Abstract
The efficiency of white lupine (Lupinus albus) to uptake and accumulate mercury from a soil polluted by mining activities was assessed in a pot experiment with chemically assisted phytoextraction. The mobilizing agents tested were ethylenediaminetetracetic acid (EDTA) and hydrochloric acid (HCl). Two doses of each amendment were used (0.5 and 1.0 g of amendment per kg of soil), and unamended pots were used as a control. Addition of HCl to the soil did not negatively affect plant biomass, while the use of EDTA led to a significant decrease in plant growth when compared to that found for non-treated pots, with plants visually showing symptoms of toxicity. The addition of hydrochloric acid increased root, shoot and total plant Hg uptake of white lupine by 3.7 times, 3.1 times and 3.5 times, respectively, in relation to non-amended plants. The greatest efficiency was obtained for the highest HCl dose. EDTA led to higher concentrations of total plant Hg than that found with the control, but, due to the aforementioned decrease in plant biomass, the Hg phytoextraction yield was not significantly increased. These results were attributed to the capability of both amendments to form stable Hg complexes. The concentration of Hg in the water of the soil pores after the phytoextraction experiment was very low for all treatments, showing that risks derived from metal leaching could be partially avoided by using doses and chemicals suitable to the concentration of metal in the soil and plant performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is regarded as one of the most toxic pollutants in the world and poses a serious threat to public health and the natural environment. Mercury pollution can result from direct contamination (spilling, landfill, mine tailings, etc.) or indirectly such as from previously volatilized mercury settling back on the soil. It has a great global impact due to its toxicity, complex dynamics in the environment and its tendency to biomagnify in ecosystems (Boening 2000).
In mercury-mining districts, soil can become heavily polluted due to the extent of mining and refining activities. Even after many years of inactivity, the soil in the areas surrounding Hg mining may contain high concentrations of mercury which are of environmental concern. Hg is much more persistent in soils than lakes, oceans and other biomes (Xu et al. 2015). Traditionally, the most common method for remediating mercury-contaminated soils has been excavation and disposal, but these methods are costly and crude. Moreover, they are only useful if the mercury is tightly localized. Therefore, more recent efforts have focused on developing more adequate remediation technologies such as stabilization/solidification, vitrification, electroremediation, soil washing, thermal desorption, immobilization and phytoremediation (Wang et al. 2012).
Phytoextraction is a type of phytoremediation process which involves the use of plants to take up pollutants from the soil and accumulate them in aboveground plant tissues. This is considered to be a cost-effective and environmentally friendly technology that could potentially be applied to soils polluted by mercury (Ali et al. 2013). One of the drawbacks this technology has is that availability of metals in soils affect root absorption and, therefore, metal accumulation in plants. How much availability there is for uptake, i.e. the phytoavailability of metals, is affected by numerous soil factors, such as the cation exchange capacity, pH and organic matter content, and the speciation of the metal, which is correlated to the factors mentioned above and the metal species itself, plays an important part (Evangelou et al. 2007). Enhancing metal accumulation in existing high yielding crop plants without diminishing their yield is one of the most feasible strategies in the development of phytoremediation.
Chemically assisted phytoextraction involves the application of chemical amendments to soil to foster the solubility of metals and thereby increase their accumulation in plant tissues. Evangelou et al. (2007) made an extensive review of the use of different chelating agents for assisted phytoextraction focusing on their effects, mechanism, toxicity and fate in the soils. Various aminopolycarboxilic acids, such as ethylenediaminetetracetic acid (EDTA), ethylene diamine disuccinate (EDDS) and nitrilotriacetic acid (NTA), to natural low molecular weight acids, such as citric and tartaric acids, are described together. Assisted phytoextraction of mercury has been reported by several researchers: Potassium iodide, sodium thiosulphate, thiourea, EDTA, urease, citric acid and compost have been used as amendments to increase the solubility of mercury and to enhance plant uptake (Moreno et al. 2005a,b; Smolińska and Cedzyńska 2007; Wang et al. 2011; Cassina et al. 2012; Smolińska and Król 2012; Smolińska 2015; Smolińska and Rowe 2015; Smolińska and Leszczynska 2015; Franchi et al. 2016).
