Abstract
Background
Lead occupational exposure is now a main concern in the modern world. Lead is a non-biodegradable element with multi-devastating effects on different organs. Acute or chronic exposure to lead is reported to be one of the most important causes of infertility both in males and females basically by inducing oxidative stress and apoptosis.
Objectives
The current study scrutinized the mitigating effects of N-acetylcysteine (NAC) on lead toxicity, oxidative stress, and apoptotic/anti-apoptotic genes in the testis tissues of male rats.
Methods
Rats were randomly divided into a control group (G1) and four study groups treated with single and continuous doses of lead with and without NAC administration. Malondialdehyde (MDA), total antioxidant capacity (TAC), and 8-hydroxy-2'-deoxyguanosine (8-OHdG) were analyzed as oxidative stress biomarkers and the expression of apoptosis-related genes was studied using RT-PCR.
Results
Continuous exposure to lead caused a significant decrease in sperm count, motility, viability, and morphology (P < 0.001). Number of germinal cells, Leydig cells, spermatocytes, and the diameter of seminiferous tubule were significantly decreased (P < 0.001) in G3 group. Continuous exposure to lead significantly decreased TAC content, but increased the levels of MDA and 8-OHdG (P < 0.001). Administration of continuous dose of lead dramatically increased expression of Bax, Caspase-3, Caspase-8, Cytochrome-C, MMP2, and MMP9 genes in testicular tissue. NAC treatments not only improved morphological changes and sperm quality, but also enhanced antioxidant balance and modulated apoptosis process in testicular tissue of rats.
Conclusion
Lead exposure strongly motivated testicular cells towards apoptosis, caused an oxidant/antioxidant imbalance, and decreased sperm quality along with morphological changes in testis cells. NAC treatments was associated with protective effects on testicular tissue mainly by rebalancing of the antioxidants capacity, as well as downregulation of apoptosis-related genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a chemical and potent environmental toxicant that naturally exists in the environment [1]. This heavy element can be absorbed and stored in bones, blood, and various tissues. Lead poisoning can be associated with serious and sometimes fatal conditions such as cardiovascular disease, renal and kidney failure, neurological disorders, hemolytic anemia, bone injury, and cancers [2,3,4,5]. Based on the previous studies, chronic exposure to lead may affect male reproductive functions [6, 7]. It has been also reported that chronic exposure to lead may negatively affect sperm quality such as sperm count, motility, and morphology and consequently increase the risk of infertility [8, 9]. Nevertheless, data around the effects of lead on male fertility are very controversial and further studies are needed for a better understanding of its cellular and molecular mechanisms of toxicity.
Some studies suggested that lead may trigger testicular cells damage and poor quality of sperm through multiple mechanisms, especially by depleting antioxidant capacity, reducing essential elements (e.g., Ca, Zn, and Cu), enhancing oxidative stress and activating of matrix metalloproteinases (MMPs) that ultimately cause apoptosis [10, 11]. Oxidative stress (OS) resulted by overproduction of reactive oxygen species (ROS) and triggering apoptosis process can be highlighted as a main mechanism of lead toxicity on reproductive organs [12, 13]. Overproduced ROS interacts with DNA, proteins, and lipids and causes severe damage to cells [14]. Furthermore, oxidative stress enhances expression and activation of MMPs that is subsequently associated with serious tissue injuries [15]. Rana et al. [16] reported that immunosuppressive mechanisms may contribute in Pb-induced apoptosis.
As a pivotal process of cell homeostasis, apoptosis can be triggered via different physiological and toxicological stimuli [17]. It is previously reported that lead can induce apoptosis mainly by interrupting ATP production and mitochondrial membrane disintegration. Consequently, cytochrome C releases from the intermembrane space into the cytosol and alters Bax/Bcl2 ratio that encourages cells towards the mitochondrial-dependent apoptosis [18, 19]. Morales et al. [20] revealed that heavy metals can noticeably affect DNA stability, Ca metabolism, P53, caspase 3, and Bcl2 expression and disrupt the antioxidant defense capacity. Given the undeniable role of mitochondria in cell death process, it seems that lead toxicity is conducted by overabundance of free radicals and their reaction with key macromolecules.
