Abstract
Informal-level electronic waste (e-waste)-processing activities are performed at hotspots in developing countries such as India, China, and Ghana. These activities increase the ambient burden of heavy metals and contribute to the toxic exposure of the general population. However, few data exist on the internal exposure of populations involved in these informal activities and in close contact with fumes from the direct combustion of electronic waste products in these countries. Therefore, in a cross-sectional study design, we analyzed blood, urine, and hair samples from 75 e-waste workers residing in and/or working on a large e-waste recycling site in Agbogbloshie, Accra, Ghana, and compared the results against those of 40 individuals living in a suburb of Accra without direct exposure to e-waste recycling activities. A comparative analysis using the Mann-Whitney U test showed significantly higher median concentrations of blood lead (88.5 vs. 41.0 μg/l, p < 0.001), cadmium (0.12 vs. 0.10 μg/gcrea, p = 0.023), chromium (0.34 vs. 0.23 μg/gcrea, p < 0.001), and nickel (3.18 vs. 2.03 μg/gcrea, p < 0.001) in the urine of e-waste workers than those of controls. There was no difference in blood cadmium concentrations between the groups (0.51 vs. 0.57 μg/l, p = 0.215) or in urine mercury levels (0.18 vs. 0.18 μg/gcrea, p = 0.820). Hair mercury levels were higher in the controls than in the e-waste workers (0.43 vs. 0.72, p < 0.001). We compared our data with those from European populations, specifically using the German reference values, and found that the internal concentrations of the participants exceeded the German reference values in 59.3 vs. 3.1% (e-waste workers vs. controls) for blood lead, 56.9 vs. 52.5% for urine nickel, 22.2 vs. 20.0% for urine chromium, and 17.8 vs. 62.2% for hair mercury. In particular, the high blood lead levels of up to several hundred micrograms per liter are a cause for concern because many of the workers in Agbogbloshie are children or adolescents who are in developmental stages and are at a particular risk for negative health effects. We conclude that exposure to some of the heavy metals tended to be a citywide phenomenon, but populations directly exposed to e-waste recycling are experiencing higher exposure levels and have concentration levels much higher than those of the general population and much higher than those found in European populations. To achieve environmental sustainability and to minimize the impact of e-waste-processing activities in developing countries, national authorities must formalize the rapidly growing informal-level e-waste management sector in these countries by deploying cleaner and easy-to-operate e-waste processing technologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
A global increase in technological change has brought in its wake a growing number of electronic products, which contributes substantially to the global solid waste stream. It is estimated that more than 40 million tons of waste from electric and electronic equipment (WEEE or e-waste) are produced annually worldwide, mainly in industrialized countries (Breivik et al. 2014; Baldé et al. 2015). The electronic products generated in large quantities in industrialized countries soon outlive their usefulness and become obsolete or semi-spent materials in resource-poor countries and are discarded as e-waste. A substantial proportion of e-waste is inappropriately recycled through crude processes and finally discarded at informal e-waste dumps/recycling sites (EWRSs) in developing countries (Jian et al. 2014), such as China (e.g., Guiyu in Shantou, Guangdong Province, and Taizhou in Zhejiang Province), India (e.g., New Delhi), Pakistan (e.g., Karachi), Nigeria (e.g., Lagos), and Ghana (e.g., Agbogbloshie), without any form of control (Puckett et al. 2005; Brigden et al. 2008; Robinson 2009; Chi et al. 2011; Wei and Liu 2012; Sthiannopkao and Wong 2013; Wang et al. 2013; Iqbal et al. 2015). These processes include, among others, heating, acid leaching, and wanton burning, and are mostly conducted by children and adolescents without regard for human or environmental health implications (Gilbert et al. 2006; Schmidt 2006; Wong et al. 2007; Robinson 2009; Asante et al. 2012; Akormedi et al. 2013; Heacock et al. 2016).
One of the largest EWRSs in Africa is in Agbogbloshie, Accra, Ghana. Here, this uncontrolled informal activity results in large-scale contamination with a multitude of toxins at extreme concentrations, resulting in exposure of these communities to toxic substances via drinking water, inhalation of gaseous fumes, and consumption of food products sold in a nearby open market. Among other toxins, large quantities of gaseous oxides of heavy metals, such as lead, mercury, cadmium, chromium, and nickel, are released during informal e-waste-recycling activities (Brigden et al. 2008; Asante et al. 2012). Our own studies provide evidence of contamination of the Agbogbloshie site with organic chemical pollutants and related human exposures for e-waste-recycling workers (Feldt et al. 2014; Wittsiepe et al. 2015).
A primary source of environmental emissions and human exposure to heavy metals is the open-air combustion of e-waste. Other major sources of emissions of inorganic substances into the environment, especially in Ghana, are gold mining, biomass fuel production, paint production, Saharan sand, sea spray, and vehicular traffic (Kylander et al. 2003; Affum et al. 2008; Aboh et al. 2009; Boamponsem et al. 2010; Atiemo et al. 2012; Acheampong et al. 2013).
