gene transcription and translation regulation (Pegg 2014, 2016), allosteric regulation of ion channel functions such as N-methyl-d-aspartate (NMDA) receptor (Johnson 1996; Sirrieh et al. 2015), Ca2+ signaling and signal transduction (Rao et al. 2012), inhibition of glucocorticoid-induced apoptosis (Hegardt et al. 2003), protection from permeability transition and membrane potential loss in isolated liver mitochondria (Belosludtsev et al. 2014), and macromolecular synthesis such as DNA, actin, and microtubules (Igarashi and Kashiwagi 2010, 2015). In addition, it has been shown that Spm can inhibit mitochondrial permeability transition (MPT) by retaining normal reduced levels of glutathione and sulfhydryl groups, as well as preventing the production of hydrogen peroxide (H2O2) via hydroxyl radical (·OH) scavenging (Sava et al. 2006). Furthermore, Spm acts as an anti-inflammatory agent (Lagishetty and Naik 2008; LØvaas and Carlin 1991) and as a biologically important antioxidant in vitro (Rider et al. 2007; Toro-Funes et al. 2013). Therefore, Spm has been receiving considerable attention as a nutritional substance to act against oxidative stress and apoptosis. To our knowledge, no information is available to date concerning the anti-apoptotic effect of Spm against hepatotoxicity induced by lead and/or γ-irradiation in any mammalian in vivo system.
The aim of the present study was to assess the plausible hepatoprotective effect of Spm against acute lead and/or gamma irradiation-induced liver toxicity in a male albino rat experimental model.
Materials and methods
Animals
Male albino rats (n = 80), weighing between 120 and 150 g, were obtained from the Nile Company for Pharmaceuticals and Chemical Industries, Cairo, Egypt. Animals were kept for 10 days for laboratory acclimatization and were supplied with standard laboratory diet and tap water ad libitum. All animal procedures were performed according to the Ethics Committee of the National Research Center conformed to the “guide for the care and use of laboratory animals” (NIH publication, No.85-23, 1996).
Irradiation process
Whole-body gamma irradiation was implemented using the facilities provided by the National Center for Radiation Research and Technology (NCRRT). Cesium-137 irradiation unit (Gammacell 40; GC40), produced by the Atomic Energy of Canada Limited (AECL), was used (0.708 rad/s). It is characterized by a unified distribution of rays for small biological materials with no external hazards for the operating persons.
Experimental design
A total of 80 rats were divided into eight groups (10 rats in each) as follows: group I—negative control group; group II—rats were subjected to whole-body gamma irradiation as a single dose (6 Gy); group III—rats received lead acetate (PbAct) trihydrate (obtained from Fluka Chemical Co.) orally (75 mg/kg bw ≡ 40 mg/kg bw Pb) for 14 consecutive days (Farrag et al. 2007); group IV—rats received spermine (Spm; purchased from Sigma-Aldrich, St. Louis., MO, USA) intraperitoneally (10 mg/kg bw) for 14 consecutive days (Sadasivan et al. 2014); group V—rats received PbAct as in group III then exposed to gamma irradiation (6 Gy) on the 14th day of Pb injection; group VI—rats received Spm as in group IV, then exposed to gamma irradiation (6 Gy) on the 14th day of treatment; group VII—rats received PbAct orally (75 mg/kg bw) and Spm intraperitoneally (10 mg/kg bw) for consecutive 14 days; group VIII—rats received PbAct and Spm as in group VII were then irradiated on the 14th day of combined treatment. Sacrifice of all rats was carried out 24 h post irradiation.
Tissue sampling
Liver tissues were divided into two parts; one for the determination of nitric oxide level and arginase activity and the second was used for the estimation of Bax, Bcl-2, and p21 gene expression and Western blot analyses. Moreover, kidney and brain tissues were used for nitric oxide estimation. After dissection, liver, kidney, and brain tissues were washed with ice-cold isotonic saline (0.9% w/v NaCl, blotted to dryness and weighed. For each tissue, a 10% (w/v) homogenate in ice-cold 0.9% NaCl was prepared using a Tri-R STIR-R model K41 homogenizer. The homogenates were centrifuged at 4000 rpm for 15 min at 4 °C using cooling centrifuge (Universal 16 R, Germany), and the supernatants were used for the estimation of tissue nitric oxide levels in all and activity of arginase in those of the liver only.
Biochemical parameters
Determination of NO
Nitric oxide (μmol/g tissue) in liver, kidney, and brain homogenates was colorimetrically estimated according to the method described by Montgomery and Dymock (1961).
Measurement of arginase activity in liver homogenates
Arginase activity (U/g tissue) was quantified using an arginase activity assay kit (Cat No. MAK112) purchased from Sigma-Aldrich (St. Louis, MO, USA) according to the manufacturer’s instructions and guidelines.
Determination of Bax, Bcl-2, and p21 gene expression
Quantitative real-time PCR (qRT-PCR) was used to measure the expression of the pro-apoptotic (Bax) and anti-apoptotic (Bcl-2 and p21) genes. Total RNA was isolated from liver tissues using the RNeasy Mini Kit (Cat No. 74104) obtained from Qiagen Inc. (Valencia, CA, USA) according to the manufacturer’s instructions. Total RNA concentration was determined spectrophotometrically by measuring the absorbance at 260 and 280 nm. The purity of the extracted RNA was estimated by calculating A260/A280 ratio and the extent of its degradation was assessed by electrophoresis on a denaturing agarose gel.
