Abstract
Many studies indicate that lead (Pb) and cadmium (Cd) exposure may alter bone development through both direct and indirect mechanisms, increasing the risk of osteoporosis later in life. The aim of this study was to investigate the association between Pb and Cd exposure, physical growth, and bone and calcium metabolism in children of an electronic waste (e-waste) processing area. We recruited 246 children (3–8 years) in a kindergarten located in Guiyu, China. Blood lead levels (BLLs) and blood cadmium levels (BCLs) of recruited children were measured as biomarkers for exposure. Serum calcium, osteocalcin, bone alkaline phosphatase, and urinary deoxypyridinoline were used as biomarkers for bone and calcium metabolism. Physical indexes such as height, weight, and head and chest circumference were also measured. The mean values of BLLs and BCLs obtained were 7.30 μg/dL and 0.69 μg/L, respectively. The average of BCLs increased with age. In multiple linear regression analysis, BLLs were negatively correlated with both height and weight, and positively correlated with bone resorption biomarkers. Neither bone nor calcium metabolic biomarkers showed significant correlation with cadmium. Childhood lead exposure affected both physical development and increased bone resorption of children in Guiyu. Primitive e-waste recycling may threaten the health of children with elevated BLL which may eventually cause adult osteoporosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background, aim, and scope
Lead (Pb) and cadmium (Cd) are significant industrial and environmental pollutants, and their ubiquity in the environment and bioaccumulation in organisms have led to a variety of adverse effects in mammals. The neurotoxicity of Pb on children is widely studied and well documented (Olympio et al. 2009; Finkelstein et al. 1998; Zahran et al. 2009) as well as its association with other diseases (Moncrieff et al. 1964). It has also been confirmed that childhood exposure to Pb can cause bone demineralization and damage, thus influencing a child’s growth. Moreover, bone carries nearly 95 % of the total Pb burden in the individual body (Barry and Mossman 1970; Kim et al. 1995). Long half-life of lead in bone provides an index of cumulative exposure over decades. The storage and release of Pb follow the general physiology of bone calcium with similar kinetics, and therefore have the potential to interfere with bone and calcium metabolism. Recent epidemiological data and experimental works showed that effects of Pb on bone cell functions could persist for a lifetime eventually leading to osteoporosis and osteopenia (Barry 1975; Goyer et al. 1994; Min et al. 2008; Shen et al. 2001).
Furthermore, high environmental Cd exposure is also associated with bone damage (osteoporosis and osteomalacia). This type of bone damage has been described in women affected by the Itai-Itai disease. Studies also indicated that even relatively low exposure concentration levels may produce adverse effects on the skeleton, such as low bone mineral density (BMD) (Alfvén et al. 2000; Järup and Alfvén 2004; Sughis et al. 2011 ).
Despite considerable research on bone damage caused by Pb and Cd toxicity, the mechanisms are still not very well characterized. Many studies described the direct cellular toxic effect of Pb and Cd on osteoblast and osteoclast function and the indirect adverse renal effects of Pb and Cd. Reports have shown that Pb or Cd can act directly on bone cells and cause decreases in bone formation and increases in bone resorption, independent of their effects on the kidney, intestine, or circulating hormone concentrations (Ohta et al. 2002; Schutte et al. 2008). Both metals are also nephrotoxic (Alfvén et al. 2002; De Burbure et al. 2006), and their low-level chronic exposure increases the excretion of low molecular weight proteins and lysosomal proteins, impairs kidney function, inhibits the uptake of dietary vitamins and calcium, and disturbs vitamin D metabolism (Chalkley et al. 1998; Rosen et al. 1980). The three most likely mechanisms by which these metals might contribute to the development of osteoporosis include alteration of (1) peak bone mass, (2) the rate of bone resorption in an older person, and/or (3) alteration of the structural integrity of the bone (Goyer et al. 1994). Although there is good support for retardation of endochondral bone growth by lead, long-term consequences of this retardation on peak skeletal mass remain yet to be established. Studies on the effects of long-term Pb and Cd exposure on bone damage to either support or to eliminate a role for Pb and Cd in the pathogenesis of osteoporosis are needed.
