Abstract
Sediment tends to accumulate inorganic and persistent hydrophobic organic contaminants representing one of the main sinks and sources of pollution. Generally, contaminated sediment poses medium- and long-term risks to humans and ecosystem health; dredging activities or natural resuspension phenomena (i.e., strongly adverse weather conditions) can remobilize pollution releasing it into the water column. Thus, ex situ traditional remediation activities (i.e., dredging) can be hazardous compared to in situ techniques that try to keep to a minimum sediment mobilization, unless dredging is compulsory to reach a desired bathymetric level. We reviewed in situ physico-chemical (i.e., active mixing and thin capping, solidification/stabilization, chemical oxidation, dechlorination, electrokinetic separation, and sediment flushing) and bio-assisted treatments, including hybrid solutions (i.e., nanocomposite reactive capping, bioreactive capping, microbial electrochemical technologies). We found that significant gaps still remain into the knowledge about the application of in situ contaminated sediment remediation techniques from the technical and the practical viewpoint. Only activated carbon-based technologies are well developed and currently applied with several available case studies. The environmental implication of in situ remediation technologies was only shortly investigated on a long-term basis after its application, so it is not clear how they can really perform.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine sediment can accumulate persistent hydrophobic or-ganic contaminants (HOCs) such as polychlorinated biphe-nyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), dichloro-diphenyl-trichloroethane (DDT), and heavy metals (Nikolaou et al. 2009a, 2009b; Lofrano et al. 2016). Sediment-bound pollutants pose major concerns for human health and the environment, showing combined effects that are still largely unknown (USEPA, US Environmental Protection Agency 2005; Libralato et al. 2009, 2010a, 2010b; Mamindy-Pajany et al. 2010; Hurel et al. 2016). As a result, remediation of contaminated sediments has raised a great deal of scientific and public concern around the world representing a huge actual challenge both under a technical and technological viewpoint.
Sediment remediation techniques are commonly classified as in situ (i.e., treatments operating where the contamination is present with no sediment dredging) and ex situ (i.e., treatments including sediment dredging or resuspension phenomena to some extent). Nevertheless, dredging still remains an important issue; like for hotspots, dredging activities can heavily remobilize sediment like as the associated pollution via washing out events (Arizzi Novelli et al. 2006; Libralato et al. 2008; Krull et al. 2014; Chakraborty et al. 2014).
From 26 dredging projects carried out by the National Research Council (NRC, National Research Council 2007), systematic difficulties were observed in achieving target cleanup thresholds in addition to the impairment of sediment-associated benthic ecosystem. During the remediation of sediment in large-scale contaminated sites like Hunters Point Naval Shipyard (San Francisco, CA, USA), ex situ techniques were ineffective: economic, environmental, and technical goals were not met (Zimmerman et al. 2004).
The development of cost-effective sediment management strategy requires a multi-approach assessment including in situ treatment alternatives, unless dredging is compulsory to reach a desired bathymetric level. Since they allow sediment remediation avoiding excavation and transport, remediation footprint and cost savings could be significantly optimized. The main disadvantages are related to long-lasting procedures (months or years), uncertainty about the treatment uniformity due to the variability of sediment and aquifer characteristics, and the overall efficiency of the process is more difficult to verify.
This paper proposed an overview of existing in situ sediment remediation treatments: (i) providing a synthetic overview of the physico-chemical performance of contaminants’ reduction, (ii) refining papers on the basis of the description of detailed experimental design and the application of standardized methods, and (iii) supporting a “mind-the-gap” approach stressing on missing data to support hazard assessment. A special focus was devoted to three main technological clusters identified for in situ remediation: (i) physical and chemical treatments, (ii) biological treatments, and (iii) hybrid solutions. Results were discussed considering the characteristics of contaminants and their removal efficiency, how long the remediation might last, the role of natural organic matter (NOM) and of potential by-products, and their ecotoxicological implications, including remediation design and general costs as well.
Techniques and technologies for in situ sediment remediation
Apart from the no-action in situ approach, like monitored matural attenuation/recovery (MNA/R) (USEPA, US Environmental Protection Agency 2014), only direct or active interventions might produce a significant reduction in sediment contamination level in a reasonable time (e.g., from months to few years).
Physical and chemical treatments
In situ amendment: active mixing and thin capping
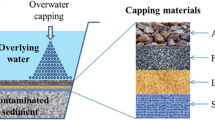
Two main approaches could be used to remediate contaminated sediment: (i) active mixing and (ii) thin capping. Active mixing consists of mixing contaminated sediments with natural substrates or other inert materials. In both cases, the bioactive surface layer of sediment is able to transfer contaminants from sediment to strongly binding sorbent particles, reducing their bioavailability to benthic organisms and contaminant flux into the water column and thus the potential general accumulation in the aquatic food web (Ghosh et al. 2011).
Thin capping consists of one or more layers of amendment (e.g., sand and NOMs) actively reducing the overall cap thickness required, for example when compared to conventional sand cap (Wessels Perelo 2010).
In the last two decades, several authors evaluated in situ amendment introducing various sorbents such as activated carbon (AC), organoclay, apatite, biochar, coke, zeolites, and zerovalent iron (ZVI) into contaminated sediments (USEPA, US Environmental Protection Agency 2013a). Amendments tend to modify sediment geochemistry increasing contaminant binding and stability in order to reduce its risk to human health and the environment. Among all, AC, organoclay, and apatite were identified as particularly promising sorptive amendments for in situ sediment remediation (USEPA, US Environmental Protection Agency 2013b). But several data about their potential side effects are still missing. Except for AC and ZVI, (eco-)toxicity data are scarce or still unavailable.
As shown in Tables 1 and 2, most studies are referred to AC administration. Several laboratory experiments and recent field studies demonstrated that AC showed significant reductions in chemical concentration and biological availability of polychlorinated biphenyls (PCBs) (Zimmerman et al. 2004; Werner et al. 2005; Cho et al. 2009; Beckingham and Ghosh 2011; Cho et al. 2012), polyaromatic hydrocarbons (PAHs) (Hale et al. 2010; Cornelissen et al. 2011; Hale et al. 2012; Meynet et al. 2012), and dichlorodiphenyltrichloroethane (DDT) (Tomaszewski et al. 2007) by active mixing and thin capping.
Considering a series of differentially polluted sediment samples, Hale et al. (2010) and Hale and Werner (2010) highlighted that 1–5% AC can reduce the pore water concentration of PCBs, PAHs, DDT, dioxins, and furans from 70 up to 99%. Organoclay effectively removed soluble organics and non-aqueous phase liquids (NAPLs) such as oils, chlorinated solvents, and PAHs (Alther 2002a, 2002b). Apatite facilitated the immobilization of metals including Cu, Pb, and Zn (Knox et al. 2008). Laboratory results demonstrated that the effectiveness of sorbents in lowering contaminant bioavailability increased with decreasing amendment particle size, growing dose, greater mixing, and contact time (Zimmerman et al. 2005; Ghosh et al. 2011), but it could vary for various amendments with similar surface areas (Tomaszewski et al. 2007).