Considering the importance of oxidative stress in cell apoptosis and tissue injury, antioxidant therapy might be a viable solution to handle this issue [21]. N-Acetylcysteine (NAC) is an acetylated derivative of L-cysteine that applied in different medical conditions [22]. Having sulfhydryl groups, this compound is an excellent candidate for glutathione (GSH) reduction and is reported to be a useful chelator of heavy metals [23]. According to Luczak et al. [24], NAC has a direct reactivity with heavy metals, a behavior that is independent of its antioxidant capacity. Based on their study, NAC can effectively scavenge heavy metals via a conjugation process that finally make them membrane-impermeable and protects cells in different organs. NAC is widely used in different in vivo and in vitro studies for its free radical scavenging and anti-apoptotic properties [25]. It is well documented that NAC can decrease MDA formation, effectively reduce DNA cleavage, and increase percentages of viability in HepG2 cells treated with lead acetate [26]. NAC treatment is also shown to be linked with a downregulation of caspase 3, 8, Bcl2 and matrix metalloproteinase 2 and 9 in rats treated with cadmium [27]. Furthermore, according to the previous documents, NAC can remarkably improve sperm parameters (volume, motility, etc.) predominantly by OS modulation [28, 29]. Therefore, this study aimed to investigate testicular toxicity of lead and supportive effects of NAC in adjusting the rate of apoptosis, oxidative stress, and improving sperm parameters in rats.
Methods
Study Samples
This study was performed on 30 male Wistar rats (weighing 150–200 g) in a period of 4 weeks. Animals were procured from Pasteur Institute of Iran (Tehran, Iran) and randomly organized into five groups (G1–G5, n = 6). A week prior to begging of the study rats were adopted to the animal’s lab condition (temperature 20–25 °C, humidity of 55–60%, and a period of 12 h of light /darkness). In control group (G1), rats were nourished with normal pellet and water. In G2 group, rats received 50 mg/kg of lead acetate (MERCK, Germany) solution on the first day of study. Animals in G3 group were treated with 2 mg/kg of lead acetate every second day. In G4 group, animals were administrated with lead acetate (50 mg/kg) and NAC (Razak Co. Iran) (50 mg/kg) solutions, and in G5, rats administrated with a continuous dose of lead (2 mg/kg) and NAC (50 mg/kg) solutions every other day. All experiments were conducted in accordance with the animal research guideline issued by Iran’s Ethical Committee and approved by the ethical committee of the Rasht Islamic Azad University. The selection of Pb concentrations, as well as NAC supplementation, was done based on previous studies [30, 31].
Sperm Analysis
Forty-eight hours after the last treatment, rats were stupefied with a blend of xylasine (10 mg/kg) and ketamine (30–50 mg/kg) and were cut at the abdominal area. The epididymis was then collected in Ham’s F10 culture medium and incubated for 30 min (37 °C, 5% CO2 pressure). Sperm parameters such as counts, motility, morphology, and viability were evaluated after centrifugation at 1000 g for 10 min. Papanicolaou’s staining method was applied for the assessment of sperms with normal morphology (26). Ten microliters of samples was placed at the center of the Makler chamber and motile sperms were counted using a light microscope (Nikon, USA). The percentage of sperm motility was documented in a sample containing 100 cells (30). Sperm viability was determined via eosin staining method (31, 32). Briefly, 10 ml of collected samples were mixed with 10 ml of 5% eosin on the sterile slides. Eosin was only absorbed by viable sperms with intact membranes.
Tissues and Blood Samples
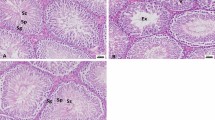
Blood samples were collected from the abdominal aorta. About 300 mg of testicular tissue of each rat was collected and then homogenized and centrifuged at 4000 × g/4 °C for 15 min [32]. The removed supernatants were stored at − 80 °C for the assessment of total antioxidant capacity (TAC), malondialdehyde (MDA), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and gene expression pattern. For histopathological study, testis tissues were kept in formalin 10% for 15 days. After dehydration and paraffin embedding, 5-µm sections were prepared using a manual microtome (Aarson, RSB-53, Japan) and stained with hematoxylin and eosin (H&E). Pathological alterations were documented by a light microscope (ECLIPSE E200, Nikon, USA).