Guiyu is an electronic waste-recycling site in southeast China. The processes and techniques used to recycle electrical waste in Guiyu are primitive. Various hazardous heavy metals are released into the environment threatening the health of local residents. Several studies conducted in Guiyu have reported the high levels of toxic heavy metals and organic contaminants in samples of dust, soil, river sediment, surface water, and groundwater of Guiyu (Deng et al. 2006; Wong et al. 2007; Yu et al. 2006). Our previous studies showed that neonates and children of Guiyu had significantly higher blood lead levels (BLLs) and blood cadmium levels (BCLs) than those from Chendian (a neighboring town) and other suburban areas (Guo et al. 2010; Huo et al. 2007; Li et al. 2008, 2011; Liu et al. 2011; Zheng et al. 2008, 2012). The aim of our present study is to examine the relationship between lead and cadmium exposure and their effects on bone development in children in e-waste recycling area.
Materials and methods
Study population and sample collection
We recruited 246 children aged 3–8 from a village kindergarten in Guiyu. Unhealthy children or those on medication were excluded. After informed consent was obtained from their parents or guardians, blood and urine samples were collected by well-trained nurses, placed on ice, transported to the laboratory, and stored at −20 °C until analysis. Physical indexes (height and weight, and head and chest circumference) were also measured. To avoid contamination, all plastic tubes and containers used for urine collection were pre-washed, soaked in dilute HNO3, and rinsed with deionized water.
A self-designed questionnaire was used to conduct a survey of parents or guardians. The questionnaire addressed factors that might influence a child’s BLLs or BCLs, including questions relating to residence, physical activity, dieting habits, nutrition, whether the parent’s occupation was related to e-waste processing, and the parent’s education level and socioeconomic status. A medical and health history was also taken into consideration.
Measurement of blood Pb and Cd
Levels of Pb and Cd in the blood were used to assess exposure. Before analysis, 100-μL blood samples were put into tubes, and 900 μL of 0.5 % nitric acid (for blood cadmium, 2 % HNO3) was added, vortexed, and subsequently digested for 10 min. The mixture was then used for lead determination. Unlike typical lead determinations, the solution had to be centrifuged for 10 min at 2,000 rpm after digestion to separate the supernatant for Cd analysis.
Both metals were determined by graphite furnace atomic absorption spectrophotometry (Jena Zenit 650, Germany), which consists of an autosampler (MPE60), with an injection volume set at 20 μL. The main parameters used for lead determination were: a wavelength of 283.3 nm, a lamp current of 4.0 mA, a slit width of 0.8 nm, drying at 90, 105, and 120 °C, ashing at 600 °C, and atomization at 1,500 °C. The standard calibration curve was plotted by the six working standard solutions which were prepared by stock Pb standard solutions diluted by nitric acid and added matrix modifier mixed with human blood. The linear correlation coefficient of the lead standard calibration curve was 0.9916. Accuracy of the method was controlled by recoveries between 95 and 107 %. The parameters for Cd analysis were: a wavelength of 228.8 nm, a current of 2.0 mA, a slit width of 1.2 nm, drying at 90, 105, and 120 °C, ashing at 300 °C, and atomization at 1,300 °C. The linear correlation coefficient of the Cd standard calibration curve was 0.9990. The recoveries for this method were 100–103 %, which were also from spiked blood samples.
Biochemical measurements
The following biochemical markers which relate to the bone metabolism were measured: serum osteocalcin, bone alkaline phosphatase (bALP), calcium (Ca), and urine deoxypyridinoline (uDPD). The immunoassays for osteocalcin, bALP, and uDPD were performed using a commercial ELISA kit (Sun Biomedical Technology, Beijing, China) and an ELISA automatic microplate reader (BIO-TEK, USA). Analysis of the sample solution was repeated to confirm the method’s precision. The CVs were <13 % for osteocalcin, <9 % for U-DPD, and <6 % for bALP. Urinary creatinine was determined by a colorimetric assay, using commercially available kit (Nanjing, Jiancheng, China). uDPD was then adjusted to urinary creatinine to account for differences in dilution of the urine. The total serum Ca was determined by compleximetry (Fosun Long March Medical Science Company, Shanghai, China) and an automatic biochemistry analyzer (Beckman DXC 800, USA).
Statistical analysis
For database management and statistical analysis, we used SPSS 13.0. We expressed the central tendency and the spread of variables by the mean or the medium and the 5th to 95th percentile interval. Spearman rank correlation (rs) was used to assess any univariate associations. Mann–Whitney U test, t test, and Chi-square test were used to compare the covariates between subjects by cumulative Pb exposure status (i.e., low vs. high). All p values were derived with a two-sided hypothesis.