Lead and other trace metals are major components of e-waste (Woo et al. 2016). One cathode ray tube (CRT) computer monitor alone can contain up to 2 kg of lead (Herat 2008; Niu et al. 2012). A list of heavy metals occurring in e-waste is shown in Table 1.
A major focus in the context of e-waste is the adverse health effects caused by exposure to lead. Well-known adverse consequences of lead exposure include neurodevelopmental disruptions caused by fetal lead exposure or exposure during childhood, which results in cognitive impairment and intellectual and behavioral deficits (WHO 1995; Olympio et al. 2009; Zahran et al. 2009). In addition, childhood exposure to lead can cause bone demineralization and stunting of growth in children. The effects on bone cell functions can persist for the entire lifetime of the exposed individual (Shen et al. 2001; Min et al. 2008). Chronic lead exposure in adults causes nerve disorders and memory or concentration problems (Sanders et al. 2009; Flora et al. 2012). Impacts on the immune system and genotoxic and endocrine effects have also been reported (Mishra 2009; Garcia-Leston et al. 2010; Rana 2014).
In China, especially in Guiyu and the surrounding areas, internal exposure to heavy metals from EWRSs, in most cases lead, with blood lead levels ranging up to several hundred micrograms per liter, have resulted in negative health effects. In addition, close relationships between elevated lead exposure, adverse health effects and the primitive e-waste recycling activities have been documented (Huo et al. 2007; Li et al. 2008a; Li et al. 2008b; Zheng et al. 2008; Guo et al. 2010; Liu et al. 2011; Mittal 2011; Wang et al. 2011b; Wang et al. 2012; Yang et al. 2013a; Yang et al. 2013b; Guo et al. 2014; Zhang et al. 2015; Zeng et al. 2016b). Furthermore, studies on the human body burden of e-waste-related exposures (Song and Li 2014) and human health consequences of metal exposures in China have been recently reviewed (Song and Li 2015; Xu et al. 2015; Zeng et al. 2016a).
In this study, we report biomonitoring data of heavy metals in e-waste workers from one of the largest e-waste dumps in Africa and compare the results to nearby control individuals.
Material and methods
Study area and population
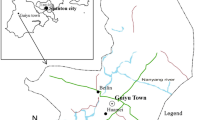
The study was conducted in two locations: at the EWRS in Agbogbloshie, Accra, the capital city of Ghana, and in Kwabenya North, a suburb on the periphery of Accra. The Agbogloshie e-waste processing site is one of the largest e-waste dumps in sub-Saharan Africa and processes millions of tons of e-wastes each year (Amankwah-Amoah 2016). Approximately 40,000 people live in this area and work under the most deplorable environmental conditions; these people largely represent a migrant population from the northern parts of Ghana. The migrant workforce comprises mostly unskilled persons who are making a transition between rural and urban environments. The Agbogbloshie area is heavily polluted, with the air laden with fumes from the combustion products of e-waste burning. Kwabenya North is a suburb of Accra, approximately 25 km northwest of Agbogbloshie, from which controls were chosen to compare living conditions to those at the e-waste dump. The population in Kwabenya North predominantly consists of migrants who are transitioning from rural areas to an urban fringe area and are mostly unskilled and of low socio-economic status, similar to the migrants working on the e-waste dumpsite.
Study design and statistical analysis
The study was a cross-sectional design involving 75 exposed individuals (residing in and/or working on the EWRS in Agbogbloshie, Accra) and a control group of 40 persons living in Kwabenya North who had neither visited the Agbogbloshie recycling site nor resided there. The statistical analysis was performed using STATISTICA software system (Version 10, Statsoft Inc., Tulsa, OK, USA, www.statsoft.com). The Mann-Whitney U test was used to compare differences between the exposed and unexposed groups. A correlation analysis was conducted using the Pearson product-moment correlation.
Recruitment of subjects
We obtained ethical clearance from the Institutional Review Board (IRB) of the Noguchi Memorial Institute for Medical Research (NMIMR), University of Ghana.
Working within these informal communities is sensitive, and several weeks of preparatory community entry activities preceded subject recruitment. At the end of the community sensitization activities, a team of qualified and trained field workers approached the potential study participants and carefully explained the purpose of study, its objectives, procedures, and possible risks associated with participating in the study in English and in the local language. Persons eligible for study participation were recruited after written informed consent had been obtained. The subjects were selected randomly once they consented to participate in the study. A qualified clinician then conducted a standardized interview, recorded biometric data and medical history, and performed a brief physical examination according to a standard protocol on each subject before blood, hair, and urine samples were collected.
Sample collection
At the site, we erected a temporary clinic and study office in November 2011, where the study procedures were performed. Blood samples were taken by a qualified phlebotomist. A full blood count was conducted on the day of sampling, and the remaining blood samples were stored at −20 °C until shipment to Germany for analysis. The participants were given clean, empty 100-ml containers into which they urinated and collected the urine themselves. Hair samples were collected by uniformly trimming the hair on the mid-upper reaches of the skull into a clean plastic receptacle using clean forceps and clean pair of scissors.
Blood and urine samples were frozen at −20 °C and shipped to Germany on dry ice and maintained at −20 °C until ready for analysis. The hair samples were stored at room temperature and also shipped to Germany.