As shown in Table 3, some patented commercial products are available yet and some of them were applied at full-scale remediation projects like for capping (Table 2), but every technological approach must be considered on a case-by-case basis. In fact, organoclay® MRM (Table 3) can enhance the production of methyl mercury in presence of sulfate-reducing bacteria. Short-, medium-, and long-term monitoring surveys should be carried out after remediation activities to verify the amendment stability in sediment within real exposure scenarios.
Considering the Hunters Point Shipyard case study (Table 1), Choi et al. (2016) focused on the importance of developing and applying decision-making frameworks for in situ sediment AC remediation, including a modeling approach supporting long-term prediction and engineering design. The modeling framework compared various design alternatives for treatment optimization and estimation of long-term effectiveness over 10–20 years under slow mass transfer condition in order to identify the best efficient and cost-effective solution for HOC-contaminated sediment treatment.
Solidification/stabilization
In situ solidification/stabilization (S/S) treatment involves the addition of chemicals and/or cements to encapsulate contaminated sediment and/or convert pollutants into less soluble, less mobile, or less toxic forms (Scanferla et al. 2009; Wang et al. 2015). Unless Portland cement and quicklime are the most commonly used materials for S/S, recently, new additives are available from the market (Table 3). The mixture of reagents and additives used for S/S is commonly referred as the binder and can range from a single compound to a multi-component system.
The S/S process proved to be efficient for treating sediments contaminated with heavy metals, PAHs, and PCBs (EPA 2009). S/S is most often selected for metals including lead, arsenic, and chromium because these contaminants form insoluble compounds when combined with appropriate additives. In applying S/S for treating organics, the use of some kind of organophilic clay and AC, either as pre-treatment or as additives in cement, can improve contaminant immobilization. Generally, the depth of contaminated sediment can limit its application. A series of bench tests should be performed stabilizing the best mix design capable of reducing the leaching of contaminants from the solidified mass. Mixing conditions, pH, water/binder ratio, and curing temperature were identified as the principal factors influencing solidified sediment strength and leaching behavior (Malviya and Chaudhary 2006). Since mixing and temperature are difficult to control in situ, the process can be less effective than other in situ treatments. Few examples of full-scale in situ S/S of contaminated sediments were reported (Robb et al. 2015). Small-scale immobilization has been used at Manitowoc harbor in Wisconsin (USA), where cement and fly ash slurry were added to sediment using a proprietary mixing tool and slurry injector (EPA 1994). The in situ mixing of cement with sediment for enhancing primarily compressive strength has not been proved or accepted for treatment of contaminated marine sediments in USA (EPA 1993). The use of in situ S/S process should be carefully evaluated since the treated site characteristics could be significantly modified. Some processes might result in substantial volume increase up to two times the original one. No data are available about the ability of the process to keep contaminants immobilized over time in real environmental conditions. A generalized lack of aging effects of the technology was evidenced.
Chemical oxidation
In situ chemical oxidation involves the introduction of chemical oxidant agents into the subsurface in order to transform sediment contaminants into less harmful chemical species. Contaminants amenable to treatment by chemical oxidation include benzene, toluene, ethylbenzene, and xylenes (BTEX); methyl tert-butyl ether (MTBE); total petroleum hydrocarbons (TPH); chlorinated solvents (ethenes and ethanes); PAHs; polychlorinated biphenyls (PCBs); chlorinated benzenes (CBs); phenols; organic pesticides (insecticides and herbicides); and munitions constituents (e.g., cyclotrimethylenetrinitramine, trinitrotoluene, octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine) (Flotron et al. 2005; Brosillon et al. 2015; Shih et al. 2016).
The most commonly employed chemical oxidants include hydrogen peroxide, ozone, sodium permanganate, and sodium persulfate (Shih et al. 2016). These oxidants have been able to cause rapid and complete chemical destruction of many toxic organic chemicals; other organics are amenable to partial degradation as an aid to subsequent bioremediation.
So far, most applications focused on ex situ treatments but presenting limited potentiality for in situ activities due to the difficulty related to direct addition of chemicals to sediment and the consequent environmental side effects.
Dechlorination
Nanoscale zero-valent iron (nZVI) can reduce some organic contaminants to less toxic by-products. Laboratory studies evidenced that also micrometer-scale ZVI effectively promoted sediment reductive dechlorination of PCBs, but including PCB congeners potentially more toxic than the parent compounds and increasing degradation time for larger congeners (Gardner 2004; USEPA, US Environmental Protection Agency 2013a). Additional limitations related to ZVI can include alterations to sediment geochemistry, passivation of iron by the formation of a thin layer of iron oxide, and the high cost of microscale and nanoscale iron. At now, the use of ZVI to treat contaminated sediment is limited to bench-scale studies with no pilot- or full-scale applications for in situ sediment remediation to the best of our knowledge.
Electrokinetic separation
Electrokinetic separation is an emerging technology relying on the application of a low-voltage direct current through sediment to separate and extract heavy metals, radionuclides, and organic contaminants (SMOCS 2012; Hahladakis et al. 2013; Iannelli et al. 2015). The promotion of electro-migration involves (i) positively charged chemical species (e.g., metals, ammonium ions, and some organic compounds) moving toward the cathode and (ii) negatively charged chemicals (e.g., chloride, cyanide, fluoride, nitrate, and negatively charged organic species) migrating toward the anode. Enhancing agents (e.g., surfactants reducing the interfacial tension) and co-solvents could be used in order to increase the reduction performance (Wan et al. 2009). The addition of solubilizing agents can change the characteristics of sediment particles as well as of pore water, potentially affecting the electro-osmotic flow and, consequently, the removal process of contaminants. Cyclodextrins are frequently used being biodegradable and non-toxic increasing the solubility of contaminants like PAHs by lowering their sorption at the same time (Hahladakis et al. 2013). Inert electrodes, such as carbon or graphite, or platinum, must be used since metallic electrodes may dissolve as a result of electrolysis introducing further contamination into sediment (Hahladakis et al. 2013).
Sediment flushing
In situ sediment flushing treatment removes harmful chemicals by injecting water or chemicals into sediment washing it out and conveying hydrophilic contaminants toward the extraction wells. Elutriates are pumped and treated in on-site wastewater treatment plants (SMOCS 2012). Environmentally sustainable surfactants could be used to increase the solubility of organic compounds, the flushing solution could significantly alter the physico-chemical properties of sediment. This technology can offer the potentiality of recovering metals mobilizing a wide range of organic and inorganic contaminants especially from coarse-grained soils, but it could be highly non-effective in presence of heterogeneous mixtures of contaminants because no universal flushing solution is available: a case-by-case basis approach is strongly required. The flushing technique could be effective, but current in situ applications are mainly related to soil (SMOCS 2012).
Bioremediation
Bioremediation consists of biologically driven processes to remove and/or detoxify environmental pollutants. Frequently, the complexity of sediment-water ecosystem can limit the effectiveness of in situ bioremediation, which is generally more successful when environmental conditions are carefully controlled and adequately adjusted to enhance biotransformation processes. Under this viewpoint, ex situ treatments are easier to manage.