Multiple linear regression models were used to analyze any association between exposure and physical growth. For each of the three models, the physical growth variable was evaluated in relation to Pb, Cd, and confounding factors (age and sex of the children). Not all variables were included in one model because physical growth variables had a high degree of collinearity. For each model, height, weight, or BMI was the dependent variable and age, sex, BLLs, and BCLs were the independent variables.
Associations between biomarkers of effect and exposure were evaluated using multiple linear regressions. We indentified covariates by a stepwise regression procedure with the p values for variables to enter and to stay in the model set at 0.15. Covariates considered for entry in the model were age, gender, body mass index, use of supplements (calcium and/or vitamin D), and socioeconomic status.
Results
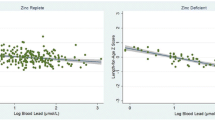
Characteristics of the study population regarding sex, age, blood cadmium, blood lead, and bone biomarkers are presented in Table 1. In the first evaluation, we assessed the univariate associations between exposure and the various bone-related variables and covariates (Table 2). A dwelling place located near or that functioned as an e-waste workshop was a risk factor that contributed to high BCLs. Good nutrition and eating habits such as consuming milk products and having a vitamin supplement were protective factors for the children. There was a significant positive correlation between BCLs and age. Having a dwelling place near or functioned as an e-waste workshop displayed a near-significant association (p =0.054) with BLLs. There was a strong positive correlation between blood lead and uDPD but not between osteocalcin and bALP. Similar effects were not seen for blood cadmium. In this analysis, serum total Ca levels showed a negative association with age, and also higher Ca levels were observed in girls compared to boys. We also found a negative association between uDPD and weight and height, and positive association between Ca levels and bALP and uDPD.
In a separate analysis of the covariates between subjects by cumulative Pb and Cd exposure status (i.e., low vs. high), we found that subjects with high cumulative Pb exposure had a higher uDPD and serum calcium than the subjects with low cumulative Pb exposure (Table 3). We further evaluated the associations between exposure and physical growth variables by adjusting for age and sex (Table 4). This was done because age and gender were shown to be strongly associated with the physical growth of the children in a multiple linear regression analysis. In this adjusted model, BLLs showed a significant negative association with height and weight. However, the association between Cd and physical growth indexes (height, weight, and BMI) became insignificant. In an exploratory analysis, we studied the associations of biomarkers of exposure. BLLs were associated with increases in the urinary excretion of DPD. The serum total Ca increased with age (Table 5).
Discussion
In this study, a higher BLL was associated with reduced height and increased bone resorption in children living in Guiyu which is consistent with our primary hypothesis. There was no association between Cd exposure and bone metabolism. However, this was the first comprehensive assessment of the association linking Pb and Cd exposure to childhood bone growth and bone and Ca metabolism following adjustment for age and sex.
It is difficult to find a perfect dose estimate for either Pb or Cd due to the prevalence of so many other toxic agents. Blood Pb is the most frequently used dose estimate for Pb and is a commonly used indicator of the total body burden by WHO (1995). The half-life of Pb in blood is short, about 36 days, which typically represents recent exposure. Methods for detecting low Pb levels in bone with in vivo X-ray fluorescence are now available (Hu et al. 1998) but are not easily accessible. The dose estimate most often used for Cd is urinary Cd. Blood Cd is considered the most valid marker for recent exposure. However, accumulated Cd in the body will influence the blood Cd concentrations. Therefore, even after exposure ceases the concentration in blood will not decrease to the pre-exposure levels. As a result, Cd in blood may serve as a good estimate of the overall accumulated body burden. The reported correlation coefficient between Cd in blood and urine was 0.6 (Järup and Kesson 2009).
Pb is considered to be one of the major heavy metal contaminants during the process of e-waste recycling (Wong et al. 2007). We found that having a dwelling place near or functioning as an e-waste workshop contributed to high BLLs in children. In this study, the mean child BLL was 7.30 μg/dL, much lower in comparison to our previous studies conducted in Guiyu (Huo et al. 2007; Zheng et al. 2008). However, local children are still under great risk considering the fact that source of the environmental contaminants still exists and no safe threshold for BLLs in young children has been identified by the Centers for Disease Control and Prevention (2005).