Laboratory analysis
If not otherwise noted, laboratory analyses were performed according to “Analyses of Hazardous Substances in Biological Materials, Part II” (Angerer and Schaller 2008), a collection of methods supported by the DFG-Commission for the investigation of health hazards of chemical compounds in the work area. All measurements were conducted in duplicate. Analyses of blank samples and standard reference materials were included regularly and meet the designated requirements.
Cadmium and nickel in urine
A total of 4.0 ml of acidified urine was extracted with 500 μl of HMA-HMDC-solution (13.6 g/l hexamethyleneammonium-hexamethylenedithiocarbamidate in xylene/isobutyl-methylketone (3 + 7), enrichment factor 8). Determination of Cd or Ni in the organic phase (20 μl) was performed using graphite furnace atomic absorption spectrometry with a Zeeman background correction (Perkin Elmer SIMAA 6000 or 6100 spectrometer) at 229.8 nm (Cd) or 232.0 nm (Ni) and the addition of a Mg/Pd-modifier. Calibration was completed using four samples of spiked natural urine in the range of 0–2.5 μg/l for Cd or 0–10 μg/l for Ni. For internal quality control, standard reference material was used (Clin Check® Control, urine control for trace elements, Level I, Recipe, Munich, Germany). The limits of quantification were 0.05 μg/l (Cd) and 0.3 μg/l (Ni).
Chromium in urine
Determination of Cr in acidified urine (20 μl, 1:1 with 2% nitric acid) was conducted using graphite furnace atomic absorption spectrometry with a Zeeman background correction (Perkin Elmer SIMAA 6000 or 6100 spectrometer) at 357.9 nm and with the addition of a Mg/Pd-modifier. Calibration was completed using aqueous standard solutions in the range of 0 to 20 μg/l. For the internal quality control, standard reference material was used (Clin Check® Control, urine control for trace elements, Level I, Recipe, Munich, Germany). The limit of quantification was 0.1 μg/l.
Mercury in urine and hair
Mercury was analyzed in acidified urine (100 μl) and hair samples (approximately 3.5 mg) using a direct mercury analyzer (MLS DMA-80) according to the manufacturer’s instructions. The unwashed and untreated hair sample was cut into small pieces and introduced into the direct mercury analyzer, where it was initially dried and then thermally decomposed in a continuous flow of oxygen. Combustion products were transported and further decomposed in a hot catalyst bed. The mercury vapor was trapped on a gold amalgamator and subsequently desorbed for quantification. The mercury content was determined using atomic absorption spectrophotometry at 254 nm. Calibration was completed using aqueous standard solutions in the absolute range of 0.25 to 1000 ng. Reference material Clin Check® Control, urine control for trace elements, Level I (Recipe, Munich, Germany), and hair reference material NCS ZC 81002b (NCS Beijing, China) were used for the internal quality control. The limits of quantification were 0.1 μg/l (urine) and 0.003 μg/g (hair).
Cadmium and lead in whole blood
For protein precipitation, 600 μl of nitric acid (≥65%, puriss. p.a.) was added to 200 μl of whole blood. The supernatant (20 μl) was analyzed using graphite furnace atomic absorption spectrometry with a Zeeman background correction (Perkin Elmer SIMAA 6000 or 6100 spectrometer) at 228.8 nm (Cd) or 283.3 nm (Pb) with the addition of a Mg/Pd-modifier. Calibration was completed with aqueous standard solutions in the range of 0 to 2.5 μg/l (Cd) or 0 to 25 μg/l (Pb). For internal quality control, standard reference material was used (Clin Check® Control, whole blood control for trace elements, Level I, Recipe, Munich, Germany). The limits of quantification were 0.4 μg/l (Cd) and 2.0 μg/l (Pb).
Creatinine in the urine
Creatinine in the urine was determined photometrically as picrate according to the Jaffé method (Larsen 1972).
External quality control
The laboratory participated regularly and successfully in the German external quality assessment scheme (EQUAS) for analyses in biological materials of the Institute and Out-Patient Clinic for Occupational, Social and Environmental Medicine of the University Erlangen-Nuremberg, Germany and at the EQUAS of INSTAND e.V., WHO Collaborating Centre for Quality Assurance and Standardization in Laboratory Medicine, Düsseldorf, Germany. For the mercury hair analysis, we participated successfully in the EQUAS of the European COPHES/DEMOCOPHES human biomonitoring study (Esteban et al. 2015).
Results
We recruited 75 exposed participants working or living at the Agbogbloshie EWRS and 40 unexposed controls and were able to collect 73 and 37 hair samples, respectively, and 72 and 40 blood and urine samples, respectively. The biometric, socio-demographic, and medical parameters of the participants and a comparison of the data have been previously published in detail (Feldt et al. 2014; Wittsiepe et al. 2015); the basic biometric data are shown in Table 2. The exposed participants and controls were not different in gender, age, height, weight, body mass index (BMI), or most other parameters (see Feldt et al. 2014). In addition, it should be noted that the e-waste recyclers at Agbogbloshie did not use any protective devices as observed during the study.