Single-stranded cDNA was synthesized from the reverse transcription of 0.5–2 μg total RNA using random hexamer primers in a total volume of 50 μl with High Capacity cDNA Reverse Transcription Kit (Cat No. K1621) purchased from Applied Biosystems (Foster City, CA, USA). Synthesis of cDNA was performed at 37 °C for 6 min and 1 h for elongation, followed by reverse transcriptase enzyme inactivation for 10 min at 95 °C. The synthesized cDNA was stored at − 20 °C pending quantitative real-time polymerase chain reaction (qRT-PCR) analyses of gene expression. PCR primers were designed with Gene Runner Software (Hasting Software Inc., Hasting, NY, USA) from RNA sequences from GenBank (Table 1).
qRT-PCR amplifications were performed using Applied Biosystems with software version 3.1 (StepOne™, USA). The qRT-PCR mixture consisted of 5 μl cDNA and 1 μl each of forward and reverse primers in a total volume of 25 μl. The amplification conditions were as follows: 2 min at 90 °C for initial denaturation, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 60 s, and elongation at 72 °C for 60 s. All qRT-PCR reactions were run in triplicate and average Ct for each gene was calculated. The relative Ct (ΔΔCt) method was used to determine the relative fold difference in expression of target genes relative to the negative control group. Quantification of the target gene was normalized to amplification of an endogenous reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and subsequently expressed as relative to negative control.
Western blot analysis of Bcl-2 and Bax proteins
Total proteins were extracted from liver tissues of the eight tested groups stored at − 80 °C for Western blotting analysis. Protein concentration was determined by the Bradford assay (Bradford 1976). Equal amounts of the protein extracts were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and the resolved proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked at room temperature for 2 h with 5% (w/v) dried skim milk in TBST (10 mM Tris-Hcl, pH 7.5, 100 mM NaCl, and (v/v) 0.1% Tween 20) to prevent non-specific binding, and then incubated at room temperature overnight in 5% (w/v) dried skim milk in TBST with anti-Bcl-2 or anti-Bax primary antibodies (Abcam, Cambridge, MA, USA, 1:1000 dilution of each). After washing with TBST buffer, the membranes were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000 dilution) for 2 h at room temperature and then washed again with four changes of TBST at room temperature. Chemiluminescence detection was performed with the Bio-Rad detection kit according to the manufacturer’s protocol. The intensity of protein bands was quantified by densitometric analysis using Bio-Rad software (Bio-Rad, USA) and the intensity of each protein was normalized to β-actin. Protein levels were expressed as a fold increase relative to negative control (Ctrl). To verify equal protein loading and transfer, the blots were probed for β-actin using an anti-β-actin primary antibody (Thermo scientific, 1:1000 dilution) using the same protocol as above.
Statistical analysis of data
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by post hoc Tukey’s HSD (Honestly Significant Difference) test by GraphPad Prism 5 software package. Data were presented as mean ± SEM (n = 6 rats/group) with an acceptable level of significance of p ≤ 0.05. The method used for the analysis of the results is that given by Milton et al. (1986).
Results
In comparison with the control group, nitric oxide (NO) levels significantly increased in lead (Pb)-intoxicated (76.18, 62.38, and 30.5%) and γ-irradiated (96.4, 69.9, and 47.8%) groups in liver, kidney, and brain homogenates, respectively. Furthermore, a remarkable increase in NO levels in liver (132%) and kidney (98.2%) homogenates was observed in animals which received Pb and γ-irradiation (Table 2).
Administration of Spm along with Pb (Spm + Pb) resulted in a non-significant change in NO levels as compared to the control group, while a significant (30.86, 29.4, and 22.88%) decrease was observed in liver, kidney, and brain homogenates, respectively, in comparison with the Pb-treated (Pb) group. Furthermore, administration of Spm and γ-irradiation (Spm + IR) resulted in a significant (33.5, 27.9, and 28.25%) decrease in NO levels in liver, kidney, and brain homogenates, respectively, compared to the γ-irradiated (IR) group. Injection of Spm along with Pb followed by whole-body γ-irradiation (Spm + Pb + IR) ameliorated NO levels in liver, kidney, and brain homogenates with a significant decrease as compared to Pb-intoxicated (31.9, 23.1, and 21.59%) and γ-irradiated (24.08, 19.55, and 12.99%) groups. On the other hand, combination of the three agents resulted in a significant increase (33.7, 30.6, and 15.9%) in NO levels in liver, kidney, and brain homogenates, respectively, as compared to the control group (Table 2).
In male albino rats, Pb and/or whole-body γ-irradiation induced a significant decrease in liver arginase activity among the intoxicated animals in comparison with the control group. However, administration of Spm followed by γ-irradiation (Spm + IR) or along with Pb (Spm + Pb) significantly increased arginase activity in comparison with γ-irradiated or Pb-intoxicated animals, respectively. Furthermore, a significant increase in arginase activity was observed in animals treated with a combination of the three agents (Spm + Pb + IR) when compared to Pb-intoxicated animals or animals subjected to Pb-intoxication and γ-irradiation, whereas a significant decrease was observed as compared to the control group (Fig. 1).
Effect of spermine (Spm) on arginase activity (U/g tissue) in liver homogenates of male albino rats intoxicated with lead (Pb) and/or γ-irradiation (IR). Data are represented as mean ± SEM, n = 6 rats/group. a, b, and c indicate significant changes from control (Ctrl), lead, and γ-irradiation, respectively at p ≤ 0.05
In order to assess the plausible anti-apoptotic role of Spm against Pb- and/or γ-irradiation-induced apoptosis, Bax (pro-apoptotic), Bcl-2 (anti-apoptotic), and p21 (a multifunctional protein capable of suppressing apoptosis) were chosen to investigate their role in the hepatoprotection of Spm against Pb- and/or γ-irradiation-induced apoptosis. Results indicated that exposure of male albino rats to either Pb or γ-irradiation resulted in a significant decrease in Bcl-2 and p21 mRNA levels and a significant increase in expression of Bax mRNA as compared to the control group. Moreover, exposure to Pb along with γ-irradiation exhibited a significant decrease in Bcl-2 and p21 mRNA expression levels and a more pronounced increase in Bax mRNA expression (Fig. 2).
Effect of spermine (Spm) on a Bcl-2, b Bax, and c p21 gene expression in liver homogenates of male albino rats intoxicated with lead (Pb) and/or γ-irradiation (IR). GAPDH was used for normalization. mRNA levels are expressed as a fold increase relative to negative control (Ctrl). Data are represented as mean ± SEM, n = 6 rats/group. a, b, and c indicate significant changes from control (Ctrl), lead, and γ-irradiation, respectively at p ≤ 0.05
Administration of Spm alone showed no significant effect on Bax, Bcl-2, or p21 mRNA expression in liver homogenates compared to those from the control group. On the other hand, its administration along with Pb (Spm + Pb) restored Bcl-2 mRNA levels to normal control value. Although combined administration of Spm with Pb (Spm + Pb) alleviated the latter’s effect on levels of Bax and p21 mRNA expression, it still showed a significant increase in Bax and a significant decrease in p21 mRNA levels as compared to the control group (Fig. 2).