Childhood Pb exposure has been attributed to adult disorders such as osteoporosis (Goyer et al. 1994; Gruber et al. 1997; Nash et al. 2004). An alternative model for the development of osteoporosis is that Pb-exposed individuals may achieve a lower peak bone mass as a young adult. This study supports prior studies showing a negative association between BLLs and height in children (Frisancho and Ryan 1991; Kim et al. 1995; Min et al. 2008; Shen et al. 2001) Similarly, Zuscik et al. found that Pb has an effect on the growth plate by the induction of chondrogenes and by reducing chondrocyte maturation, resulting in delayed endochondral bone formation and suggested that the possible mechanism for this process was through the modulation and integration of multiple signaling pathways including TGF-β, BMP, AP-1, and NFκB (Zuscik et al. 2007, 2002). Animal experiments demonstrated that Pb exposure can inhibit fracture healing in mice, with complex effects noted on chondrogenesis and chondrocyte maturation (Carmouche et al. 2005).
Another potential mechanism is that lead affects bone at a cellular level and induces bone loss. Reports have shown that Pb may exert both direct and indirect actions on bone turnover; indirectly through changes in the circulating levels of hormones, particularly 1,25-dihydroxyvitamin D3, which modulate bone cell function, and directly on osteoblast and osteoclast function (Berglund et al. 2000; Pounds et al. 1991). In line with these experimental studies, we found there is an association between BLL and bone resorption. We suggest that lead affects bone resorption (osteoclasts), resulting in increased uDPD and serum calcium levels. Such stimulation of bone resorption has been demonstrated in both animal and in vitro studies (Escribano et al. 1997; Gruber et al. 1997). It has been observed in bone cultures that Pb increases bone resorption by a mechanism involving PGE2 which increases the intracellular levels of cAMP and calcium ions (Miyahara et al. 1995).
In this investigation, no association between bone formation biomarkers and Pb exposure was found. The possible explanation is that Pb exposure at this level was not significant enough to have that effect. In our study, the mean BLL for the local children was 7.30 μg/dl (range, 3.30–24.90). Epidemiological studies have shown that children with increased Pb absorption have a reduction in 1α, 25 (OH)2 D3 concentration which returned to normal once blood Pb decreased to <30 μg/dl (1.4 μmol/l; Rosen et al. 1980).
Despite the extensive research on the effects of Pb on bone metabolism, population studies are limited. In children, high blood Pb was associated with higher BMD (Campbell et al. 2004). Contrary to this, another study found no correlation between blood Pb and BMD (Alfvén et al. 2002). We also found no association between blood Cd and bone turnover biomarkers. Two recent studies also reported no convincing association between low blood Cd exposure and bone (Rignell-Hydbom et al. 2009; Trzcinka-Ochocka et al. 2010). Cd accumulates with age, thus elderly women constitute a high risk group (Järup and Kesson 2009). This study showed that the children may be too young to have bone problems resulting from low cadmium exposure.
Conclusion
In this population study, no association between Cd exposure and bone metabolism biomarkers was found. However, considering Cd accumulates with age, childhood exposure might pose a risk for these children later in life. An adverse effect of Pb exposure on bone growth was demonstrated in this study. An exposure-dependent relationship was found between BLL and bone resorption biomarkers. Childhood Pb exposure may cause adult osteoporosis. Limitations of our study include the cross-sectional design and the difficulty in adjusting for potential confounders. Many other pollutants, such as POPs, also exist in this environment and in the human body, and have the potential to act as confounding factors. A prospective study would be required to confirm this causal finding.