In all samples, mercury in hair, lead in blood, and chromium and nickel in urine were detectable. Part of the levels of cadmium in blood (29% (exposed) and 10% (control)), cadmium in urine (15.3% (exposed) and 12.5% (control)), and mercury in the urine (16.7% (exposed) and 10% (control)) were below the limits of quantification (0.4 μg Cd/l blood, 0.1 μg Cd/l urine, and 0.1 μg Hg/l urine). Under these circumstances and for statistical calculations, the levels were set to one-half of the limit of quantification. The levels in urine were also calculated on a creatinine basis to adjust for the hydration status of the participants.
Descriptive statistics of the levels of heavy metals measured in hair (Hg), blood (Cd, Pb), and urine (Cd, Cr, Hg, and Ni), and of creatinine in urine are presented in Table 3. As usual in human biomonitoring studies, the concentrations showed a lognormal-like distribution. Using the Mann-Whitney U test, we found differences (p < 0.05) between the two subgroups for mercury levels in hair (median: 0.43 vs. 0.72 μg/g) and in volume-based levels in urine (median: 0.22 vs. 0.33 g/l), with higher levels in the control group. The blood lead levels were higher in the exposed group (median: 88.5 vs. 41 μg/l). Clear differences were found for creatinine-based levels of Cd (median: 0.12 vs. 0.10 μg/g creatinine), Cr (median: 0.34 vs. 0.23 μg/g creatinine), and Ni (3.18 vs. 2.03 μg/g creatinine). The creatinine levels in urine were higher in the control group (p = 0.015). For levels of Cd in blood; volume-based levels of Cd, Cr, and Ni in urine; and Hg in urine on a creatinine basis, there were no differences between the exposed and control groups. For all metals except mercury in hair, the highest values were in the exposed group.
An inter-correlation (Pearson) of the levels of heavy metals in different matrices is shown in Table 4. We found statistically significant (p < 0.05) dependencies for Cd in urine and blood, Cd (blood or urine), and Ni or Cr in urine, and Ni and Cr in urine.
For comparison with the US and European populations, Table 5 shows the levels of heavy metals in hair, blood, and urine found in this study against P95 values of the NHANES survey (US Department of Health and Human Services—Centers for Disease Control and Prevention 2014) and German background levels of unexposed persons and official German reference values. While the levels of Cd in blood and urine and Hg in urine were within the same range (not recognizable or only slightly more than 5% of the values above the P95 values), considerably higher levels were found for Hg in hair and Ni in urine, both in the exposed and control groups and for lead blood levels of exposed males (German data) or both groups compared to the NHANES survey. In this case, approximately 60% of all samples of the exposed group were above the German reference value of 90 μg/l, and 92% of all samples (both groups) were above the P95 value of the NHANES survey.
Discussion
General observations
Internal exposure to Hg, Pb, Cr, and Ni in Ghana is clearly higher than German background levels, as indicated by the high proportion of participants in the control group with concentrations above the German reference values. We found elevated levels of Hg (hair) in 62%, Ni (urine) in 55%, Pb (blood) in 40%, and Cr (urine) in 20% of the individuals in the control group (Table 5).
There might be several reasons for this higher background exposure, with nutrition being an important factor. Hg intake occurs mainly via the consumption animal food, such as fish/shellfish (Castano et al. 2016), and Ni is obtained from the intake of vegetable foods such as tea, cocoa, and nuts. Another relevant source might be drinking water. Asante et al. (2012) found great variations in heavy metal concentrations in drinking water from Accra and reported the corrosion of household plumbing systems and the use of metal (galvanized iron) pipes for water distribution in Ghana to be relevant sources. In the case of Hg in hair, external exposure sources, such as hair-care products and others, cannot be excluded.
The issue of the relatively high levels of metals observed in the controls can further be explained as probably due to the use of the biomass fuel and quarrying activities of the residents of Kwabenya North (Feldt et al. 2014). The residents are engaged in quarrying activities, which produce dust, the inhalation of which may contribute to the elevation of heavy metals above general population levels. Detailed data on those possible confounders were not analyzed in this study.
Working and living on the EWRS of Agbogbloshie was associated with significantly higher levels of Pb in the blood and Cd, Cr, and Ni, in some instances reaching excessive concentrations. Surprisingly, the concentrations of Hg in hair and urine were clearly higher in the control group than in individuals exposed to e-waste activities.
As previously discussed (Feldt et al. 2014), the urine creatinine concentration, as an indicator of urine concentration and thus the hydration status of participants, was significantly lower in the exposed group than in the control group (Table 3). An adjustment for creatinine was performed to enable a comparison between the study groups.
Lead
The most obvious differences between the exposed group and the controls were found for lead. Approximately 60% of the lead levels of the exposed volunteers exceeded German reference values. Although a causal relationship between e-waste and elevated lead levels cannot be proven by our cross-sectional study design, we believe that the high lead content in e-waste materials together with different dismantling techniques, such as pickling, manual dismantling, and burning, are responsible for lead intake, mostly by oral and dermal pathways but also by air and secondary contamination. Our findings are in accordance with biomonitoring studies from e-waste dismantling areas in China, where high lead levels in exposed persons were also found (see above).