Administration of Spm along with Pb followed by γ-irradiation (Spm + Pb + IR) caused a significant decrease in hepatic Bax mRNA compared to Pb-intoxicated (Pb) or γ-irradiated (IR) groups, whereas no significant difference was observed in comparison with the control group. Furthermore, Bcl-2 mRNA expression level in liver homogenates of animals injected with Spm + Pb + IR was restored to normal control level and was significantly increased as compared to levels detected in Pb-intoxicated or γ-irradiated groups. In spite of the observed increase in expression level of p21 in the group administered with Spm + Pb + IR in comparison with Pb-intoxicated or γ-irradiated groups, the expression level was significantly decreased as compared to the control group (Fig. 2).
To further assess the anti-apoptotic role of Spm against acute Pb- and/or γ-irradiation-induced hepatotoxicity in the tested groups of animals, protein levels of Bcl-2 and Bax were evaluated by Western blot. Compared to the control group, exposure of male albino rats to either Pb or γ-irradiation (IR) resulted in a significant decrease in Bcl-2 protein levels. On the other hand, a significant increase in the expression of Bax protein was observed in the Pb-intoxicated or γ-irradiated animals. Moreover, exposure to Pb along with γ-irradiation (Pb + IR) exhibited a significant decrease in protein level of Bcl-2 and a highly significant increase in Bax protein expression (Fig. 3). The decrease of Bcl-2 protein expression and the increase in that of Bax in the Pb-intoxicated (Pb) and/or γ-irradiated (IR) male albino rats led to a highly significant decrease in the ratio of Bcl-2 to Bax, a finding that supports the involvement of the intrinsic apoptotic pathway in such experimental animals (Fig. 3).
Effect of spermine (Spm) on a Bcl-2, b Bax protein expression, and c Bcl-2/Bax ratio in liver homogenates of male albino rats intoxicated with lead (Pb) and/or γ-irradiation (IR). Β-actin was used for normalization. Protein levels are expressed as a fold increase relative to negative control (Ctrl). Data are represented as mean ± SEM, n = 6 rats/group. a, b, and c indicate significant changes from control (Ctrl), lead, and γ-irradiation, respectively at p ≤ 0.05. d Representative Western blots showing expression of Bax and Bcl-2 in liver tissues of the tested groups
In the present study, treatment with Spm exhibited no significant difference in either the expression pattern of Bcl-2 and Bax proteins or the Bcl-2/Bax ratio when compared to negative control. However, a highly significant increase in the expression of Bcl-2 protein and Bcl-2/Bax ratio together with a highly significant decrease in the expression of Bax protein was observed when compared to Pb-intoxicated (Pb) or γ-irradiated (IR) animals. In addition, it was observed that the combined administration of Spm with Pb (Spm + Pb) or with γ-irradiation (Spm + IR) alleviated their effects on the expression level of Bcl-2 protein and Bcl-2/Bax ratio. However, a significant decrease as compared to the control group is still observed (Fig. 3).
Administration of Spm along with Pb followed by γ-irradiation (Spm + Pb + IR) caused a significant increase in hepatic Bcl-2 protein level compared to Pb-intoxicated (Pb) or γ-irradiated (IR) groups, while a significant decrease was observed in comparison with the control group. A similar pattern of Bcl-2/Bax ratio was observed, except for an observed non-significant difference when compared to the γ-irradiated (IR) group (Fig. 3). On the other hand, administration of Spm along with Pb followed by γ-irradiation (Spm + Pb + IR) caused a significant decrease in hepatic Bax protein level compared to the Pb-intoxicated (Pb) group.
Collectively, the data obtained from both gene expression and Western blot analyses revealed that Spm reduced Pb- and/or γ-irradiation-induced apoptosis through downregulation of Bax, upregulation of Bcl-2, and consequent increase of the Bcl-2/Bax ratio.
Discussion
The acquired results indicated that the levels of nitric oxide (NO) in liver, kidney, and brain homogenates were significantly increased in rats exposed to lead and/or γ-irradiation. In agreement with our findings, other studies suggested the involvement of NO and reactive nitrogen species (RNS) in Pb-induced oxidative stress (Abdel Moneim 2016; Abdel-Moneim et al. 2011; El-Tantawy 2016; Samarghandian et al. 2013). The observed increase in levels of NO induced by exposure to γ-irradiation might be related to lethal injury. Consistently, several studies, including animal ones, have shown an increased formation of NO after exposure to IR (Babicova et al. 2011; Chu et al. 2015; Mansour et al. 2014; Ohta et al. 2007). It has been previously reported that NO, through the induction of DNA damage, stimulates the expression and accumulation of the tumor suppressor p53 (Ambs et al. 1997; Forrester et al. 1996). As well, the synergy between NO and ionizing radiation (IR) was shown to activate p53 by posttranslational protein modification via phosphorylation (Cook et al. 2004). In addition, NO stabilizes the tumor-suppressor protein p53 by disrupting its ubiquitylation and proteasomal degradation (Hess et al. 2005). Actually, expression of the pro-apoptotic genes, such as Bax (Miyashita et al. 1994) and that of the cyclin-dependent kinase inhibitor p21 (el-Deiry et al.1993), is transactivated by p53, whereas that of the anti-apoptotic protein Bcl-2 is downregulated (Miyashita et al. 1994). Hence, accumulation of p53 mediated by NO induces apoptosis by upregulation of Bax or cell cycle arrest by p21 upregulation (Kolb 2000). Under conditions of NO overproduction, NO reacts with superoxide anion (O2 ·−) leading to the generation of the strong biological oxidant, peroxynitrite (ONOO−). Peroxynitrite is much more reactive than its parent molecules NO and O2 ·− (Beckman and Koppenol 1996). It has been shown that peroxynitrite induces the MPT, which contributes to both apoptotic and necrotic cell death in various human pathologies (Packer et al. 1997; Virag et al. 2003). Moreover, NO is known to activate the intrinsic apoptotic pathway to induce cell death. It has been shown that the integral members of the intrinsic apoptotic pathway, Bax and Bak, are activated by NO and that cytochrome c is released from the mitochondria. The combined loss of Bax and Bak, or the individual loss of Caspase-9, completely prevents NO-induced cell death indicating that these proteins are required for this pathway (Snyder et al. 2009).