Oxidative Stress Biomarkers
Levels of TAC and MDA were measured according to method previously explained by Benzie [33] and Rao [34] respectively. 8-OHdG levels were determined using an ELISA kit (CSB-E10140h, 96 T, Cusabio, Wuhan, China). The absorbance was measured by a micro-plate ELISA reader (Azure Biosystems, Inc, USA) at 450 nm, and the concentration of 8-OHdG was calculated from a standard curve.
Metal Analysis
Blood specimens were centrifuged at 600 × g for 10 min. Supernatants were then diluted tenfold using double-distilled water and an atomic absorption spectroscopy (AAS 7000 Shimadzu, Japan) was applied for the assessment of the levels of lead, Ca, and Zn.
Gene Expression Analysis
Tissue RNA extraction was performed using an RNX-Plus Kit (SinaClon; RN7713C) and its quantity and quality were assessed by a Nanodrop spectrophotometer (Thermo Sci., Newington, NH). cDNA synthesis and amplifications were performed according to study by Alizadeh et al. [30]. Primer sequences are demonstrated in Table 1. GAPDH was hired as the reference gene. 2−ΔCt method was considered for the rate of gene expressions [35].
Statistical Analysis
One-way ANOVA-post hoc Tukey test was selected in SPSS software (version 20) for data analysis. Data were presented as means ± SD and P < 0.05 was considered as significant.
Results
Comparison of histological parameters between the groups is depicted in Table 2. The numbers of germinal cells, Leydig cells, and spermatocytes, as well as the diameter of seminiferous tubule, were significantly decreased upon continuous exposure to lead (P < 0.001). In comparison with G1, mature form of spermatozoas are dramatically decreased in G2 and specifically in G3 (yellow arrows). The number of Leydig and interstitial cells is raised following exposure to single and continuous doses of lead; however, changes in the latter group were more noticeable. Interestingly, NAC supplementation in G4 and G5 significantly increased the number of mature sperms and spermatids in comparison with those only treated with single and continuous doses of lead in G2 and G3 respectively (Fig. 1).
Comparison of sperm parameters between different groups is presented in Table 3. The mean of sperm parameters, sperm count, percentage of motile sperm, and viable and abnormal sperm between groups was significantly different (P < 0.001). While there was no significant difference in the mean of all sperm parameters between control and rats in G2 and G4 groups, rats exposed to continuous dose of lead (G3) exhibited poor quality of sperm count, motility, morphology, and viability compared to the other groups (P < 0.001). NAC supplementation dramatically enhanced sperm quality parameters in G5 rats; however, the quality of sperm parameters was still significantly lower than animals in G1 and G2 groups (P < 0.01; Table 3). This result indicated that lead in single doses had no remarkable effects on sperm parameters.
Table 4 illustrates the comparison of the content of OS biomarkers between control and study groups. In rats of G3 group, mean values of FRAP (193.59 ± 17.61 µg/mL) were noticeably lower than other groups (P < 0.001). NAC therapy observably surged FRAP values in G5 rats (from 193.59 ± 17.61 µg/mL to 318.37 ± 62.9 µ/mL; p = 0.018). Conversely, animals in G3 group showed a remarkable difference in MDA (50.51 ± 2.55 µg/mL; P < 0.001) and 8-OHdG (2.66 ± 0.26 ng/ml; P < 0.001) contents compared to other groups. NAC treatments markedly reversed these values in G5 group (P < 0.01).
Table 5 reflects mean levels of lead, Zn, and Ca in the serum samples of rats. As it can be seen, continuous administration of lead effectively decreased mean levels of Zn (12.66 ± 1.96 mg/l) and Ca (2.14 ± 0.22 mmol/l) in G3 group in comparison with normal group. Interestingly, these values were reversely improved in G5 group where animals were treated with NAC. The mean level of lead in serum sample of animals that were fed with continuous dose of lead (G3) was significantly increased by about 67% compared to those in control group. NAC treatments significantly decreased lead levels in serum of rats that continuously exposed to lead (G5) by nearly 50% (from 2.03 ± 0.18 µg/L to 1.05 ± 0.11 µg/l; p < 0.01).