In addition, until 2007, only leaded petrol was sold in Ghana, and an impact of automobile emissions on dust (365 ± 93 μg/g) and soil (291 ± 76 μg/g) lead concentrations was found in a study conducted in Ghana in 2001 (Kylander et al. 2003). Contamination by leaded petrol used in the past may be an additional source of lead exposure but cannot explain the higher lead levels of the e-waste workers.
Comparison with other studies
Table 6 compares various studies from the past decade according to the levels of heavy metals in the hair, blood, and urine of children and adults involved with or living at sites with informal e-waste recycling activities. Viewed regionally, most studies were performed in Guiyu, Taizhou, and other Chinese EWRSs. Within the assessment of human e-waste-related exposure to heavy metals, measurements of lead levels in blood have attracted the most attention, and have been the focus of lead exposure studies due to their particular sensitivity in children and neonates (studies on placenta and umbilical cord blood levels are not included in Table 6).
The comparison shows that higher cadmium exposure levels have been found in other studies, but the results were not consistent and varied regionally. In most studies, higher internal exposures to lead (in comparison to the control groups) were confirmed for workers and inhabitants of e-waste exposure areas and the time of working/exposure was relevant to increased blood lead levels. Although the techniques used in different EWRSs varied, the elevated blood lead levels of the exposed workers and children were within a similar range of up to several hundred micrograms per liter. Globally, the studies suggest that both living and working at EWRSs leads to high internal lead exposures and that children (and neonates) are at an especially high health risk.
Limitations of the study
As previously discussed (Feldt et al. 2014), the cross-sectional study design is not suitable for establishing causal relationships between internal heavy metal exposure and e-waste activities. Differences between the two study groups exist in religious affiliation (exposed group: Muslim migrants; control group: Christians) and ethnicity, which might indicate differences in diets and methods of food preparation. In the least, these differences might influence heavy metal exposure and thus represent potential confounders. Detailed data on food consumption behavior and individual drinking water intake and heavy metal concentrations were not recorded. Previous exposure due to road traffic and occupation may also be confounders and were not controlled for in this study.
It must be noted that in Agbogbloshie, many of the workers are children or adolescents. Our study group is not representative of all ages of e-waste workers at the dumpsite because only adults were included in our cohort study. Especially in the case of lead exposure, children are at particular risk for negative health effects due to higher exposure and susceptibility during childhood development (Dapul and Laraque 2014).
Conclusion
In conclusion, exposure to trace metals tends to be a citywide phenomenon, but e-waste workers and the populations in the vicinity of e-waste processing sites tend to experience higher levels of exposure to many of the trace metals we investigated. In particular, lead was found in alarmingly high concentrations in a number of participants and represents a major health hazard. As a next step, we call for future studies that take into account the possibility of determining all e-waste-originated pollutants and quantify their burden in e-waste workers. We recommend to include the detailed identification of different sources of e-waste related exposure and other relevant exposure factors, especially diet, for workers, and inhabitants of the area. In consideration of the long-term action plans to achieve environmental sustainability objectives and reduce, the impact of e-waste processing activities on the environment and human health in rapidly growing cities in low-to-middle-income countries, national authorities may take steps to formalize the rapidly growing informal e-waste management sector by deploying cleaner and easy-to-operate e-waste processing technologies. The use of modern technologies will help prevent the burning of e-waste in Ghana (Asante et al. 2016).
References
Aboh IJK, Henriksson D, Laursen J, Lundin M, Ofosu FG, Pind N, Lindgren ES, Wahnstrom T (2009) Identification of aerosol particle sources in semi-rural area of Kwabenya, near Accra, Ghana, by EDXRF techniques. X-Ray Spectrom 38:348–353
Acheampong MA, Paksirajan K, Lens PNL (2013) Assessment of the effluent quality from a gold mining industry in Ghana. Environ Sci Pollut R 20:3799–3811