As previously reported, the significant increase in NO levels following exposure to lead or/γ-irradiation might be ascribed to the increased activity of the inducible form of nitric oxide synthase (iNOS, EC 1.14.13.39) which is largely considered to be absent under physiological conditions (Chi et al. 2006; Ibuki and Goto 1997). Increased activity of iNOS has been related to cell toxicity, as it enhanced inflammatory deleterious processes (Korhonen et al. 2005). In addition, γ-irradiation may increase NO by supporting the entrance of Ca2+ ions into the membrane in addition to the cytosol of NO-producing cells through membrane lesions induced by irradiation (Gorbunov et al. 2000).
In the current study, administration of Spm alone prompted a significant increase in NO levels in brain tissues compared to controls. It has been reported that polyamines, including Spm, modulate the activity of NMDA receptors, a subtype of the ionotropic glutamate receptors that primarily mediates calcium-permeable excitatory neurotransmission in the central nervous system (Johnson 1996; Sirrieh et al. 2015). Crespi and Rossetti (2004) have shown that the formation and release of NO is correlated with the functional activation of NMDA receptor-mediated glutamate transmission. The observed increase in NO levels by Spm alone might be accountable for glutamate-mediated excitotoxicity, i.e., glutamate killing of neurons, where NO leads to inhibition of mitochondrial respiration and causes glutamate release, which together with the decrease in membrane potential leads to the activation of NMDA receptor, causing excitotoxicity (Brown 2010; Manucha 2017). Administration of Spm along with lead or γ-irradiation group significantly (p ≤ 0.05) restored NO levels to control values, suggesting that Spm may be useful in combating the damage induced by free radicals resulting from lead or γ-irradiation toxicity and maintaining the cellular redox balance. It is believed that polyamines, including Spm, confer DNA protection by minimizing the indirect effects of radiation damage via their ability to induce DNA compaction and aggregation as well as acting as scavengers of free radicals (Douki et al. 2000; Iacomino et al. 2014).
In the present study, a significant (p ≤ 0.05) decline in arginase activity was observed after exposure to lead exposure and/or γ-irradiation, which may be due to the fact that lead and/or γ-irradiation exposure increased NO by enhancing inducible iNOS. Consequently, sufficient quantity of NO is produced which could limit the availability of L-arginine for ornithine synthesis. The competition between arginase and NOS for the common substrate, L-arginine, caused its depletion and resulted in a significant decrease in liver arginase activity and a subsequent decline in ornithine decarboxylase (ODC, EC 4.1.1.17) activity and depletion of ornithine (Wu and Morris 1998). A clear-cut recovering of liver arginase enzymatic activity in the Spm-treated group may be due to its antioxidant properties that resulted in the inhibition of iNOS activity. Hence, reduction in levels of NO with the subsequent increase in L-arginine availability resulted in a marked recovery in altered arginase activity to normal control values. The results obtained were not in agreement with those of Avtandilyan (2013) who reported in a similar study on brain and kidney tissues that arginase activity was inhibited by Spm via its effect on the allosteric regulatory site of the enzyme.
In addition to oxidative stress, apoptosis is considered a well-characterized phenomenon in lead-induced toxicity (Jia et al. 2012; Wang et al. 2015). The current study revealed a significant (p ≤ 0.05) increase in mRNA and protein levels of Bax and a decline in those of Bcl-2 in the lead-intoxicated group, suggesting that oxidative stress consequent to lead exposure resulted in mitochondria-mediated apoptosis. Previously, several studies have indicated that lead poisoning could induce apoptosis in a number of experimental systems, including rat liver (Agarwal et al. 2009; Banijamali et al. 2016; Haouas et al. 2014; Iavicoli et al. 2001; Kaczynska et al. 2011; Liu et al. 2012; Sharifi et al. 2002). Recently, it has been shown that the expression level of Bax was significantly upregulated in the liver of acute PbAc-intoxicated rats, at both mRNA and protein levels, while the levels of Bcl-2 mRNA and protein were significantly downregulated (Abdel Moneim 2016). A similar significant imbalance of Bax/Bcl-2 was reported either consequent to activation of p53 by DNA damage (Xu et al. 2006, 2008) or activation of histone hyperacetylation which reduced Bcl-2 levels and increased those of Bax (Xu et al. 2015).
Previously, it has been reported that the p53-mediated apoptotic pathway is the most praised mechanism of ionizing radiation-induced DNA damage (Lowe et al. 1993). Activation of p53 subsequent to irradiation exposure leads to the activation of Bax, a well-known downstream mediator of the transcription factor p53, which contributes to the initiation of the caspase cascade and cellular death (Haupt et al. 2003; Sang et al. 1995). In the current study, a significant increase in mRNA and protein levels of the pro-apoptotic marker Bax and a significant decrease in those of the anti-apoptotic marker Bcl-2 were observed in the γ-irradiated experimental animals. Comparable findings have been reported in a number of either in vitro or in vivo studies (Bing et al. 2013; Chen et al. 2015; Han et al. 2011; Lee et al. 2007; Yang et al. 2017).
Notably, a more pronounced change in mRNA and protein expression profiles of the apoptotic markers Bax and Bcl-2 was seen in rats co-exposed to lead and whole-body gamma irradiation as compared to individual exposures. It has been shown that both agents caused the generation of free radicals which activate apoptotic stimuli leading to the release of cytochrome c and henceforth activation of downstream caspases (Kiang et al. 2012; Liu et al. 2016; Wang et al. 2016). In addition, MPT has been shown to be involved in Pb-induced mitochondrial apoptosis (Liu et al. 2016).