Gene expression analysis of apoptosis is shown in Fig. 2. There was a meaningful difference in the expression figures of selected genes between groups (P < 0.001). Results represented that opposed to Bcl2 genes that significantly downregulated (2.95-fold), expression of Bax (4.30-fold), Caspase 3 (3.16-fold), Caspase 9 (3.69-fold), Cyto C (3.62-fold), and MMP2 (5.38-fold) and MMP9 (3.92-fold) genes was dramatically upregulated in response to continuous concentrations of lead (Fig. 2; P < 0.001). In contrast, NAC treatment significantly improved the expression of these genes. While the expression of Bcl2 was enhanced by 1.52-fold, NAC supplementation significantly decreased the expression of Bax, Caspase-3, Caspase-8, Cyto-C, MMP2, and MMP9 by 1.81-fold, 1.78-fold, 1.55-fold, 1.67-fold, 1.92-fold, and 1.44-fold, respectively.
Discussion
Toxicity of lead on male reproductive function and fertility has increased worldwide concern. In the current study, we explored the supportive effects of NAC on tissue alterations, sperm parameters quality, oxidative stress biomarkers, and gene expression of apoptosis pathway in the testicular tissue of rats exposed to lead. Our results showed that lead exposure, particularly at continuous dose, was significantly correlated to poor quality of sperm parameters. NAC supplementation remarkably improved the sperm count, motility, normal morphology, and viability of mature sperms in exposed rats. Microscopic examinations revealed that continual exposure to lead impressively declined the number of germinal cells, spermatocytes, Leydig cells, and the diameter of seminiferous tubules. In this study, group comparisons illustrated that members treated in G3 experienced more adverse effects than those in G1 and G2. Furthermore, even after continuous treatment with NAC in G5, rats that were introduced to the chronic doses of lead experienced the worst effects. On the other hand, our findings revealed that constant treatment with NAC dramatically modulated toxic effects of lead in G5 in comparison with the G4 group that received only a single dose of NAC. In other words, both the NAC and lead showed to be extremely effective specially in long-term administration. Similarly, Almansour et al. [36] showed that long exposure to lead can cause serious microscopic damage in testis tissue, including Leydig cells’ destruction, seminiferous tubules thicknesses, spermatocyte degeneration, tubular atrophy, and spermatogenesis arrest. Offor et al. [37] reported that exposure to lead acetate (30 mg/kg) for 60 days caused a marked reduction in sperm count, viability, and normal morphology Similarly, Elgawish et al. [38] demonstrated that exposure to lead acetate (60 mg/kg) for 28 days significantly dwindled sperm reserves, percentage of sperm motility, and viability.
We also found that lead exposure is highly connected with antioxidant deficiency, lipid peroxidation redundancy, and increased DNA oxidation contents in the gonads of exposed animals. Level of lead in the serum samples of animals treated with lead at chronic doses was markedly increased while serum levels of Zn and Ca were significantly dropped. Moreover, lead consumption at chronic doses meaningfully overexpressed the apoptosis-related genes such as Bax, Caspases, Cyt-C, and MMPs in the testicular tissue of exposed rats. It might be a main reason for decreased number of mature sperms in lead-exposed rats. Recent studies have demonstrated that lead exposure is significantly contributed to higher percentage of cells apoptosis [39, 40]. Our results can be a backbone idea that lead toxicity in testis tissue is dominantly mediated through oxidative stress and apoptosis. A growing number of studies confirmed toxic effects of lead on different tissues and highlighted lead for its oxidant and inflammatory identity. For instance, Elgawish found that chronic exposure to lead caused a significant reduction in the activity of superoxide dismutase (SOD) and catalase. They also reported overproduction of caspase-3 protein in the testis of lead-treated rats [38]. Other studies reported that lead exposure significantly increased the MDA content, but significantly decreased SOD and GPX activities and the level of glutathione [41, 42]. Another research conducted in poultries revealed high MDA contents and low level of glutathione in the liver [43]. More recently, Shraideh et al. worked on the occupational lead exposure and plasma OS biomarkers. According to them, exposed people have higher level of plasma lead (~ 4–5 times) and decreased levels of TAC and GSH compared to normal individuals [44]. A previous study reported that chronic exposure to lead triggers neuronal cells apoptosis in the brain tissue of mice [39]. Xu et al. [45] found that lead exposure induces histone acetylation and subsequently vascular and cardiac cells apoptosis. Altogether, these findings indicate that morphological alterations of testis, oxidative stress, and overexpression of apoptotic mediators are likely a main mechanism of lead toxicity on testicular tissue and poor quality of sperms.