Affum HA, Oduro-Afriyie K, Nartey VK, Adomako D, Nyarko BJB (2008) Biomonitoring of airborne heavy metals along a major road in Accra, Ghana. Environ Monit Assess 137:15–24
Akormedi M, Asampong E, Fobil JN (2013) Working conditions and environmental exposures among electronic waste workers in Ghana. Int J Occup Env Heal 19:278–286
Amankwah-Amoah J (2016) Global business and emerging economies: towards a new perspective on the effects of e-waste. Technol Forecast Soc 105:20–26
Angerer J, Schaller K-H (2008) Analyses of hazardous substances in biological materials. German Research Foundation, Bonn
Asante KA, Agusa T, Biney CA, Agyekum WA, Bello M, Otsuka M, Itai T, Takahashi S, Tanabe S (2012) Multi-trace element levels and arsenic speciation in urine of e-waste recycling workers from Agbogbloshie, Accra in Ghana. Sci Total Environ 424:63–73
Asante KA, Pwamang JA, Amoyaw-Osei Y, Ampofo JA (2016) E-waste interventions in Ghana. Reviews on environmental health
Atiemo SM, Ofosu FG, Aboh IJK, Oppon OC (2012) Levels and sources of heavy metal contamination in road dust in selected major highways of Accra, Ghana. X-Ray Spectrom 41:105–110
Baldé CP, Wang F, Kuehr R, Huisman J (2015) The global e-waste monitor – 2014,. United Nations University, IAS – SCYCLE, Bonn, Germany. http://i.unu.edu/media/unu.edu/news/52624/UNU-1stGlobal-E-Waste-Monitor-2014-small.pdf
Boamponsem LK, Adam JI, Dampare SB, Nyarko BJB, Essumang DK (2010) Assessment of atmospheric heavy metal deposition in the Tarkwa gold mining area of Ghana using epiphytic lichens. Nucl Instrum Meth B 268:1492–1501
Breivik K, Armitage JM, Wania F, Jones KC (2014) Tracking the global generation and exports of e-waste: do existing estimates add up? Environ Sci Technol 48:8735–8743
Brigden K, Labunska I, Santillo D, Johnston P (2008) Chemical contamination at e-waste recycling and disposal sites in Accra and Korforidua, Ghana. Greenpeace Research Laboratories, Technical Note 10/2008 10
Castano A, Cutanda F, Esteban M, Part P, Navarro C, Gomez S, Rosado M, Lopez A, Lopez E, Exley K, Schindler BK, Govarts E, Casteleyn L, Kolossa-Gehring M, Fiddicke U, Koch H, Angerer J, Den Hond E, Schoeters G, Sepai O, Horvat M, Knudsen LE, Aerts D, Joas A, Biot P, Joas R, Jimenez-Guerrero JA, Diaz G, Pirard C, Katsonouri A, Cerna M, Gutleb AC, Ligocka D, Reis FM, Berglund M, Lupsa IR, Halzlova K, Charlier C, Cullen E, Hadjipanayis A, Krskova A, Jensen JF, Nielsen JK, Schwedler G, Wilhelm M, Rudnai P, Kozepesy S, Davidson F, Fischer ME, Janasik B, Namorado S, Gurzau AE, Jajcaj M, Mazej D, Tratnik JS, Larsson K, Lehmann A, Crettaz P, Lavranos G, Posada M (2015) Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environ Res 141:58-68
Chi XW, Streicher-Porte M, Wang MYL, Reuter MA (2011) Informal electronic waste recycling: a sector review with special focus on China. Waste Manag 31:731–742
Dapul H, Laraque D (2014) Lead poisoning in children. Adv Pediatr 61:313–333
Esteban M, Schindler BK, Jimenez JA, Koch HM, Angerer J, Rosado M, Gomez S, Casteleyn L, Kolossa-Gehring M, Becker K, Bloemen L, Schoeters G, Den Hond E, Sepai O, Exley K, Horvat M, Knudsen LE, Joas A, Joas R, Aerts D, Biot P, Borosova D, Davidson F, Dumitrascu I, Fischer ME, Grander M, Janasik B, Jones K, Kasparova L, Larssen T, Naray M, Nielsen F, Hohenblum P, Pinto R, Pirard C, Plateel G, Tratnik JS, Wittsiepe J, Castano A, Labs ER (2015) Mercury analysis in hair: comparability and quality assessment within the transnational COPHES/DEMOCOPHES project. Environ Res 141:24–30
ewasteguide.info (2009) e-waste. Hazardous Substances in e-Waste. A Knowledge Base for the Sustainable Recycling of E-Waste. E-Waste: A Swiss E-Waste Guide
Feldt T, Fobil JN, Wittsiepe J, Wilhelm M, Till H, Zoufaly A, Burchard G, Goen T (2014) High levels of PAH-metabolites in urine of e-waste recycling workers from Agbogbloshie, Ghana. Sci Total Environ 466-467:369–376
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Garcia-Leston J, Mendez J, Pasaro E, Laffon B (2010) Genotoxic effects of lead: an updated review. Environ Int 36:623–636
Gilbert E, Dodoo DK, Okai-Sam F, Essuman K, Quagraine EK (2006) Characterization and source assessment of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in sediments of the Fosu lagoon, Ghana. J Environ Sci Heal A 41:2747–2775
Guo P, Xu X, Huang B, Sun D, Zhang J, Chen X, Zhang Q, Huo X, Hao Y (2014) Blood lead levels and associated factors among children in Guiyu of China: a population-based study. PLoS One 9:e105470
Guo YY, Huo X, Li Y, Wu KS, Liu JX, Huang JR, Zheng GN, Xiao QN, Yang H, Wang YP, Chen AM, Xu XJ (2010) Monitoring of lead, cadmium, chromium and nickel in placenta from an e-waste recycling town in China. Sci Total Environ 408:3113–3117