Several treatment approaches are included in contaminated sediment bioremediation (Wessels Perelo 2010) like (i) monitored natural attenuation/recovery (NA/NR) (i.e., the action is “no action” meaning that no other activities than environmental monitoring are required leaving natural remediation to occur), (ii) biostimulation (i.e., indigenous populations eliminating pollutants are stimulated removing the factors limiting their growth), (iii) bioaugmentation (i.e., introduction of allocthonous species for the degradation of contaminants, and (iv) phytoremediation (i.e., using macrophytes and/or algae to degrade and/or remove contaminants from sediment). Wessels Perelo (2010) reviewed in situ bioremediation of organic pollutants in sediment evidencing how it is promising (i.e., lower impact and more cost-efficient) than traditional management strategies, but with some major drawbacks. In situ bioremediation takes longer (from months to several years) and is less predictable than traditional methods being valuable only to treat low-risk sites with low level or diffuse contamination not immediately impairing human health and the environment (Magar and Wenning 2006). Akcil et al. (2015) stated that when considering how to reduce the risk of contaminated sediment, it is important to recognize the capacity of natural processes to achieve remediation objectives without human intervention.
NA processes could reduce pollutants under different environmental conditions (i.e., pH and nutrient levels) (Röling and van Verseveld 2002). Frequently, NA is driven by physical mechanisms such as mixing and in-place burial of contaminated sediment with progressively cleaner sediment delivered by the watershed. Natural sedimentation process can reduce contaminants’ (bio)availability of environmental concern (CEC) limiting their downstream transport as well.
Other potentially significant mechanisms include chemical processes such as adsorption and redox reactions coupled with biodegradation. According to Wang and Tam (2012), no significant decline in total PAHs, TBT, heavy metals, and contamination “hotspots” could be detected 1 year after the removal of an old floating dock in Hong Kong SAR (South China). However, the profile of 16 PAHs changed 6 months in the impacted stations after the dock removal showing a decrease of some low-molecular-weight PAHs suggesting the presence of on-going in situ biodegradation events.
In confined hydrocarbon-rich environments, anoxic conditions tend to prevail already few centimeters below the water-sediment and the increase of oxygen availability would support biodegradation processes. The use of oxygen-releasing compounds (ORC) based on Ca or Mg peroxide has been proposed to ensure long-lasting release of oxygen in contaminated subsurface environments (Bellagamba et al. 2016). But drawbacks are present such as (i) the poor control of the oxygen rate released and the resulting oxygen availability, (ii) the need for repeated injections due to oxygen consumption/scavenging by biotic and abiotic side reactions, and (iii) ORC could have secondary harmful effects on aquatic biota (Abdallah et al. 2009). Apart from several case studies (Yu et al. 2011; Akcil et al. 2015; Matturro et al. 2015), no great additional information is available after Wessels Perelo (2010) investigation of in situ sediment bioremediation.
Hybrid solutions
Nanocomposite reactive capping
In order to enhance current capping technologies, Choi et al. (2009) focused on the development of granular AC (GAC) impregnated with reactive iron/palladium (Fe/Pd) bi-metallic nanoparticles (NPs) (i.e., reactive AC (RAC)). RAC is a smart composite for dechlorination of PCBs. Due to its high adsorption capacity, RAC actively attracts hydrophobic PCBs from sediment matrix. The concept of a “reactive” cap/barrier composed of RAC pellets contained between thin geo-textile membranes is proposed in Fig. 1.
Conceptual diagram of “reactive” capping barrier composed of GAC impregnated with Fe/Pd bimetallic particles (noted as RAC) for the adsorption and simultaneous dechlorination of PCBs in sediment (Choi et al. 2009)
In 2 days, 5 g/L of RAC achieved 86% dechlorination of 2-chlorobiphenyl (2-ClBP, 4.08 mg/L), while almost complete dechlorination was achieved in just 1 day at 20 and 100 g/L of RAC. Its scale-up and field application will take some time due to the high cost of RAC mainly due to Pd doping and synthesis time. In general, GAC showed PCB adsorption capacity in presence of ZVI as a strong reducer, while Pd speeds up the dechlorination kinetic. Critical issues like Fe and Pd leaching, adsorption, and dechlorination capacity and yield of RAC, as well as their aging and oxidation, are still under investigation along with the initial demonstration of PCB-contaminated sediment remediation using RAC (Choi et al. 2009).
Bioreactive capping
The use of physico-chemical active caps is a promising option, but possible limitations (e.g., high material costs, sorption, and reaction capacities) lead to the consideration of in situ bio-reactive caps. Specifically, biotransformation of contaminants is designed to occur within the cap matrix producing environmentally friendly reaction by-products. Biologically based active caps have the potential to keep reactivity over long time periods serving as potentially sustainable remedial options especially when degrading microorganisms are present and the necessary metabolic requirements are met (Himmelheber et al. 2011). Previous studies about the activity of microbial populations within a sediment cap demonstrated that indigenous microorganisms in the underlying sediment, including organisms capable of contaminant biotransformation, could colonize the overlying cap and, possibly, participate to contaminant bioattenuation process (Himmelheber et al. 2009).
Some recent examples of bioreactive capping were related to ZVI (Sun et al. 2010) and AC (Wang et al. 2014). An integrated ZVI–sorbent–microorganism remediation system as an in situ active capping technique was proposed to remediate nitrobenzene-contaminated (NB-contaminated) sediment; NB was reduced by ZVI to aniline that is more biodegradable than the parental compound (Sun et al. 2010). A novel bio-reactive capping barrier composed of polysulfone/GAC (PS/GAC) hybrid membrane immobilized with microorganism was developed to remediate NB-polluted sediment (Wang et al. 2014).
Microbial electrochemical technologies
Microbial electrochemical technologies (METs) are increasingly considered for remediating contaminated sediment. They rely on the capability of microorganisms to use (directly or indirectly) solid electrodes; suitably deployed in the contaminated environment, they seemed to require low maintenance and being virtually an inexhaustible source of electron donors/acceptors for the reductive/oxidative degradation of contaminants (Aulenta et al. 2011; Li and Yu 2015; Wen-Wei and Han-Qing 2015; Majone et al. 2015; Bellagamba et al. 2016) suggesting the creation of benthic microbial fuel cells (BMFC) (Wen-Wei and Han-Qing 2015).
Apart from creating an efficient electron transport route for bridging the natural redox reactions, BMFC can be used to directly supply electron donors or acceptors to sediment through tuning the local electrode potential. Thus, in some cases, the cathode can also be buried into the sediment, while an external voltage can be applied to provide the desired electrode potential for contaminant removal (Aulenta et al. 2007; Chun et al. 2013).
Bellagamba et al. (2016) explored the possibility to electrochemically manipulate the redox potential of a crude oil-contaminated marine sediment establishing in situ conductive conditions to contaminants’ biodegradation by autochthonous microbial communities. They showed that low-voltage electrolysis (2 V) applied continuously or intermittently accelerated (up to 3 times) the biodegradation of hydrocarbons from crude oil in marine sediment.
Discussion
Factors affecting technology selection should generally include (i) contaminant characteristics; (ii) removal of target contaminants (i.e., performance criteria); (iii) contaminant transformation and release; (iv) material/reagent placement, time, mechanisms, physical and chemical stability, and monitoring; (v) ecotoxicological implications; and (vi) costs.