References
Alfvén T, Elinder CG, Carlsson MD, Grubb A, Hellström L, Persson B, Pettersson C, Spang G, Schütz A, Järup L (2000) Low-level cadmium exposure and osteoporosis. J Bone Miner Res 15:1579–1586
Alfvén T, Järup L, Elinder C (2002) Cadmium and lead in blood in relation to low bone mineral density and tubular proteinuria. Environ Health Perspect 110:699–702
Barry P (1975) A comparison of concentrations of lead in human tissues. Br J Ind Med 32:119–139
Barry PSI, Mossman DB (1970) Lead concentration in human tissues. Br J Ind Med 27:339–351
Berglund M, Akesson A, Bjellerup P, Vahter M (2000) Metal–bone interactions. Toxicol Lett 112:219–225
Campbell J, Rosier R, Novotny L, Puzas J (2004) The association between environmental lead exposure and bone density in children. Environ Health Perspect 112:12001203
Carmouche JJ, Puzas JE, Zhang X, Tiyapatanaputi P, Cory-Slechta DA, Gelein R, Zuscik M, Rosier RN, Boyce BF, O'Keefe RJ, Schwarz EM (2005) Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect 113:749–755
Centers for Disease Control and Prevention (2005) Preventing lead poisoning in young children. CDC, Atlanta
Chalkley SR, Richmond J, Barltrop D (1998) Measurement of vitamin D3 metabolites in smelter workers exposed to lead and cadmium. Occup Environ Med 55:446–452
De Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A (2006) Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect 114:584–590
Deng WJ, Louie PKK, Liu WK, Bi XH, Fu JM, Wong MH (2006) Atmospheric levels and cytotoxicity of PAHs and heavy metals in TSP and PM2.5 at an electronic waste recycling site in southeast China. Atmos Environ 40:6945–6955
Escribano A, Revilla M, Hernandez ER, Seco C, González-Riola J, Villa LF, Rico H (1997) Effect of lead on bone development and bone mass: a morphometric, densitometric, and histomorphometric study in growing rats. Calcif Tissue Int 60:200–203
Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev 27:168–176
Frisancho A, Ryan A (1991) Decreased stature associated with moderate blood lead concentrations in Mexican-American children. Am J Clin Nutr 54:516–519
Goyer R, Epstein S, Bhattacharyya M, Korach KS, Pounds J (1994) Environmental risk factors for osteoporosis. Environ Health Perspect 102:390–394
Gruber H, Gonick H, Khalil-Manesh F, Sanchez TV, Motsinger S, Meyer M, Sharp CF (1997) Osteopenia induced by long-term, low-and high-level exposure of the adult rat to lead. Miner Electrolyte Metab 23:65–73
Guo Y, Huo X, Li Y, Wu K, Liu J, Huang J, Zheng G, Xiao Q, Yang H, Wang Y, Chen A, Xu X (2010) Monitoring of lead, cadmium, chromium and nickel in placenta from an e-waste recycling town in China. Sci Total Environ 408:3113–3117
Hu H, Rabinowitz M, Smith D (1998) Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect 106:1–8
Huo X, Peng L, Xu X, Zheng L, Qiu B, Qi Z, Zhang B, Han D, Piao Z (2007) Elevated blood lead levels of children in Guiyu, an electronic waste recycling town in China. Environ Health Perspect 115:1113–1117
Järup L, Alfvén T (2004) Low level cadmium exposure, renal and bone effects—the OSCAR study. Biometals 17:505–509
Järup L, Kesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H (1995) A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ Health Perspect 103:952–957
Li Y, Huo X, Liu J, Peng L, Li W, Xu X (2011) Assessment of cadmium exposure for neonates in Guiyu, an electronic waste pollution site of China. Environ Monit Assess 177:343–351
Li Y, Xu X, Wu K, Chen G, Liu J, Chen S, Gu C, Zhang B, Zheng L, Zheng M, Huo X (2008) Monitoring of lead load and its effect on neonatal behavioral neurological assessment scores in Guiyu, an electronic waste recycle town in China. J Environ Monit 10:1233–1238
Liu J, Xu X, Wu K, Piao Z, Huang J, Guo Y, Li W, Zhang Y, Chen A, Huo X (2011) Association between lead exposure from electronic waste recycling and child temperament alterations. Neurotoxicology 32:458–464
Min KB, Min JY, Cho SI, Kim R, Kim H, Paek D (2008) Relationship between low blood lead levels and growth in children of white-collar civil servants in Korea. Int Hyg Environ Health 211:82–87
Miyahara T, Komiyama H, Miyanishi A, Takata M, Nagai M, Kozuka H, Hayashi T, Yamamoto M, Ito Y, Odake H et al (1995) Stimulative effects of lead on bone resorption in organ culture. Toxicology 97:191–197