Hydrochloric acid may lead to the formation of stable Hg-chloride complexes (Gabriel and Williamson 2004), and moreover, it is well known that metals are usually more available at low pH values. However, to date, its use as amendment in chemically assisted phytoextraction of mercury has not been reported. EDTA was also used in this research because it has been shown to be able to significantly increase the total amount of Hg taken up by plants in phytoextraction experiments conducted by using soils artificially contaminated with HgCl2, HgSO4 or Hg(NO3)2 (Smolińska and Cedzyńska 2007). So, the objective of this research was to investigate the capability HCl and EDTA have to enhance phytoextraction of mercury in a soil polluted by historical mining activities by using lupine plants under laboratory conditions. White lupine (Lupinus albus) was used in this research taking into account the previously reported results about its use in Hg phytoextraction (Rodríguez et al. 2007; Zornoza et al. 2010).
Materials and methods
Soil
The Hg-polluted soil used in the experiment was randomly collected from an agricultural plot (UTM 30S 0352018, 4289465) located near a former Hg metallurgy plant in the Almadén district (Ciudad Real, Spain), a historical mercury-mining centre located in central Spain, approximately 300 km southwest of Madrid. As a consequence of the prolonged mining activities (more than 2000 years) together with natural emissions, high levels of mercury have been reported in the soils, waters and air of the surrounding areas (Gray et al. 2004). Superficial samples (0–20 cm) of cultivated soil were taken. The physicochemical characteristics of the soil were determined by standard methods used by the Spanish Ministry of Agriculture, Fisheries and Food (MAPA 1994). The soil was classified as loamy with 12.2 % clay, 45.0 % silt and 42.9 % sand, with a pH (in water) of 6.4, 2.48 % organic matter (OM), a cation exchange capacity (CEC) of 16.2 cmolc kg−1 and with electrical conductivity of 249 mS cm−1. The soil was air-dried, crushed and screened through a 5-mm sieve to remove stones, plant roots and other large particles prior to its use in the pot experiments.
Phytoextraction experiment
White lupine, L. albus L., was selected for mercury phytoextraction experiments. Lupine seeds (cultivar ‘Marta’) were soaked in a saturated CaSO4 solution for 1 h and then placed on wet filter paper for 4 days in the dark to germinate. After this period, the seedlings were transferred to plastic pots (11.4 cm high and 9.6 cm in diameter) containing 500 g DW of a substrate made by a mixture of the polluted soil and perlite (2:1 v/v). Perlite was used as it improved drainage in the pots to some extent. A total of 16 seeds were sown per pot. The substrate moisture was initially adjusted to field water capacity (80 %), and water losses were compensated for by adding deionized water every 2 days throughout the experiment. Additionally, the substrates were occasionally supplemented with a commercial fertilizer applied by foliar feeding (Peter Professional Scotts; NPK 20 + 20 + 20).
The pot experiment was conducted for 3 months in a greenhouse, under natural light conditions. The day/night temperature of the air ranged from 28 to 10 °C, respectively. Sixty-five days after planting, 200 mL of EDTA or HCl aqueous solution was applied to the surface of the pots at rates of 0 (control), 0.5 and 1 g of mobilizing agent per kg of soil substrate. All treatments were carried out in triplicate.
Lupine shoots and roots were harvested at days 32, 60, 74, 80 and 95 (two individual plants at each sampling). The three last sampling corresponded to 9, 15 and 30 after adding the soil amendments. The shoots and roots were separated and washed thoroughly with deionized water, placed on filter paper, air-dried for 72 h, ground into a fine powder by a ball mill (Retsch MM200, Haan, Germany) and sealed in plastic bags for subsequent determination of mercury. Growth substrate was sampled both at the beginning and at the end of the experiment. It was air-dried, disaggregated, sieved to <2 mm and, finally, ground into a fine powder by a ball mill (Retsch MM200, Haan, Germany) prior to be analysed for pH and total and available mercury content. All soil samples were analysed in triplicate for their general soil properties and total mercury content.
Samples of soil pore water were taken on days 60 (5 days before amendment addition), 70, 80, 86 and 95 (5, 10, 21 and 30 days after amendment addition) by using Rhizon soil moisture samplers (Rhizosphere Research Products, Wageningen, Holland). Water samples were filtered by using 0.45-μm syringe filters and acidified with diluted nitric acid prior to mercury analysis.