Characteristics of contaminants
Metals and metalloids according to sediment geochemical fraction can significantly change their mobility. Since sediment composition varies from site to site due to geological, hydrographical, climatic, and socio-economic characteristics, metals and metalloids can distribute among various geochemical phases with various grades of adsorption and retention. Thus, their partitioning must be determined on a case-by-case basis before any intervention (Akcil et al. 2015). Organic pollutants may associate temporarily to the particulate matter, establishing equilibrium relations at the water-sediment interface. These sorption and desorption processes can substantially influence compound bioavailability (Viganò 2000). The direct transfer of chemicals from sediments to organisms is now considered to be a major route of exposure for many species. To evaluate the contaminant release from sediment through desorption processes, both the characteristics of sediment and overlying water column must be considered (Zoumis et al. 2001).
Over limited concentration ranges, the partitioning of HOCs between sediment and water can be described in simple terms according to a solid-water distribution coefficient (K d) based on the assumption that partitioning depends primarily upon sediment total organic carbon (TOC) as stated in Eq. 1:
where C s = sediment contaminant concentration (mg/kg), C w = water contaminant concentration (mg/L), K d = solid-water distribution coefficient (L/kg), f oc = fraction of sediment OC, and K oc = OC-normalized partition coefficient (L/kg OC). For HOCs, K oc is often correlated with octanol-water partition coefficient (K ow) of pollutants.
Removal of target contaminants
Remediation technologies should be evaluated on the basis of their compliance with site-specific goals on a site-specific basis. Due to the complexity of field activities and the lack of proper controls, like as temporal or spatial replicates, incomplete results are available for in situ treatment of contaminated marine sediment (Mayer-Pinto et al. 2010). Additional focused field-scale demonstrations would be helpful to evaluate site-specific HOCs such as dioxins, furans, and methyl mercury whose treatment effectiveness has been either variable or slow to develop (Patmont et al. 2014).
Technological efficiency
The effectiveness of in situ remediation technologies can vary on the basis of the selected technological options. Generally, physico-chemical processes are quicker (from months to years), but with a potential immediate environmental impact, while bioremediation is slower (from years to decades) having a little impact that is diluted over time.
Sediment treated with 3.4% AC showed a decrease of the total aqueous PCB concentrations up to 87 and 92% for 1- and 6-month contact times, respectively. With active mixing, the effect of AC addition to sediment on PCB aqueous equilibrium concentration is manifested relatively quickly and is not lost with time. Similarly, adding AC to sediment reduced the water equilibrium of total PAH by 74 and 84% for 1- and 6-month contact periods, respectively (Zimmerman et al. 2004).
Adsorption kinetic studies of NB onto pure polysulfone and polysulfone/GAC hybrid membranes at 20 mg/L initial concentration showed that most NB adsorption rapidly occurs in the first 2 h, reaching the adsorption equilibrium after 6 h (Wang et al. 2014). This was due to the fact that adsorbents get adsorbed into the meso-pores during the initial stages; adsorbents need to move deeper into the pores encountering larger resistance (Himmelheber et al. 2011).
Microbial reductive dechlorination of PCBs in contaminated sediment is characterized by long lag periods and low rates ranging from months to years (Wiegel and Wu 2000). This is a significant difficulty to use anaerobic bioremediation as sediment cleanup technology. However, it has been proved that the direct addition of controlled amounts of ZVI to sediment could be an effective way to reduce the lag period prior to dechlorination at PCB-impacted sites (Wessels Perelo 2010).
Influence of natural organic matter
The content of NOM can seriously affect the performance of in situ treatments. In laboratory trials, some authors demonstrated a decrease in sorbent capacity due to NOM (Koelmans et al. 2009; To et al. 2008; Cho et al. 2012). Moreover, there could be an influence of NOM from the surrounding test area such as from overlying water and deposited sediment (Cho et al. 2009). Additional NOM could also be formed in intertidal sediment under field conditions from algae biomass decay. The adsorption of pollutant molecules by sorbent could result in greater competition for the finite number of sorption sites over time. Long-term effects and fouling mechanisms need to be further evaluated especially under field conditions.
Chemical oxidation treatments are also sensitive to NOM. Ozone decomposes back to oxygen rapidly in presence of OM. In general, OM reduces the efficiency of chemical oxidation processes scavenging considerable amounts of non-selective oxidants (Cho et al. 2009).
By-products
All in situ chemical methods have the potential to generate secondary impacts with the application of treatment reagents considering their direct related toxicity and generated post-treatment by-products. Consequently, in situ chemical treatments could be applied when the contaminated area is contained while operating or water flow can be diverted for the whole treatment duration.
Ecotoxicological assessments
Current knowledge about in situ sediment remediation techniques lacks principally of their ecotoxicological implications (Libralato et al. 2008; Han et al. 2015; Libralato et al. 2016).
Several bioindicators were used to assess the potential ecotoxicological implications of AC like bacteria, annelids, molluscs, and crustaceans. Van der Mei et al. (2008) exposed Escherichia coli and Raoultella terrigena observing cell viability as endpoint. After 30 min, mortality ranged between 83 and 96 and 54–56% for acid and basic AC, respectively, and between 76 and 78 and 32–37% for positively and negatively charged AC, in that order. Jonker et al. (2009) did not observe any adverse effect on the structure of microbiological communities after exposing them to freshwater and marine sediment treated with AC (2, 4, 10, and 20% w/w). Jonker et al. (2009) demonstrated that powdered ACs can exert adverse effects to aquatic invertebrates (Lumbriculus variegatus, Daphnia magna, and Corophium volutator) based on different mechanisms stating that it should be preferably washed prior to their application as well as removed, when exhausted, preventing pollutants to be released back to the environment.
Millward et al. (2005) investigated AC (3.4 and 8.5%) effect to the marine polychaete Neanthes arenaceodentata observing an average body weight reduction of approximately 50% caused by the ingested AC interfering with nutrients’ uptake due to its affinity to lipids, carbohydrates, and proteins. At the same time, AC showed a great ability to sequester PCB. Indeed, the average PCB content in N. arenaceodentata was reduced by 82% after 1 month and 87% after 6 months of contact time. In the case of the marine polychaete Nereis diversicolor, Cornelissen et al. (2006a,b) evidenced that 2% AC reduced the PAH biota-sediment accumulation factor by 6.7-fold after 28 days. Similarly, no or slight effects were evidenced by Ho et al. (2004), McLeod et al. (2007, 2008), and Janssen et al. (2012) considering other species.
The potential effects of organo-clay, apatite, and biopolymers were investigated with several biological models like annelids (Paller and Knox 2010; Rosen et al. 2011), molluscs (Paller and Knox 2010), crustaceans (Paller and Knox 2010; Rosen et al. 2011), and fish (Rosen et al. 2011). Adverse effects were absent or slight, but sometimes measurable when in presence of biopolymers.
Also, nZVI was investigated several times (Libralato et al. 2016), including bacteria (Lee et al. 2008; Diao and Yao 2009), fungi, yeasts (Diao and Yao 2009; Otero-González et al., 2013), algae (Keller et al. 2012), rotifers (Nogueira et al. 2015), molluscs (Kadar et al. 2009, 2010), crustacean (Libralato 2014), and fish (Li et al. 2009), but effect data on in situ treated soil or sediment are substantially absent. Results are very case specific, generally with slight effects at the considered exposure concentrations.