Ha NN, Agusa T, Ramu K, Tu NPC, Murata S, Bulbule KA, Parthasaraty P, Takahashi S, Subramanian A, Tanabe S (2009) Contamination by trace elements at e-waste recycling sites in Bangalore, India. Chemosphere 76:9–15
Heacock M, Kelly CB, Asante KA, Birnbaum LS, Bergman AL, Brune MN, Buka I, Carpenter DO, Chen AM, Huo X, Kamel M, Landrigan PJ, Magalini F, Diaz-Barriga F, Neira M, Omar M, Pascale A, Ruchirawat M, Sly L, Sly PD, Van den Berg M, Suk WA (2016) E-waste and harm to vulnerable populations: a growing global problem. Environ Health Persp 124:550–555
Herat S (2008) Recycling of cathode ray tubes (CRTs) in electronic waste. Clean-Soil Air Water 36:19–24
Huo X, Peng L, Xu XJ, Zheng LK, Qiu B, Qi ZL, Zhang B, Han D, Piao ZX (2007) Elevated blood lead levels of children in Guiyu, an electronic waste recycling town in China. Environ Health Persp 115:1113–1117
Iqbal M, Breivik K, Syed JH, Malik RN, Li J, Zhang G, Jones KC (2015) Emerging issue of e-waste in Pakistan: a review of status, research needs and data gaps. Environ Pollut 207:308–318
Jian Z, Jian-Feng Z, Xian-Mei W, Nkrumah PN (2014) Comparative studies on e-waste disposal practices in developing countries and their environmental effects: an example between Guiyu, China and Agbogbloshie, Ghana. Civil, Structural and Environmental Engineering 838-841(1–4):2701–2706
Krause C, Babisch W, Becker K, Bernigau W, Helm D, Hoffmann K, Nöllke P, Schulz C, Schwabe R, Seifert B, Thefeld W (1996) Umwelt-Survey 1990/92, Band Ia: Studienbeschreibung und Human-Biomonitoring: Deskription der Spurenelementgehalte in Blut und Urin der Bevölkerung der Bundesrepublik Deutschland. WaBoLu-Hefte 1
Kylander ME, Rauch S, Morrison GM, Andam K (2003) Impact of automobile emissions on the levels of platinum and lead in Accra, Ghana. J Environ Monitor 5:91–95
Larsen K. (1972) Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217
Li Y, Xu XJ, Liu JX, Wu KS, Gu CW, Shao G, Chen SJ, Chen GJ, Huo X (2008a) The hazard of chromium exposure to neonates in Guiyu of China. Sci Total Environ 403:99–104
Li Y, Xu XJ, Wu KS, Chen GJ, Liu JX, Chen SJ, Gu CW, Zhang B, Zheng LK, Zheng MH, Huo X (2008b) Monitoring of lead load and its effect on neonatal behavioral neurological assessment scores in Guiyu, an electronic waste recycling town in China. J Environ Monitor 10:1233–1238
Liu JX, Xu XJ, Wu KS, Piao ZX, Huang JR, Guo YY, Li WQ, Zhang YL, Chen AM, Huo X (2011) Association between lead exposure from electronic waste recycling and child temperament alterations. Neurotoxicology 32:458–464
Min KB, Min JY, Cho SI, Kim R, Kim H, Paek D (2008) Relationship between low blood lead levels and growth in children of white-collar civil servants in Korea. Int J Hyg Environ Health 211:82–87
Mishra KP (2009) Lead exposure and its impact on immune system: a review. Toxicology in vitro: An International Journal published in Association with BIBRA 23:969–972
Mittal A (2011) High blood lead levels in e-waste recyclers. Epidemiology 22:S293–S293
Morf LS, Tremp J, Gloor R, Schuppisser F, Stengele M, Taverna R (2007) Metals, non-metals and PCB in electrical and electronic waste actual levels in Switzerland. Waste Manag 27:1306–1316
Ni W, Chen Y, Huang Y, Wang X, Zhang G, Luo J, Wu K (2014) Hair mercury concentrations and associated factors in an electronic waste recycling area, Guiyu, China. Environ Res 128:84–91
Niu RX, Wang ZS, Song QB, Li JH (2012) LCA of scrap CRT display at various scenarios of treatment. Procedia Environ Sci 16:576–584
Olympio KPK, Gonçalves C, Günther WMR, Bechara EJH (2009) Neurotoxicity and aggressiveness triggered by low-level lead in children: a review. Revista Panamericana De Salud Publica-Pan American Journal of Public Health 26:266–275
Puckett J, Westervelt S, Gutierrez R, Takamiya Y (2005) The digital dump - exporting re-use and abuse to Africa. The Basel Action Network
Rana SV (2014) Perspectives in Endocrine Toxicity of Heavy Metals-A Review. Biol Trace Elem Res 160:1–14
Robinson BH (2009) E-waste: an assessment of global production and environmental impacts. Sci Total Environ 408:183–191
Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24:15–45
Schmidt CW (2006) Unfair trade: e-waste in Africa. Environ Health Persp 114:A232–A235
Shen XM, Wu SH, Yan CH (2001) Impacts of low-level lead exposure on development of children: recent studies in China. Clin Chim Acta 313:217–220
Song Q, Li J (2014) A systematic review of the human body burden of e-waste exposure in China. Environ Int 68:82–93
Song QB, Li JH (2015) A review on human health consequences of metals exposure to e-waste in China. Environ Pollut 196:450–461