According to our results and based on the previous studies, lead toxicity is probably driven by induction of oxidative stress. Thus, antioxidant therapy might be a wise strategy to protect testicular cells from oxidative damage and apoptosis. In this current study, preservative effects of NAC were studied on morphological changes, ROS production, apoptosis, and consequently poor sperm quality caused by lead. Here, we found that NAC therapy effectively reduces cell injuries and improved sperm quality parameters. Furthermore, MDA and 8-OHdG levels were declined in the testis cells of rats treated with NAC. On the other hand, our findings demonstrated that NAC not only modulated the expression of apoptotic genes but also adjusted blood levels of Zn and Ca in lead-exposed rats. Although these mitigating effects were not observed in an ideal level in the group treated with continual doses of lead but underpinned the theory that NAC supplementation can be a viable procedure to diminish damage caused by lead intoxication. Large bodies of documents have previously reported the healing features of NAC supplementation in various tissues. For example, an in vitro study documented that over-production of ROS and overexpression of apoptotic genes related to intrinsic and extrinsic pathway can be exotically controlled by NAC treatment of cells intoxicated with Zearalenone [46]. Cay et al. [47] stated a potential role of NAC therapy in decreasing the MDA level and increasing GPX activity in testis tissue. Another study claimed that NAC can decrease the rate of apoptosis and ischemic injuries and protects testicular tissue basically via inhibition of reductive process of endoplasmic reticulum [48]. Malmir et al. [49] established that NAC supplementation is a potent antioxidant that compensates the adverse effects of paranonylphenol on spermatogenesis, testis, and levels of testosterone and MDA. Shieh et al. [50] unveiled the fact that NAC application repressed expression of Bax, Bcl2, Caspases-3, and Caspases-9 in human astrocytes treated with malathion. According to them, NAC acted as a free radical scavenger and modulated oxidative stress. In another study, Kumar et al. [51] reported that Pb exposure causes reproductive toxicity through the excess generation of free radicals and impairment of antioxidant defense, especially GSH pool. On the other hand, NAC supplementation inversed the adverse effects of Pb-induced reproductive toxicity, indicating its antioxidant potential. Chen et al. [52] considered therapeutic properties of NAC in managing of oxidative damage and inhibition of apoptosis in the brain tissue of cadmium exposed mouse. They observed a positive correlation between the NAC administration and increased activities of Cu/Zn-superoxide dismutase and catalase together with surged levels of glutathione and GPX in the brain tissue. By summarizing the results of the previous and current results, it can be stated that lead mediates its toxicity mainly by inducing oxidative stress and promoting cell death in the testis tissue. Alternately, NAC supplementation declines cytotoxic effects of lead and enhances the sperm parameters.
Conclusion
In conclusion, our findings revealed an interconnection of tissue injuries and long exposure to lead predominantly because of accelerating the generation of reactive oxygen species and detracting of the antioxidant defense system that finally encouraged cells towards the apoptosis. NAC, as an efficient compound in replenishing the glutathione reservoirs, preserves cells against the oxidative stress and enervates rate of apoptosis by increasing the integrity of mitochondrial cell membrane and scavenging of free radicals.