Technology footprint and general costs
The way in which amending agents are administered in situ is of extreme importance to optimize the whole remediation performance (Cho et al. 2012). Amendments can be contained in a mat, applied in bulk onto the sediment surface, mixed into the sediment, or added as part of a sand cap or as a layer within a sand cap (USEPA, US Environmental Protection Agency, 2013a, b). When amendments are mixed into the sediment, heavy equipment has to be used, potentially producing stressing events to benthic communities along with high handling costs during placement. Analogously, when applying in situ chemical treatments, it is necessary to ensure that treatment reagents are completely mixed with the contaminated sediment layer by the introduction of one or more reagents, additives, and/or nutrients onto the sediment by spreading and settling or injecting them inside it through tubes, pipes, or other devices. Further methods to administer in situ treatments consist in isolating sediment from the surrounding environment especially when reagents or process conditions used can be harmful to the environment.
In general, using in situ treatment appears to be less expensive than ex situ treatment or disposal of contaminated sediment. It has already been estimated that the cost of a typical AC treatment is at least an order of magnitude lower than sediment dredging and disposal (Ghosh et al. 2011). However, the full technological cost must be evaluated through pilot studies on a case-by-case basis.
The cost of passive and reactive supplies depends on the type of material, purity, size, delivery, source, material processing needs, and means of application. McDonough et al. (2007) reported that cap placement costs for large-scale site (circa 405 ha) at about 25/0.836 $/m2, excluding the material cost. The breakup of cap placement costs is approximately as follows: (a) mobilization/demobilization 1/0.836 $/m2, (b) cap placement 10/0.836 $/m2, (c) project management 2/0.836 $/m2, (d) monitoring 10/0.836 $/m2, and (e) miscellaneous (site preparation, construction management, design, and permit) 2/0.836 $/m2.
Conclusions
Apart from the kind of amendment potentially useful in active capping or mixing, little is known regarding amendment application techniques, application rates, and amendment combinations that could maximize the immobilization of contaminants. As with other remedial alternatives (such as capping and dredging), the long-term permanence of amendments and their ability to retain contaminants over time are not well understood. Significant gaps remain between the current understanding of the in situ technology and the level of engineering know-how necessary for widespread implementation as an alternative remedial action mainly related to contaminant reduction efficiency under a spatial and temporal scale. Most of the times, due to the lack of real scale applications, costs are not available like as the full range of drawbacks.
The effectiveness of some amendments, such as AC and Organoclay™, has been demonstrated just in a small number of field applications, while other amendments, such as zerovalent iron (ZVI), phosphate additives, and biopolymers, are still in the bench-scale or pilot-testing phase. Even though some of these materials have been used in other environmental applications, such as groundwater and off-gas treatments, there are a limited number of projects and available performance data on their effectiveness in treating contaminated sediments.
The use of physico-chemical-based active barriers seems to be promising; however, the adsorption and/or reaction capacities of reactive materials are limited, and the contaminants cannot be completely removed from the environment. Capped sediment can also represent a future risk by excessive contaminant breakthrough due to diffusion or advection. Hence, the enhancement of biodegradation by effective microorganisms into the capping system is necessary. Biologically based active caps could have the potential to maintain reactivity over long periods of time serving as a sustainable remedial option also hosting microorganisms able to biotransform contaminants.
Future research efforts should (i) investigate long-term effects of treatment activities (i.e., years after the end of the remediation); (ii) develop database about pros and cons of adsorptive materials considering both their physico-chemical and ecotoxicological implications coupling contaminant removal and environmental compatibility issues; potentially, the database should be extended to all new proposed in situ remediation technologies; and (iii) provide cost-benefit analysis considering life cycle impact analysis as well.
References
Abdallah E, Goncalves AA, Gagnon GA (2009) Oxygen release compound as a chemical treatment for nutrient rich estuary sediments and water. J Environ Sci Health A Tox Hazard Subst Environ Eng 44(7):707–713
Alther G (2002a) Using organoclays to enhance carbon filtration. Waste Manag 22(5):507–513
Alther G (2002b) Organoclays remove organics and metals from water. In: Kostecki PT, Calabrese EJ, Dragun J (eds) Contaminated soils, vol 7. Amherst Scientific Publishers, Amherst, MA, pp. 223–231
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Arizzi Novelli A, Losso C, Libralato G, Tagliapietra D, Pantani C, Volpi Ghirardini A (2006) Is the 1:4 elutriation ratio reliable? Ecotoxicological comparison of four different sediment: water proportions. Ecotoxicol Environ Saf 65:306–313
Aulenta F, Tocca L, Verdini R, Reale P, Majone M (2011) Dechlorination of trichloroethene in a continuous-flow bioelectrochemical reactor: effect of cathode potential on rate, selectivity, and electron transfer mechanisms. Environ Sci Technol 45:8444–8451
Aulenta F, Pera A, Rossetti S, Petrangeli Papini M, Majone M (2007) Relevance of side reactions in anaerobic reductive dechlorination microcosms amended with different electron donors. Water Res 41(1):27–38
Beckingham B, Ghosh U (2011) Field-scale reduction of PCB bioavailability with activated carbon amendment to river sediments. Environ Sci Technol 45(24):10567–10574
Bellagamba M, Viggi CC, Ademollo N, Rossetti S, Aulenta F (2016) Electrolysis-driven bioremediation of crude oil-contaminated marine sediments. New Biotechnol. doi:10.1016/j.nbt.2016.03.003
Brosillon S, Bancon-Montigny C, Mendret J (2015) Study of photocatalytic degradation of tributyltin, dibutylin and monobutyltin in water and marine sediments. Chemosphere 109:173–179
Chakraborty S, Bhattacharya T, Singh G, Maity JP (2014) Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: a biomonitoring approach for pollution assessment. Ecotoxicol Environ Saf 100:61–68
Cho YM, Werner D, Choi Y, Luthy RG (2012) Long-term monitoring and modeling of the mass transfer of polychlorinated biphenyls in sediment following pilot-scale in-situ amendment with activated carbon. J Contam Hydrol 129:25–37
Cho YM, Smithenry DW, Ghosh U, Kennedy AJ, Millward RN, Bridges TS, Luthy RG (2007) Field methods for amending marine sediment with activated carbon and assessing treatment effectiveness. Mar Environ Res 64:541–555
Cho YM, Ghosh U, Kennedy AJ, Grossman A, Ray G, Tomaszewski JE, Smithenry DW, Bridges TS, Luthy RG (2009) Field application of activated carbon amendment for in-situ stabilization of polychlorinated biphenyls in marine sediment. Environ Sci Technol 43(10):3815–3823
Choi H, Agarwal S, Al-Abed SR (2009) Adsorption and simultaneous dechlorination of PCBs on GAC/Fe/Pd: mechanistic aspects and reactive capping barrier concept. Environ Sci Technol 43(2):488–493
Choi Y, Cho YM, Luthy RG (2014) In situ sequestration of hydrophobic organic contaminants in sediments under stagnant contact with activated carbon. 1. Column studies. Environ Sci Technol 48(3):1835–1842
Choi Y, Cho YM, Gala WR, Hoelen TP, Werner D, Luthy RG (2016) Decision-making framework for the application of in-situ activated carbon amendment to sediment. J Hazard Mater 306:184–192
Chun CL, Payne RB, Sowers KR, and May HD (2013) Electrical stimulation of microbial PCB degradation in sediment. Water Res 47:141–152
Cornelissen G, Amstaetter K, Hauge A, Schaanning M, Beylich B, Gunnarsson JS, Breedveld GD, Oen AM, Eek E (2012) Large-scale field study on thin-layer capping of marine PCDD/F-contaminated sediments in Grenlandfjords, Norway: physicochemical effects. Environ Sci Technol 46(21):12030–12037
Cornelissen G, Kruså ME, Breedveld GD, Eek E, Oen AM, Arp HP, Raymond C, Samuelsson G, Hedman JE, Stokland O, Gunnarsson JS (2011) Remediation of contaminated marine sediment using thin-layer capping with activated carbon--a field experiment in Trondheim harbor, Norway. Environ Sci Technol 45(14):6110–6116
Cornelissen G, Breedveld GD, Naes K, Oen AMP, Ruus A (2006a) Bioaccumulation of native polycyclic aromatic hydrocarbons from sediment by a polychaete and a gastropod: freely dissolved concentrations and activated carbon amendment. Environ Toxicol Chem 25:2349–2355
Cornelissen G, Breedveld GD, Kalaitzidis S, Christanis K, Kibsgaard A, Oen AMP (2006b) Strong sorption of native PAHs to pyrogenic and unburned carbonaceous geosorbents in sediments. Environ Sci Technol 40:1197–1203
Diao M, Yao M (2009) Use of zero-valent iron nanoparticles in inactivating microbes. Water Res 43(20):5243–5251
Eek E, Cornelissen G, Kibsgaard A, Breedveld GD (2008) Diffusion of PAH and PCB from contaminated sediments with and without mineral capping; measurement and modelling. Chemosphere 71(9):1629–1638
EPA (2009) Technology performance review: selecting and using solidification/stabilization treatment for site remediation. EPA/600/R-09/148.