Sthiannopkao S, Wong MH (2013) Handling e-waste in developed and developing countries: initiatives, practices, and consequences. Sci Total Environ 463:1147–1153
U.S. Department of Health and Human Services - Centers for Disease Control and Prevention (2014) Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables August 2014
Umweltbundesamt (2012a) Europäische Studie zur Umweltbelastung von Müttern und Kindern - Erste Ergebnisse aus DEMOCOPHES
Umweltbundesamt (2012b) Referenzwerte für Antimon, Arsen und Metalle (Pd, Cd, Ni, Hg, Pt, Tl, U) im Urin oder im Blut
Wang F, Kuehr R, Ahlquist D, Li J (2013) E-waste in China: A country report
Wang H, Han M, Yang S, Chen Y, Liu Q, Ke S (2011a) Urinary heavy metal levels and relevant factors among people exposed to e-waste dismantling. Environ Int 37:80–85
Wang Q, He AM, Gao B, Chen L, Yu QZ, Guo H, Shi BJ, Jiang P, Zhang ZY, Li PL, Sheng YG, Fu MJ, Wu CT, Chen MX, Yuan J (2011b) Increased levels of lead in the blood and frequencies of lymphocytic micronucleated binucleated cells among workers from an electronic-waste recycling site. J Environ Sci Heal A 46:669–676
Wang XF, Miller G, Ding GQ, Lou XM, Cai DL, Chen ZJ, Meng J, Tang J, Chu C, Mo Z, Han JL (2012) Health risk assessment of lead for children in tinfoil manufacturing and e-waste recycling areas of Zhejiang Province, China. Sci Total Environ 426:106–112
Wei L, Liu YS (2012) Present status of e-waste disposal and recycling in China. Procedia Environ Sci 16:506–514
WHO (1995) Inorganic lead. World Health Organization, Geneva
Wittsiepe J, Fobil JN, Till H, Burchard GD, Wilhelm M, Feldt T (2015) Levels of polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/Fs) and biphenyls (PCBs) in blood of informal e-waste recycling workers from Agbogbloshie, Ghana, and controls. Environ Int 79:65–73
Wong MH, Wu SC, Deng WJ, Yu XZ, Luo Q, Leung AOW, Wong CSC, Luksemburg WJ, Wong AS (2007) Export of toxic chemicals—a review of the case of uncontrolled electronic-waste recycling. Environ Pollut 149:131–140
Woo SH, Lee DS, Lim SR (2016) Potential resource and toxicity impacts from metals in waste electronic devices. Integr Environ Asses 12:364–370
Xu X, Zeng X, Boezen HM, Huo X (2015) E-waste environmental contamination and harm to public health in China. Front Med 9:220–228
Yang H, Huo X, Yekeen TA, Zheng QJ, Zheng MH, Xu XJ (2013a) Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ Sci Pollut R 20:4441–4447
Yang Y, Lu XS, Li DL, Yu YJ (2013b) Effects of environmental lead pollution on blood lead and sex hormone levels among occupationally exposed group in an E-waste dismantling area. Biomed Environ Sci 26:474–484
Zahran S, Mielke HW, Weiler S, Berry KJ, Gonzales C (2009) Children's blood lead and standardized test performance response as indicators of neurotoxicity in metropolitan New Orleans elementary schools. Neurotoxicology 30:888–897
Zeng X, Xu X, Boezen HM, Huo X (2016a) Children with health impairments by heavy metals in an e-waste recycling area. Chemosphere 148:408–415
Zeng X, Xu X, Zheng X, Reponen T, Chen A, Huo X (2016b) Heavy metals in PM and in blood, and children's respiratory symptoms and asthma from an e-waste recycling area. Environ Pollut 210:346–353
Zhang R, Xu C, Shen H, Tang Y, Meng J, Lu W, Wang X, Lou X, Song Y, Han G, Cai D, Ding G (2012) Oxidative damage and immunotoxicity effect of people who exposed to electronic waste. Wei sheng yan jiu = Journal of Hygiene Research 41:199–203
Zhang RB, Huo X, Ho GY, Chen XJ, Wang HW, Wang TY, Ma L (2015) Attention-deficit/hyperactivity symptoms in preschool children from an E-waste recycling town: assessment by the parent report derived from DSM-IV. BMC Pediatr:15
Zhang Y, Huo X, Cao J, Yang T, Xu L, Xu X (2016) Elevated lead levels and adverse effects on natural killer cells in children from an electronic waste recycling area. Environ Pollut 213:143–150
Zheng LK, Wu KS, Li Y, Qi ZL, Han D, Zhang B, Gu CW, Chen GJ, Liu JX, Chen SJ, Xu XJ, Huo X (2008) Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ Res 108:15–20
Acknowledgements
The authors are very grateful to the staff of GIZ-ReCHT for the organizational and logistic support, the community sensitization group, medical and laboratory team, and above all, the participants who voluntarily agreed to take part in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wittsiepe, J., Feldt, T., Till, H. et al. Pilot study on the internal exposure to heavy metals of informal-level electronic waste workers in Agbogbloshie, Accra, Ghana. Environ Sci Pollut Res 24, 3097–3107 (2017). https://doi.org/10.1007/s11356-016-8002-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8002-5