References
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164
Alquezar C, Felix JB, McCandlish E, Buckley BT, Caparros-Lefebvre D, Karch CM, Golbe LI, Kao AW (2020) Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci Rep 10:569
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972
Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M (2016) Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr Environ Health Rep 3:416–433
Zhang S, Sun L, Zhang J, Liu S, Han J, Liu Y (2020) Adverse impact of heavy metals on bone cells and bone metabolism dependently and independently through anemia. Adv Sci (Weinh) 7:2000383
Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA et al (2016) Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 44:275–278
Benoff S, JacobI A, Hurley R (2000) Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update 6:107–121
Colagar AH, Marzony ET, Chaichi MJ (2009) Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res 29:82–88
Li C, Zhao K, Zhang H, Liu L, Xiong F, Wang K, Chen B (2018) Lead exposure reduces sperm quality and DNA integrity in mice. Environ Toxicol 33:594–602
Adhikari N, Sinha N, Narayan R, Saxena DK (2001) Lead-induced cell death in testes of young rats. J Appl Toxicol 21:275–277
Mirnamniha M, Faroughi F, Tahmasbpour E, EbrahimiA P, Harchegani B (2019) An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev Environ Health 34:339–348
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Marzouni ET, HarcheganiI AB, Layali (2021) Chromosomal aneuploidies and associated rare genetic syndromes involved in male infertility. J Men’s Health 17:7–17
Colagar AHMET (2009) Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr 45:144–149
Khamisabadi A, Tahmasbpour E, Ghanei MSA (2020) Roles of matrix metalloproteinases (MMPs) in SM-induced pathologies. Toxin Reviews 39:24–33
Rana SV (2008) Metals and apoptosis: recent developments. J Trace Elem Med Biol 22:262–284
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
BaSalamah MA, Abdelghany AH, El-Boshy M, Ahmad J, Idris S, Refaat B (2018) Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep 8:1–13
Flora SJ, Saxena G, Mehta A (2007) Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca2+. J Pharmacol Exp Ther 322:108–116
Morales ME, Derbes RS, Ade CM, Ortego JC, Stark J, Deininger PL, Roy-Engel AM (2016) Heavy metal exposure influences double strand break DNA repair outcomes. PLoS ONE 11:e0151367
Zeisel SH (2004) Antioxidants suppress apoptosis. J Nutr 134:3179S-3180S
Schwalfenberg GK (2021) N-Acetylcysteine: a review of clinical usefulness (an old drug with new tricks). J Nutr Metab 9949453
Kelly GS (1998) Clinical applications of N-acetylcysteine. Altern Med Rev 3:114–127
Luczak MWAZ (2013) Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium(VI), cadmium(II), and cobalt(II). Free Radic Biol Med 65:262–269
Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A (2017) A review on various uses of N-acetyl cysteine. Cell J 19:11–17
Yedjou CG, Waters D, Tchounwou PB (2008) N-Acetyl-cysteine protection against lead-induced oxidative stress and genotoxicity in human liver carcinoma (HepG(2)) cells. Metal Ions Biol Med 10:419–424
Rahmani Talatappeh N, RanjiA N, Harchegani B (2021) The effect of N-acetyl cysteine on oxidative stress and apoptosis in the liver tissue of rats exposed to cadmium. Arch Environ Occup Health 76:518–525
Ciftci H, Verit A, Savas M, Yeni E, Erel O (2009) Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology 74:73–76
Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH (2019) Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol 17:1–9
Alizadeh B, Salehzadeh A, Ranji N, Arasteh A (2022) Effects of N-acetyl cysteine on genes expression of c-myc, and Ask-1, histopathological, oxidative stress, inflammation, and apoptosis in the liver of male rats exposed to cadmium. Biol Trace Elem Res 200:661–668
Jaafarzadeh, M., R. Mahjoob Khaligh, Z. Mohsenifar, A. Shabani, M. Rezvani Gilkalaei, S. Rajabi KeleshteriA. Beigi Harchegani (2021) Protecting effects of N-acetyl cysteine supplementation against lead and cadmium-induced brain toxicity in rat models. Biol Trace Elem Res.