EPA (1994) Assessment and Remediation of Contaminated Sediments (ARCS) Program. Remediation guidance document. Great Lakes National Program Office. EPA 905-R94-003. Chicago: EPA.
EPA (1993) Selecting remediation techniques for contaminated sediment. Office of Water. EPA-823-B93-001. Washington, D.C.: EPA.
Flotron V, Delteil C, Padellec Y, Camel V (2005) Removal of sorbed polycyclic aromatic hydrocarbons from soil, sludge and sediment samples using the Fenton’s reagent process. Chemosphere 59(10):1427–1437
Gardner K (2004) In-situ treatment of PCBs in marine and freshwater sediments using colloidal zero-valent iron. A final report submitted to the NOAA/UNH Cooperative Institute for Coastal and Estuarine Environmental Technology. February 17. http://rfp.ciceet.unh.edu/display/report.php?chosen=51
Ghosh U, Luthy RG, Cornelissen G, Werner D, Menzie CA (2011) In-situ sorbent amendments: a new direction in contaminated sediment management. Environ Sci Technol 45:1163–1168
Gidley PT, Kwon S, Yakirevich A, Magar VS, Ghosh U (2012) Advection dominated transport of polycyclic aromatic hydrocarbons in amended sediment caps. Environ Sci Technol 46(9):5032–5039
Hahladakis J, Smaragdaki E, Vasilaki G, Gidarakos E (2013) Use of sediment quality guidelines and pollution indicators for the assessment of heavy metal and PAH contamination in Greek surficial sea and lake sediments. Environ Monit Assess 185:2843–2853
Hale SE, Elmquist M, Brändli R, Hartnik T, Jakob L, Henriksen T, Werner D, Cornelissen G (2012) Activated carbon amendment to sequester PAHs in contaminated soil: a lysimeter field trial. Chemosphere 87(2):177–184
Hale SE, Kwon S, Ghosh U, Werner D (2010) Polychlorinated biphenyl sorption to activated carbon and the attenuation caused by sediment. G Nest J 12(3):318–326
Hale SE, Werner D (2010) Modelling the mass transfer of hydrophobic organic pollutants in briefly and continuously mixed sediment after amendment with activated carbon. Environ Sci Technol 44(9):3381–3387
Han Z, Sani B, Akkanen J, Abel S, Nybom I, Karapanagioti HK, Werner D (2015) A critical evaluation of magnetic activated carbon’s potential for the remediation of sediment impacted by polycyclic aromatic hydrocarbons. J Hazard Mater 286:41–47
Himmelheber DW, Pennell KD, Hughes JB (2011) Evaluation of a laboratory-scale bioreactive in situ sediment cap for the treatment of organic contaminants. Water Res 45:5365–5374
Himmelheber DW, Thomas SH, Löffler FE, Taillefert MT, Hughes JB (2009) Microbial colonization of an in situ sediment cap and correlation to stratified redox zones. Environ Sci Technol 43:66–74
Hjartland T, Jersak J, Collins J, Soldal O (2013) Using carbon-enriched materials for capping contaminated sediments at the Kirkebukten Site in Bergen, Norway. In: Proceedings Seventh International Conference on Remediation of Contaminated Sediments, 4–7 February, Dallas (TX) USA. Battelle. C–060.
Ho KT, Burgess RM, Pelletier MC, Serbst JR, Cook H, Cantwell MG, Ryba SA, Perron MM, Lebo J, Huckins J, Petty J (2004) Use of powdered coconut charcoal as a toxicity identification and evaluation manipulation for organic toxicants in marine sediments. Environ Toxicol Chem 23(9):2124–2131
Hurel C, Taneez M, Volpi Ghirardini A, Libralato G (2016) Effects of mineral amendments on trace elements leaching from pre-treated marine sediment after simulated rainfall events. Environ Pollut. doi:10.1016/j.envpol.2016.09.072
Iannelli R, Masi M, Ceccarini A, Ostuni MB, Lageman R, Muntoni A, Spiga D, Polettini A, Marini A, Pomi R (2015) Electrokinetic remediation of metal-polluted marine sediments: experimental investigation for plant design. Electrochim Acta 181:146–159
Janssen EM, Choi Y, Luthy RG (2012) Assessment of nontoxic, secondary effects of sorbent amendment to sediments on the deposit-feeding organism Neanthes arenaceodentata. Environ Sci Technol 46(7):4134–4141
Johnston R, Kirtay V, Chadwick D, Rosen G, Guerrero J, Collins J, Ortega C, Webb R, May R, Germano J, Browning D, Beaver E, Wicklein M, Pittz J, Leisle D, Doyle L, Hsu L (2013) Installing an activated carbon sediment amendment at the Puget Sound Naval Shipyard and Intermediate Maintenance Facility, Bremerton, WA. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 4–7 February, Dallas (TX) USA. Battelle. B–024.