Besides, our results showed that the expression of p21 mRNA was significantly (p ≤ 0.05) decreased in groups exposed to lead and/or γ-irradiation compared with the control group. Although p21WAF1/Cip1 is induced in a p53-dependent manner via which p53 promotes apoptosis and cell cycle arrest (el-Deiry et al. 1994), it has been shown that p53-dependent apoptosis occurs normally in absence of p21WAF1/Cip1 and that cells null for p21WAF1/Cip1 gene show very high apoptosis after γ-irradiation (Caelles et al. 1994; Deng et al. 1995). In accordance, it has been shown that inactivation or depletion of p21 sensitizes cells to apoptosis (Javelaud and Besançon 2002; Martinez et al. 2002). In addition, it has been indicated that the cyclin-dependent kinase (CDK) inhibitor p21 inhibits apoptosis via multiple mechanisms, which include CDK-dependent and independent events. The CDK-dependent mechanisms include inhibition of CDKs required downstream of caspases for the generation of characteristic apoptotic alterations (Sohn et al. 2006). On the other hand, the CDK-independent events include transcriptional regulation and direct binding to pro-apoptotic gene products in the cytoplasm (Delavaine and La Thangue 1999; Perkins 2002; Vigneron et al. 2006). Moreover, it has been demonstrated that the deficiency of p21 resulted in an elevated expression of an alternative reading frame p14 (ARF), which promotes p53 stability through binding to its negative regulator, mouse double minute 2 homolog (MDM-2), thus enhancing the apoptotic pathway (Javelaud and Besançon 2002). Thus, proliferation seemed to be suppressed due to the loss of p21 via a mechanism that works through a sensitized apoptotic response. Administration of Spm along with lead and/or γ-irradiation ameliorated p21 gene expression, but with a significant (p ≤ 0.05) decrease in comparison with the control group.
Spermine (Spm) in combination with lead or γ-irradiation had an ameliorating effect on Bax, Bcl-2, p21, arginase activity, and level of nitric oxide, which might be due to an antioxidant effect against reactive oxygen and nitrogen species. Furthermore, its protective effect on mitochondrial membrane occurs by electrostatic interaction of positively charged Spm with negative polar head of phospholipids, inhibition of DNA from radiation-induced strand breaks and crosslinks to proteins, which results in p53 mRNA expression decrease and subsequently p21 gene expression normalization (Igarashi et al. 1982; Rider et al. 2007). In isolated liver mitochondria, it has been reported that the inhibition of permeability transition by Spm was dose-related and occurred as a result of changes in binding properties (Dalla Via et al. 1996).
The observed anti-apoptotic role of Spm was in agreement with the findings of Sava et al. (2006) who reported that Spm can act as an inhibitor of MPT by inhibiting H2O2 production and retaining normal reduced levels of glutathione and sulfhydryl groups. Consequently, the critical thiols responsible for pore opening are prevented from being oxidized. Moreover, electrostatic interaction of Spm with anionic charges located on pore-forming structures can prevent swelling and loss of membrane potential in the rat liver mitochondria. Recently, it has been shown that exogenous Spm effectively prevented myocyte cell death by blocking the mitochondrial apoptotic pathway through regulation of MPTP and associated pathways (Wei et al. 2016).
In conclusion, the obtained results clearly indicated that the anti-apoptotic efficacy of Spm towards hepatic injury caused by lead and/or gamma irradiation is due to the fact that Spm acts as an antioxidant, which can consistently tackle the generated free radical-induced oxidative stress. The mechanism underlying these anti-apoptotic properties of Spm involves the downregulation of Bax, a member of the pro-apoptotic Bcl-2 family, upregulation of Bcl-2, a suppressor of apoptosis, and the subsequent shift of the Bcl-2/Bax ratio towards anti-apoptosis. Taken together, our results point to the capability of Spm to protect male albino rats against lead and/or irradiation-induced apoptosis through the inhibition of mitochondria-dependent apoptotic pathway. Spermine alone was found to be more profound in increasing nitric oxide levels in brain compared to normal control, which reflected the fact that Spm alone might have the capacity to cause brain excitotoxicity, a finding that needs to be further explored. Collectively, we have shown that Spm has a remarkable therapeutic property when used in combination with lead and/or gamma irradiation.