Ma Z, Chu L, Liu H, Wang W, Li J, Yao W, Yi J, Gao Y (2017) Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats. Sci Rep 7:44819
Benzie IF (1996) Lipid peroxidation: a review of causes, consequences, measurement and dietary influences. Int J Food Sci Nutr 47:233–261
Rao B, Soufir JC, Martin M, David G (1989) Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res 24:127–134
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Almansour MI (2009) Histological alterations induced by lead in the testes of the quail Coturnix coturnix. Res J Environ Toxicol 3:24–30
Offor SJ, Mbagwu HO, Orisakwe OE (2019) Improvement of lead acetate-induced testicular injury and sperm quality deterioration by Solanum anomalum Thonn. ex. schumach fruit extracts in albino rats. J Family Reprod Health 13:98–108
Elgawish RAR, Abdelrazek HMA (2014) Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicol Rep 1:795–801
Dribben WH, Creeley CE, Farber N (2013) Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol 33:473–480
Yedjou CG, Tchounwou CK, Haile S, Edwards F, Tchounwou PB (2010) N-acetyl-cysteine protects against DNA damage associated with lead toxicity in HepG2 cells. Ethn Dis 20: S1–101–3.
AnnabiBerrahal A, Nehdi A, Hajjaji N, Gharbi N, El-Fazâa S (2007) Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C R Biol 330:581–588
Navabpour S, Yamchi A, Bagherikia S, Kafi H (2020) Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol Mol Biol Plants 26:793–802
Kumar R, Reddy AG, Anjaneyulu Y, Reddy GD (2010) Oxidative stress induced by lead and antioxidant potential of certain adaptogens in poultry. Toxicol Int 17:45–48
Shraideh Z, Badran Z, Hunaiti A, Battah A (2018) Association between occupational lead exposure and plasma levels of selected oxidative stress related parameters in Jordanian automobile workers. Int J Occup Med Environ Health 31:517–525
Li-Hui Xu, Fang-Fang Mu, Zhao J-H, He Q, Yang C-L (2015) Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS ONE 10:e0129091
Wang J, Li M, Zhang W, Gu A, Dong J, Li J et al (2018) Protective effect of N-acetylcysteine against oxidative stress induced by zearalenone via mitochondrial apoptosis pathway in SIEC02 cells. Toxins (Basel) 10:407
Cay A, Alver A, Küçük M, Işik O, Eminağaoğlu MS, Karahan SC, Değer O (2006) The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res 131:199–203
Kazaz IO, Demir S, Yulug E, Colak F, Bodur A, Yaman SO, Karaguzel E, Mentese A (2019) N-acetylcysteine protects testicular tissue against ischemia/reperfusion injury via inhibiting endoplasmic reticulum stress and apoptosis. J Pediatr Urol 15:253.e1-253.e8
Malmir M, SoleimaniMehranjani M, NaderiNoreini S, Faraji T (2018) Protective antioxidant effects of N-acetylcysteine against impairment of spermatogenesis caused by paranonylphenol. Andrologia 50:e13114
Shieh P, Jan CR, Liang WZ (2019) The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology 417:1–14
Kumar BA, Reddy AG, Kumar PR, Reddy YR, Rao TM, Haritha C (2013) Protective role of N-Acetyl L-Cysteine against reproductive toxicity due to interaction of lead and cadmium in male Wistar rats. J Nat Sci Biol Med 4:414–419
Chen S, Ren Q, Zhang J, Ye Y, Zhang Z, Xu Y et al (2014) N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol Appl Neurobiol 40:759–777
Acknowledgements
This work is part of PhD thesis which is performed at Islamic Azad University, Rasht, Iran (162373285). We would like to express our special thanks of gratitude to all the staff and members who helped us through this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedini Bajgiran, F., Khazaei Koohpar, Z. & Salehzadeh, A. Effects of N-Acetylcysteine Supplementation on Oxidative Stress and Expression of Apoptosis-Related Genes in Testicular Tissue of Rats Exposed to Lead. Biol Trace Elem Res 201, 2407–2415 (2023). https://doi.org/10.1007/s12011-022-03325-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03325-0