Jonker MTO, Suijkerbuijk MPW, Schmitt H, Sinnige TL (2009) Ecotoxicological effects of activated carbon addition to sediments. Environ Sci Technol 43(15):5959–5966
Josefsson S, Schaanning M, Samuelsson GS, Gunnarsson JS, Olofsson I, Eek E, Wiberg K (2012) Capping efficiency of various carbonaceous and mineral materials for in situ remediation of polychlorinated dibenzo-p-dioxin and dibenzofuran contaminated marine sediments: sediment-to-water fluxes and bioaccumulation in boxcosm tests. Environ Sci Technol 46:3343–3351
Kadar E, Simmance F, Martin O, Voulvoulis N, Widdicombe S, Mitov S, Lead JR, Readman JW (2010) The influence of engineered Fe2O3 nanoparticles and soluble (FeCl3) iron on the developmental toxicity caused by CO2-induced seawater acidification. Environ Pollut 158(12):3490–3497
Kadar E, Lowe DM, Solé M, Fisher AS, Jha AN, Readman JW, Hutchinson TH (2009) Uptake and biological responses to nano-Fe versus soluble FeCl3 in excised mussel gills. Anal Bioanal Chem 396(2):657–666
Keller AA, Garner K, Miller RJ, Lenihan HS (2012) Toxicity of nano-zero valent iron to freshwater and marine organisms. PLoS One 7(8):e43983
Knox AS, Dixon KL, Paller MH, Reible DD, Roberts JJ, Petrisor IG (2008) Innovative in situ remediation of contaminated sediments for simultaneous control of contamination and erosion. Annual Report SRNL-RP-2008-01216.
Koelmans AA, Kaag K, Sneekes A, Peeters ET (2009) Triple domain in situ sorption modeling of organochlorine pesticides, polychlorobiphenyls, polyaromatic hydrocarbons, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans in aquatic sediments. Environ Sci Technol 43(23):8847–8853
Krull M, Abessa DM, Hatje V, Barros F (2014) Integrated assessment of metal contamination in sediments from two tropical estuaries. Ecotoxicol Environ Saf 106:195–203
Lee C, Kim JY, Lee WI, Nelson KL, Yoon J, Sedlak DL (2008) Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ Sci Technol 42(13):4927–4933
Li WW, Yu HQ (2015) Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol Adv 33:1–12
Li H, Zhou Q, Wu Y, Fu J, Wang T, Jiang G (2009) Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Saf 72(3):684–692
Libralato G, Costa Devoti A, Zanella M, Sabbioni E, Mičetić I, Manodori L, Pigozzo A, Manenti S, Groppi F, Volpi Ghirardini A (2016) Phytotoxicity of ionic, micro- and nano-sized iron in three plant species. Ecotoxicol Environ Saf 123:81–88
Libralato G (2014) The case of Artemia spp. in nanoecotoxicology. Mar Environ Res 101:38–43
Libralato G, Volpi Ghirardini A, Francesco A (2010a) How toxic is toxic? A proposal for wastewater toxicity hazard assessment. Ecotoxicol Environ Saf 73(7):1602–1611
Libralato G, Volpi Ghirardini A, Avezzù F (2010b) Seawater ecotoxicity of monoethanolamine, diethanolamine and triethanolamine. J Hazard Mater 176(1–3):535–539
Libralato G, Losso C, Arizzi Novelli A, Citron M, Della Sala S, Zanotto E, Cepak F, Volpi Ghirardini A (2008) Ecotoxicological evaluation of industrial port of Venice (Italy) sediment samples after a decontamination treatment. Environ Pollut 156:644–650
Libralato G, Losso C, Avezzù F, Volpi Ghirardini A (2009) Influence of the salinity adjustment methods, salts and brine, on the toxicity of wastewater samples to mussel embryos. Environ Technol 30(1):85–91
Lofrano G, Libralato G, Alfieri A, Carotenuto M (2016) Metals and tributyltin sediment contamination along the Southeastern Tyrrhenian Sea coast. Chemosphere 144:399–407
Lundh T, Hansen T, Nordvik H (2013) Demonstrating in situ remedies for contaminated sediment in Norway: applicability to Sandefjord Harbor and beyond. In: Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments, 4–7 February, Dallas (TX) USA. Battelle. C–031.
Magar VS, Wenning RJ (2006) The role of monitored natural recovery in sediment remediation. Integr. Environ. Assess. Manage 2:66–74
Malviya R, Chaudhary R (2006) Factors affecting hazardous waste solidification/stabilization: a review. J Hazard Mater 137(1):267–276
Majone M, Verdini R, Aulenta F, Rossetti S, Tandoi V, Kalogerakis N, Agathos S, Puig S, Zanaroli G, Fava F (2015) In situ groundwater and sediment bioremediation: barriers and perspectives at European contaminated sites. New Biotechnol 32(1):133–146
Mamindy-Pajany Y, Libralato G, Roméo M, Hurel C, Losso C, Volpi Ghirardini A, Marmier N (2010) Ecotoxicological evaluation of Mediterranean dredged sediment ports based on elutriates with oyster embryotoxicity tests after composting process. Water Res 44(6):1986–1994
Matturro B, Ubaldi C, Grenni P, Barra Caracciolo A, Rossetti S (2015) Polychlorinated biphenyl (PCB) anaerobic degradation in marine sediments: microcosm study and role of autochthonous microbial communities. Environ Sci Pollut Res 1–11.
Mayer-Pinto M, Underwood AJ, Tolhurst T, Coleman RA (2010) Effects of metals on aquatic assemblages: what do we really know? J Exp Mar Biol Ecol 391:1–9
McDonough KM, Murphy P, Olsta J, Zhu Y, Reible D, Lowry GV (2007) Development and placement of a sorbent-amended thin layer sediment cap in the Anacostia River. Soil Sediment Contam Int J 16:313–322
McLeod PB, Luoma SN, Luthy RG (2008) Biodynamic modeling of PCB uptake by Macoma balthica and Corbicula fluminea from sediment amended with activated carbon. Environ Sci Technol 42:484–490
McLeod PB, van den Heuvel-Greve MJ, Luoma SN, Luthy RG (2007) Biological uptake of polychlorinated biphenyls by Macoma balthica from sediment amended with activated carbon. Environ Toxicol Chem 26(5):980–987
Meynet P, Hale SE, Davenport RJ, Cornelissen G, Breedveld GD, Werner D (2012) Effect of activated carbon amendment on bacterial community structure and functions in a PAH impacted urban soil. Environ Sci Technol 46(9):5057–5066
Millward RN, Bridges TS, Ghosh U, Zimmerman JR, Luthy RG (2005) Addition of activated carbon to sediments to reduce PCB bioaccumulation by a polychaete (Neanthes arenaceodentata) and an amphipod (Leptocheirus plumulosus). Environ Sci Technol 39(8):2880–2887
Nikolaou A, Kostopoulou-Karadanelli M, Lofrano G, Meriç S (2009a) Levels and toxicity of polycyclic aromatic hydrocarbons in marine sediments. Trends Anal Chem 28:653–664
Nikolaou A, Kostopoulou M, Lofrano G, Meriç S (2009b) Determination of PAHs in marine sediments: analytical methods and environmental concerns. G Nest J 11:391–405
Nogueira V, Lopes I, Rocha-Santos TA, Rasteiro MG, Abrantes N, Gonçalves F, Soares AM, Duarte AC, Pereira R (2015) Assessing the ecotoxicity of metal nano-oxides with potential for wastewater treatment. Environ Sci Pollut Res Int 22(17):13212–13224
NRC (National Research Council) (2007) Sediment dredging at superfund megasites: assessing the effectiveness. National Academies Press, Washington, DC