References
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One 11:e0158965
Abdelhalim MAK, Moussa SAA (2013) The biochemical changes in rats’ blood serum levels exposed to different gamma radiation doses. Afr J Pharm Pharmacol 7:785–792
Abdel-Moneim AE, Dkhil MA, Al-Quraishy S (2011) The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res 143:457–467
Agarwal S, Roy S, Ray A, Mazumder S, Bhattacharya S (2009) Arsenic trioxide and lead acetate induce apoptosis in adult rat hepatic stem cells. Cell Biol Toxicol 25:403–413
Ahamed M, Siddiqui MK (2007) Low level lead exposure and oxidative stress: current opinions. Clin Chem Acta 383:57–64
Ambs S, Hussain SP, Harris CC (1997) Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J 11:443–448
Ashry OM, Hussein EM, Abd El-Azime AS (2017) Restorative role of persimmon leaf (Diospyros kaki) to gamma irradiation-induced oxidative stress and tissue injury in rats. Int J Radiat Biol 93:324–329
Avtandilyan N (2013) The research of the role of nonureotelic arginases in polyamines biosynthesis in brain and kidney in vitro. Biol J Arm 65:26–32
Aykin-Burns N, Laegeler A, Kellogg G, Ercal N (2003) Oxidative effects of lead in young and adult Fisher 344 rats. Arch Environ Contam Toxicol 44:417–420
Babicova A, Havlinova Z, Pejchal J, Tichy A, Rezacova M, Vavrova J, Chladek J (2011) Early changes in L-arginine-nitric oxide metabolic pathways in response to the whole-body gamma irradiation of rats. Int J Radiat Biol 87:1067–1073
Banijamali M, Rabbani-Chadegani A, Shahhoseini M (2016) Lithium attenuates lead induced toxicity on mouse non-adherent bone marrow cells. J Trace Elem Med Biol 36:7–15
Bao Y, Chen H, Hu Y, Bai Y, Zhou M, Xu A, Shao C (2012) Combination effects of chronic cadmium exposure and gamma-irradiation on the genotoxicity and cytotoxicity of peripheral blood lymphocytes and bone marrow cells in rat. Mutat Res 743:67–74
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol 271:C1424–C1437
Belosludtsev KN, Belosludtseva NV, Dubinin MV, Gudkov SV, Pen'kov NV, Samartsev VN (2014) The influence of spermine on Ca(2+)-dependent permeability transition in mitochondria and liposomes induced by palmitic and α,Ω-hexadecanedioic acids. Biofizika 59:895–901 (in Russian)
Bing SJ, Ha D, Kim MJ, Park E, Ahn G, Kim DS, Ko RK, Park JW, Lee NH, Jee Y (2013) Geraniin down regulates gamma radiation-induced apoptosis by suppressing DNA damage. Food Chem Toxicol 57:147–153
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brown GC (2010) Nitric oxide and neuronal death. Nitric Oxide 23:153–165
Caelles C, Helmberg A, Karin M (1994) p53-Dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370:220–223
Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G (2016) Lead toxicity, antioxidant defense and environment. Rev Environ Contam Toxicol 238:45–67
Chen L, Liu Y, Dong L, Chu X (2015) Edaravone protects human peripheral blood lymphocytes from γ-irradiation-induced apoptosis and DNA damage. Cell Stress Chaperones 20:289–295
Chi C, Ozawa T, Anzai K (2006) In vivo nitric oxide production and iNOS expression in x-ray irradiated mouse skin. Biol Pharm Bull 29:348–353
Chu J, Zhang X, Jin L, Chen J, Du B, Pang Q (2015) Protective effects of caffeic acid phenethyl ester against acute radiation-induced hepatic injury in rats. Environ Toxicol Pharmacol 39:683–689
Cook T, Wang Z, Alber S, Liu K, Watkins SC, Vodovotz Y, Billiar TR, Blumberg D (2004) Nitric oxide and ionizing radiation synergistically promote apoptosis and growth inhibition of cancer by activating p53. Cancer Res 64:8015–8021
Cosentino K, García-Sáez AJ (2014) Mitochondrial alterations in apoptosis. Chem Phys Lipids 181:62–75
Crespi F, Rossetti ZL (2004) Pulse of nitric oxide release in response to activation of N-methyl-D-aspartate receptors in the rat striatum: rapid desensitization, inhibition by receptor antagonists, and potentiation by glycine. J Pharmacol Exp Ther 309:462–468
Dalla Via L, Di Noto V, Siliprandi D, Toninello A (1996) Spermine binding to liver mitochondria. Biochim Biophys Acta 1284:247–252
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
el-Deiry WS, Harper JW, O’connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y et al (1994) WAF-1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54:1169–1174
Delavaine L, La Thangue NB (1999) Control of E2F activity by p21Waf1/Cip1. Oncogene 18:5381–5392
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P (1995) Mice lacking p21Cip1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675–684
Douki T, Bretonniere Y, Cadet J (2000) Protection against radiation-induced degradation of DNA bases by polyamines. Radiat Res 153:29–35
El-Tantawy WH (2016) Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J Tradit Complement Med 6:327–331
Farrag AR, Mahdy KA, Abdel Rahman GH, Osfor MM (2007) Protective effect of Nigella sativa seeds against lead-induced hepatorenal damage in male rats. Pak J Biol Sci 10:2809–2816
Fioresi M, Furieri LB, Simões MR, Ribeiro RF Jr, Meira EF, Fernandes AA, Stefanon I, Vassallo DV (2013) Acute exposure to lead increases myocardial contractility independent of hypertension development. Braz J Med Biol Res 46:178–185
Flora SJS (2002) Nutritional components modify metal absorption, toxic response and chelation therapy. J Nut Environ Med 12:53–67
Flora SJ, Flora G, Saxena G, Mishra M (2007) Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol (Noisy˗le˗grand) 53:26–47
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC (1996) Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci USA 93:2442–2447
Gorbunov NV, Pogue-Geile KL, Epperly MW, Bigbee WL, Draviam R, Day BW, Wald N, Watkins SC, Greenberger JS (2000) Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Radiat Res 154:73–86
Gurer H, Ercal N (2000) Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med 29:927–945
Halestrap AP, Pasdois P (2009) The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta 1787:1402–1415
Han YT, Chen XH, Xie J, Zhan SM, Wang CB, Wang LX (2011) Purple sweet potato pigments scavenge ROS, reduce p53 and modulate Bcl-2/Bax to inhibit irradiation-induced apoptosis in murine thymocytes. Cell Physiol Biochem 28:865–872