Moncrieff AA, Koumides OP, Clayton BE, Patrick AD, Renwick AGC, Roberts GE (1964) Lead poisoning in children. Arch Dis Childh 39:1–13
Nash D, Magder LS, Sherwin R, Rubin RJ, Silbergeld EK (2004) Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: the third national health and nutrition examination survey, 1988–1994. Am J Epidemiol 160:901–911
Ohta H, Ichikawa M, Seki Y (2002) Effects of cadmium intake on bone metabolism of mothers during pregnancy and lactation. Tohoku J Exp Med 196:33–42
Olympio KP, Gonçalves C, Günther WM, Bechara EJ (2009) Neurotoxicity and aggressiveness triggered by low-level lead in children: a review. Rev Panam Salud Publica 26:266–275
Pounds J, Long G, Rosen J (1991) Cellular and molecular toxicity of lead in bone. Environ Health Perspect 91:17–32
Rignell-Hydbom A, Skerfving S, Lundh T, Lindh CH, Elmståhl S, Bjellerup P, Jünsson BA, Strümberg U, Akesson A (2009) Exposure to cadmium and persistent organochlorine pollutants and its association with bone mineral density and markers of bone metabolism on postmenopausal women. Environ Res 109:991–996
Rosen JF, Chesney RW, Hamstra A, DeLuca HF, Mahaffey KR (1980) Reduction in 1, 25-dihydroxyvitamin D in children with increased lead absorption. N Engl J Med 302:1128–1131
Schutte R, Nawrot TS, Richart T, Thijs L, Vanderschueren D, Kuznetsova T, Van Hecke E, Roels HA, Staessen JA (2008) Bone resorption and environmental exposure to cadmium in women: a population study. Environ Health Perspect 116:777–783
Shen X, Wu S, Yan C (2001) Impacts of low-level lead exposure on development of children: recent studies in China. Clin Chim Acta 313:217–220
Sughis M, Penders J, Haufroid V, Nemery B, Nawrot TS (2011) Bone resorption and environmental exposure to cadmium in children: a cross-sectional study. Environ Health 10:104
Trzcinka-Ochocka M, Jakubowski M, Szymczak W, Janasik B, Brodzka R (2010) The effects of low environmental cadmium exposure on bone density. Environ Res 110:286–293
WHO (1995) Inorganic lead. World Health Organization, Geneva
Wong CS, Wu SC, Duzgoren-Aydin NS, Aydin A, Wong MH (2007) Trace metal contamination of sediments in an e-waste processing village in China. Environ Pollut 145:434–442
Yu XZ, Gao Y, Wu SZ, Zhang HB, Cheung KC, Wong MH (2006) Distribution of polycyclic aromatic hydrocarbons in soils at Guiyu area of China, affected by recycling of electronic waste using primitive technologies. Chemosphere 65:1500–1509
Zahran S, Mielke HW, Weiler S, Berry KJ, Gonzales C (2009) Children's blood lead and standardized test performance response as indicators of neurotoxicity in metropolitan New Orleans elementary schools. Neurotoxicology 30:888–897
Zheng G, Xu X, Li B, Wu K, Yekeen TA, Huo X (2012) Association between lung function in school children and exposure to three transition metals from an e-waste recycling area. J Expo Sci Environ Epidemiol. doi:10.1038/jes.2012.84
Zheng L, Wu K, Li Y, Qi Z, Han D, Zhang B, Gu C, Chen G, Liu J, Chen S, Xu X, Huo X (2008) Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ Res 108:15–20
Zuscik MJ, Ma L, Buckley T, Puzas JE, Drissi H, Schwarz EM, O'Keefe RJ (2007) Lead induces chondrogenesis and alters transforming growth factor-beta and bone morphogenetic protein signaling in mesenchymal cell populations. Environ Health Perspectives 115:1276–1282
Zuscik MJ, Pateder DB, Puzas JE, Schwarz EM, Rosier RN, O'Keefe RJ (2002) Lead alters parathyroid hormone-related peptide and transforming growth factor-beta1 effects and AP-1 and NF-kappaB signaling in chondrocytes. J Orthop Res 20:811–818
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21177080). We wish to thank Dr. Stanley Li Lin for his constructive comments.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Hui Yang and Xia Huo contributed equally to this work and are Co- First authors.
Rights and permissions
About this article
Cite this article
Yang, H., Huo, X., Yekeen, T.A. et al. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ Sci Pollut Res 20, 4441–4447 (2013). https://doi.org/10.1007/s11356-012-1366-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1366-2