Oen AM, Beckingham B, Ghosh U, Kruså ME, Luthy RG, Hartnik T, Henriksen T, Cornelissen G (2012) Sorption of organic compounds to fresh and field-aged activated carbons in soils and sediments. Environ Sci Technol 46(2):810–817
Otero-González L, García-Saucedo C, Field JA, Sierra-Álvarez R (2013) Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere 93(6):1201–1206
Paller MH, Knox AS (2010) Amendments for the in situ remediation of contaminated sediments: evaluation of potential environmental impacts. Sci Total Environ 408(20):4894–4900
Patmont CR, Ghosh U, LaRosa P, Menzie CA, Luthy RG, Greenberg MS, Cornelissen G, Eek E, Collins J, Hull J, Hjartland T, Glaza E, Bleiler J, Quadrini J (2014) In situ sediment treatment using activated carbon: a demonstrated sediment cleanup technology. In press. Integr Environ Assess Manag 11(2):195–207
Robb AC, deGrood TJ, Weber R (2015) In situ stabilization/solidification (ISS): another tool for remediation of contaminated sediments. Presented at the Western Dredging Association, Midwest Chapter Meeting March 11–13, Milwaukee, Wisconsin
Röling WFM, van Verseveld HW (2002) Natural attenuation: what does the subsurface have in store? Biodegradation 13:53–64
Rosen G, Leather J, Kan J, Arias-Thode YM (2011) Ecotoxicological response of marine organisms to inorganic and organic sediment amendments in laboratory exposures. Ecotoxicol Environ Saf 74(7):1921–1930
Samuelsson GS, Hedman JE, Elmquist Kruså M, Gunnarsson JS, Cornelissen G (2015) Capping in situ with activated carbon in Trondheim harbor (Norway) reduces bioaccumulation of PCBs and PAHs in marine sediment fauna. Mar Environ Res 109:103–112
Scanferla P, Ferrari G, Pellay R, Volpi Ghirardini A, Zanetto G, Libralato G (2009) An innovative stabilization/solidification treatment for contaminated soil remediation: demonstration project results. J Soils Sediments 9:229–236
Shih YJ, Binh NT, Chen CW, Chen CF, Dong CD (2016) Treatability assessment of polycyclic aromatic hydrocarbons contaminated marine sediments using permanganate, persulfate and Fenton oxidation processes. Chemosphere 150:294–303
SMOCS (2012) Sustainable management of contaminated sediments. Project No: Baltic Sea Region Programme Project No #39
Sun H, Xu X, Gao G, Zhang Z, Yin P (2010) A novel integrated active capping technique for the remediation of nitrobenzene-contaminated sediment. J Hazard Mater 182:184–190
To PC, Mariñas BJ, Snoeyink VL, Ng WJ (2008) Effect of pore-blocking background compounds on the kinetics of trace organic contaminant desorption from activated carbon. Environ Sci Technol 42(13):4825–4830
Tomaszewski JE, Werner D, Luthy RG (2007) Activated carbon amendment as a treatment for residual DDT in sediment from a superfund site in San Francisco Bay, Richmond, California, USA. Environ Toxicol Chem 26:2143–2150
USEPA, US Environmental Protection Agency (2005) Contaminated sediment remediation guidance for hazardous waste sites. EPA-540-R-05-012
USEPA, US Environmental Protection Agency (2013a) Use of amendments for in situ remediation at superfund sediment sites. Office of Superfund Remediation and Technology Innovation. OSWER Directive 9200.2–128FS. April. [cited 2014 April]. http://www.epa.gov/superfund/health/conmedia/sediment/pdfs/in_situ_AmendmentReportandAppendix_FinalApril2013.pdf
USEPA, US Environmental Protection Agency (2013b) Superfund remedial program review action plan. Washington DC. November. [cited 2014 April]. Available from: http://www.epa.gov/superfund/cleanup/pdfs/Final_SPR_Action_Plan-11_26_2013_(2).pdf
USEPA, US Environmental Protection Agency (2014) Technical resource document on monitored natural recovery. EPA/600/R-14/083
Van der Mei HC, Atema-Smit J, Jager D, Langworthy DE, Collias DI, Mitchell MD, Busscher HJ (2008) Influence of adhesion to activated carbon particles on the viability of waterborne pathogenic bacteria under flow. Biotechnol Bioeng 100(4):810–813
Viganò L (2000) Assessment of the toxicity of River Po sediments with Ceriodaphnia dubia. Aquat Toxicol 47(3):191–202
Wan JZ, Yuan SH, Chen J, Li TP, Lin L, XH L (2009) Solubility-enhanced electrokinetic movement of hexachlorobenzene in sediments: a comparison of cosolvent and cyclodextrin. J Hazard Mater 166:221–226
Wang L, Tsang DC, Poon CS (2015) Green remediation and recycling of contaminated sediment by waste-incorporated stabilization/solidification. Chemosphere 122:257–264
Wang Q, Li Y, Wang C, Wu Y, Wang P (2014) Development of a novel multi-functional active membrane capping barrier for the remediation of nitrobenzene-contaminated sediment. J Hazard Mater 276:415–421
Wang YF, Tam NFY (2012) Natural attenuation of contaminated marine sediments from an old floating dock part II: changes of sediment microbial community structure and its relationship with environmental variables. Sci Total Environ 423:95–103
WDOE (Washington State Department of Ecology) (2012) Custom Plywood Interim Action: thin-layer capping pilot study, Anacortes, Washington. Work plan prepared by Hart Crowser, Inc. July. Available from: https://fortress.wa.gov/ecy/gsp/CleanupSiteDocuments.aspx?csid=4533
Wen-Wei L, Han-Qing Y (2015) Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol Adv 33(1):1–12
Werner D, Higgins CP, Luthy RG (2005) The sequestration of PCBs in Lake Hartwell sediment with activated carbon. Water Res 39:2105–2113
Wessels Perelo L (2010) Review: in situ and bioremediation of organic pollutants in aquatic sediments. J Hazard Mater 177(1):81–89
Wiegel J, Wu Q (2000) Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microbiol Ecol 32(1):1–15
Yu HY, Bao LJ, Liang Y, Zeng EY (2011) Field validation of anaerobic degradation pathways for dichlorodiphenyltrichloroethane (DDT) and 13 metabolites in marine sediment cores from China. Environ Sci Technol 45(12):5245–5252
Zimmerman JR, Werner D, Ghosh U, Millward RN, Bridges TS, Luthy RG (2005) Effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environ Toxicol Chem 24(7):1594–1601
Zimmerman JR, Ghosh U, Millward RN, Bridges TS, Luthy RG (2004) Addition of carbon sorbents to reduce PCB and PAH bioavailability in marine sediments: physicochemical tests. Environ Sci Technol 38(20):5458–5464
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266(1):195–202
Acknowledgments
This work was carried out in the framework of the Cooperation Agreement between the Special Commissioner of the Italian Government for urgent measures of remediation and environmental requalification of Taranto area (South Italy) and the Technical University of Bari.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
Lofrano, G., Libralato, G., Minetto, D. et al. In situ remediation of contaminated marinesediment: an overview. Environ Sci Pollut Res 24, 5189–5206 (2017). https://doi.org/10.1007/s11356-016-8281-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8281-x