Haouas Z, Sallem A, Zidi I, Hichri H, Mzali I, Mehdi M (2014) Hepatotoxic effects of lead acetate in rats: histopathological and cytotoxic studies. J Cytol Histol 5:256
Haupt S, Berger M, Goldberg Z, Haupt Y (2003) Apoptosis—the p53 network. J cell Sci 116:4077–4085
Hegardt C, Andersson G, Oredsson SM (2003) Spermine prevents cytochrome c release in glucocorticoid-induced apoptosis in mouse thymocytes. Cell Biol Int 27:115–121
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166
Hurst S, Hoek J, Sheu SS (2017) Mitochondrial Ca2+ and regulation of the permeability transition pore. J Bioenerg Biomembr 49:27–47
Iacomino G, Picariello G, Stillitano I, D’Agostino L (2014) Nuclear aggregates of polyamines in a radiation-induced DNA damage model. Int J Biochem Cell Biol 47:11–19
Iagunov AS, Tokalov SV, Potiavina EV, Chukhlovin AB, Kiseleva LN, Kartashev AV (2006) The combined effects of the long-term gamma-irradiation and heavy metal ions on the haematopoietic system of rats. Radiats Biol Radioecol 46:23–26 (in Russian)
Iavicoli I, Sgambato A, Carelli G, Ardito R, Cittadini A, Castellino N (2001) Lead˗related effects on rat fibroblasts. Mol Cell Biochem 222:35–40
Ibuki Y, Goto R (1997) Enhancement of NO production from resident peritoneal macrophages by in vitro gamma-irradiation and its relationship to reactive oxygen intermediates. Free Radic Biol Med 22:1029–1035
Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42:39–51
Igarashi K, Kashiwagi K (2015) Modulation of protein synthesis by polyamines. IUBMB Life 67:160–169
Igarashi K, Sakamoto I, Goto N, Kashiwagi K, Honma R, Hirose S (1982) Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys 219:438–443
Islam MT (2017) Radiation interactions with biological system. Int J Radiat Biol 93:487–493
Javelaud D, Besançon F (2002) Inactivation of p21 WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J Biol Chem 277:37949–37954
Jia Q, Ha X, Yang Z, Hui L, Yang X (2012) Oxidative stress: a possible mechanism for lead-induced-apoptosis and nephrotoxicity. Toxicol Mech Methods 22:705–710
Johnson TD (1996) Modulation of channel function by polyamines. Trends Pharmacol Sci 17:22–27
Kaczynska K, Walski M, Szereda-Przestaszewska M (2011) Ultrastructural changes in lung tissue after acute lead intoxication in the rat. J Electron Microsc (Tokyo) 60:289–294
Kiang JG, Fukumoto R, Gorbunov NV (2012) Lipid peroxidation after ionizing irradiation leads to apoptosis and autophagy. In: Catala A (ed) Lipid peroxidation. InTech Open Access Publisher, Rijeka, pp 261–278
Kolb JP (2000) Mechanisms involved in the pro-and anti-apoptotic role of NO in human leukemia. Leukemia 14:1685–1694
Konopliannikov AG, Proskuriakov SLA, Konopliannikova OA, Trishkina AL, Shteǐn LV, Verkhovskiǐ LUG, Kolesnikova AL, Trofimova TP, Mandrugin AA, Fedoseev VM, Bachurin SO, Proshin AN, Skvortsov VG (2007) The influence of some retarding agents NOS of dihydrothiazine-thiazoline rank on postradiational of recovery endogenous CFU-S-8 of mice. Radiats Biol Radioecol 47:5–9 (in Russian)
Korhonen R, Lahti A, Kankaanranta H, Moilanen E (2005) Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 4:471–479
Lagishetty CV, Naik SR (2008) Polyamines: potential anti-inflammatory agents and their possible mechanism of action. Indian J Pharmacol 40:121–125
Lee JH, Kim SY, Kil IS, Park JW (2007) Regulation of ionizing radiation-induced apoptosis by mitochondrial NADP+−dependent isocitrate dehydrogenase. J Biol Chem 282:13385–13394
Liu CM, Ma JQ, Sun YZ (2012) Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp Toxicol Pathol 64:575–582
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90:1193–1209
Løvaas E, Carlin G (1991) Spermine: an anti-oxidant and anti-inflammatory agent. Free Radic Biol Med 11:455–461
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T (1993) p53 Is required for radiation-induced apoptosis in mouse thymocytes. Nature 362:847–849
Luckey TD (2008) The health effects of low-dose ionizing radiation. J Am Phys Surg 13:39–42
Maier P, Hartmann L, Wenz F, Herskind C (2016) Cellular pathways in response to ionizing radiation and their targetability for tumor radiosensitization. Int J Mol Sci 17:102
Mansour HH, Ismael N-S, Hafez HF (2014) Ameliorative effect of septilin, an ayurvedic preparation against γ-irradiation induced oxidative stress and tissue injury in rats. Indian J Biochem Biophys 51:135–141
Manucha W (2017) Mitochondrial dysfunction associated with nitric oxide pathways in glutamate neurotoxicity. Clin Investig Arterioscler 29:92–97
Martinez LA, Yang J, Vazquez ES, Rodriguez-Vargas Mdel C, Olive M, Hsieh JT, Logothetis CJ, Navone NM (2002) p21 Modulates threshold of apoptosis induced by DNA-damage and growth factor withdrawal in prostate cancer cells. Carcinogenesis 23:1289–1296
Mikkelsen RB, Wardman P (2003) Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 22:5734–5754
Milton JS, Corbert JJ, McTeer PM (1986) Introduction to statistics, 3rd edn. D.C. Heath and Company, Toronto
Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC (1994) Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9:1799–1805
Montgomery HAC, Dymock JF (1961) Colorimetric determination of nitric oxide. Analyst (London) 86:414–416
Mothersill C, Smith RW, Heier LS, Teien HC, Lind OC, Seymour CB, Oughton D, Salbu B (2014) Radiation-induced bystander effects in the Atlantic salmon (Salmo salar L.) following mixed exposure to copper and aluminium combined with low-dose gamma radiation. Radiat Environ Biophys 53:103–114
Mudipalli A (2007) Lead hepatotoxicity and potential health effects. Indian J Med Res 126:518–527
Ohta S, Matsuda S, Gunji M, Kamogawa A (2007) The role of nitric oxide in radiation damage. Biol Pharm Bull 30:1102–1107
Olsvik PA, Heier LS, Rosseland BO, Teien HC, Salbu B (2010) Effects of combined γ-irradiation and metal (Al+Cd) exposures in Atlantic salmon (Salmo salar L.) J Environ Radioact 101:230–236
Omotoso BR, Abiodun AA, Ijomone OM, Adewole SO (2015) Lead-induced damage on hepatocytes and hepatic reticular fibres in rats; protective role of aqueous extract of Moringa oleifera leaves (Lam). J Biosci Med 3:27–35
Osman NN (2013) The role of antioxidant properties of celery against lead acetate induced hepatotoxicity and oxidative stress in irradiated rats. Arab J Nuclear Sci Appl 46:339–346
Packer MA, Scarlett JL, Martin SW, Murphy MP (1997) Induction of the mitochondrial permeability transition by peroxynitrite. Biochem Soc Trans 25:909–914
Pearce JM (2007) Burton’s line in lead poisoning. Eur Neurol 57:118–119
Pegg AE (2014) The function of spermine. IUBMB Life 66:8–18
Pegg AE (2016) Functions of polyamines in mammals. J Biol Chem 291:14904–14912
Pegg AE, Michael AJ (2010) Spermine synthase. Cell Mol Life Sci 67:113–121
Perkins ND (2002) Not just a CDK inhibitor: regulation of transcription by p21 (WAF1/CIP1/ SDI1). Cell Cycle 1:39–41
Qiu J, Zhu G, Chen X, Shao C, Gu S (2012) Combined effects of γ-irradiation and cadmium exposures on osteoblasts in vitro. Environ Toxicol Pharmacol 33:149–157
Rao JN, Rathor N, Zhuang R, Zou T, Liu L, Xiao L, Wang JY (2012) Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. Am J Physiol Cell Physiol 303:C308–C317
Rider JE, Hacker A, Mackintosh CA, Pegg AE, Woster PM, Casero RA Jr (2007) Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 33:231–240
Sadasivan SK, Vasamsetti B, Singh J, Marikunte VV, Oommen AM, Jagannath MR, Pralhada Rao R (2014) Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur J Pharmacol 729:94–99
Samarghandian S, Borji A, Afshari R, Delkhosh MB, Gholami A (2013) The effect of lead acetate on oxidative stress and antioxidant status in rat bronchoalveolar lavage fluid and lung tissue. Toxicol Mech Methods 23:432–436
Sang N, Baldi A, Giordano A (1995) The roles of tumor suppressors pRb and p53 in cell proliferation and cancer. Mol Cell Diff 3:1–29
Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A (2006) Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med 41:1272–1281
Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A (2005) Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 206:1–15
Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M (2002) Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo. Neurosci Lett 329:45–48
Simões MR, Ribeiro RF Jr, Vescovi MVA, de Jesus HC, Padilha AS, Stefanon I, Vassallo DV, Salaices M, Fioresi M (2011) Acute lead exposure increases arterial pressure: role of the renin-angiotensin system. PLoS One 6:e18730
Sirrieh RE, MacLean DM, Jayaraman V (2015) Subtype-dependent N-methyl-d-aspartate receptor amino-terminal domain conformations and modulation by spermine. J Biol Chem 290:12812–12820
Snyder CM, Shroff EH, Liu J, Chandel NS (2009) Nitric oxide induces cell death by regulating anti-apoptotic Bcl-2 family members. PLoS One 4:e7059
Sohn D, Essmann F, Schulze-Osthoff K, Janicke RU (2006) p21 Blocks irradiation-induced apoptosis downstream of mitochondria by inhibition of cyclin-dependent kinase-mediated caspase-9 activation. Cancer Res 66:11254–11262
Toro-Funes N, Bosch-Fusté J, Veciana-Nogués MT, Izquierdo-Pulido M, Vidal-Carou MC (2013) In vitro antioxidant activity of dietary polyamines. Food Res Int 51:141–147
Vargas H, Castillo C, Posadas F, Escalante B (2003) Acute lead exposure induces renal haeme oxygenase-1 and decreases urinary Na+ excretion. Hum Exp Toxicol 22:237–244
Vigneron A, Cherier J, Barre B, Gamelin E, Coqueret O (2006) The cell cycle inhibitor p21waf1 binds to the myc and cdc25A promoters upon DNA damage and induces transcriptional repression. J Biol Chem 281:34742–34750
Virag L, Szabo E, Gergely P, Szabo C (2003) Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett 140–141:113–124
Wang B (2014) Analyzing cell cycle checkpoints in response to ionizing radiation in mammalian cells. Methods Mol Biol 1170:313–320
Wang H, Wang ZK, Jiao P, Zhou XP, Yang DB, Wang ZY, Wang L (2015) Redistribution of subcellular calcium and its effect on apoptosis in primary cultures of rat proximal tubular cells exposed to lead. Toxicology 333:137–146
Wang ZK, Zhou XL, Song XB, Zhuang DM, Wang ZY, Yang DB, Wang L (2016) Alleviation of lead-induced apoptosis by Puerarin via inhibiting mitochondrial permeability transition pore opening in primary cultures of rat proximal tubular cells. Biol Trace Elem Res 174:166–176
Wei C, Li H, Wang Y, Peng X, Shao H, Li H, Bai S, Xu C (2016) Exogenous spermine inhibits hypoxia/ischemia-induced myocardial apoptosis via regulation of mitochondrial permeability transition pore and associated pathways. Exp Biol Med (Maywood) 241:1505–1515
Witzmann FA, Fultz CD, Grant RA, Wright LS, Kornguth SE, Siegel FL (1999) Regional protein alterations in rat kidney induced by lead exposure. Electrophoresis 20:943–951
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Xu J, Ji LD, Xu LH (2006) Lead-induced apoptosis in PC 12 cells: involvement of p53, Bcl-2 family and caspase-3. Toxicol Lett 166:160–167
Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH (2008) Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol 46:1488–1494
Xu LH, Mu FF, Zhao JH, He Q, Cao CL, Yang H, Liu Q, Liu X, Sun SJ (2015) Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS One 10:e0129091
Yang H, Huang F, Tao Y, Zhao X, Liao L, Tao X (2017) Simvastatin ameliorates ionizing radiation-induced apoptosis in the thymus by activating the AKT/sirtuin 1 pathway in mice. Int J Mol Med 40:762–770
Zaichkina SI, Rozanova OM, Aptikaeva GF, Akhmadieva AK, Klokov DI, Smirnova EN (2001) Induction of cytogenetic damages by combined action of heavy metal salts, chronic and acute gamma irradiation in bone marrow cells of mice and rats. Radiats Biol Radioecol 41:514–518 (in Russian)
Acknowledgements
This study was performed in the National Centre for Radiation Research and Technology-Atomic Energy Authority. Special thanks to the Radiation Research Department and Central Laboratory members for providing us with chemicals needed to accomplish this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Abu-Khudir, R., Habieb, M.E., Mohamed, M.A. et al. Anti-apoptotic role of spermine against lead and/or gamma irradiation-induced hepatotoxicity in male rats. Environ Sci Pollut Res 24, 24272–24283 (2017). https://doi.org/10.1007/s11356-017-0069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0069-0