The bioaccumulation factor (BAF) of mercury by L. albus was calculated in this work by using the following equations (Wang et al. 2011):
Total and CaCl2-extractable Hg concentration in soils were the final values at the end of the experiment.
The translocation factor (TF) was calculated as the ratio of mercury concentration in the shoots relative to that in the roots (Smolińska and Leszczynska 2015):
Soil and plant mercury analysis
To determine the total concentration of Hg (<2 mm fraction), a 0.5 g sample was digested with a mixture of acids (9 mL of concentrated HNO3 + 3 mL of concentrated HCl) in a microwave unit (CEM MARS 5, Matthews, USA), according to the EPA 3051A method. Plant samples were digested in the same microwave unit by using a mixture of HNO3/HCl/H2O2 (3052 EPA method). All the samples were analysed in triplicate, and the Hg concentrations were given on a dry weight basis. Soil available mercury was extracted by using 0.01 M CaCl2, according to the method described by Novozamsky et al. (1993).
The mercury content of both soil water samples and soil and plant extracts was measured by the cold vapour technique by using an atomic absorption spectrophotometer Varian SpectrAA 240FS (Varian Inc., CA, USA) equipped with the hydride generator VGA-77. An acid solution of stannous chloride (SnCl2 25 % w/v in HCl 20 % v/v) was used as reductant for the samples. The Hg concentration values reported here were the mean of three measurements (with a variation of less than a 5 % among them).
The analytical method for soil Hg was assessed by using the 2711 Standard Reference Material (Montana soil, from LGC Promochem) with which there was 95–103 % agreement between the certified value and the concentration we obtained (n = 3). CTA-VTL-2 Reference Material (Virginia tobacco leaves, from LGC Promochem) was used to assess the analytical method for plant Hg; 91–97 % agreement was found between the Hg concentration obtained by us and the certified one (n = 3).
Statistical analyses
All statistical analyses were carried out with the IBM SPSS Statistics program version 19.0. One-way ANOVA was used to assess the effect of the amendments on plant biomass and the concentrations and phytoextraction yields of mercury in the L. albus tissues. Pearson’s correlation coefficient was used to measure the correlation between Hg plant concentrations and CaCl2-extractable Hg in the soils after the experiment. The data normality was checked by using the Kolmogorov-Smirnov test.
Results and discussion
Effect of amendments on the soil mercury
The values of pH and total and CaCl2-extractable Hg concentrations in the growth substrates before and after the phytoextraction experiments are all shown in Table 1. The initial pH of the substrate was moderately acid (6.4), and it slightly decreased with plant growth when no amendments were used and with both EDTA treatments. However, it significantly decreased with the addition of hydrochloric acid; the extent of the decrease was higher for the highest HCl dose. It agrees with the evolution of pH in the soil water samples taken throughout the phytoextraction experiment (Fig. 1). The pH of the water coming from the control pots showed values around 5.2 on the different sampling days. EDTA addition did not cause significant variations to soil water pH in relation to that of the control pots. However, on adding HCl, the pH of the soil water decreased considerably, i.e. with values of 4.2 and 3.7 with the 0.5 and 1.0 g kg−1 HCl doses, respectively, 5 days after application of the treatment. Later, the pH of the soil pore water gradually increased until the end of the experiment reaching final pH values which were slightly lower than those of the control pots.
Total mercury concentration in the initial growth substrate decreased by 21–37 % after plant growth (treated and non-treated). Moreover, both EDTA and HCl led to statistically significant (p < 0.05) lower total Hg concentrations in the soil in relation to that in the non-treated substrate. The effect was more pronounced with the highest doses of both mobilizing agents. Because the lupine plants were removed from the pots throughout the phytoextraction experiment, an exact mass balance cannot be done to calculate the Hg removed by the plants. However, an approximate calculation by using the best phytoextraction yields reached in this work (see below) let us to conclude that most of the initial mercury in the growth substrate was lost through other pathways. If we take into account that water leaching through the bottom of the pot was prevented, we can suggest that mercury may have been lost to the air. The role of rhizosphere processes play in Hg volatilization in plants has been reported by Moreno et al. (2005a) and Wang et al. (2011). Root-induced Hg volatilization would be the result of a biological reduction in Hg from Hg2+ to Hg0 carried out by Hg-resistant bacteria living in the rhizosphere or inside the roots; moreover, mercury volatilization was increased when mobilizing agents, such as thisulphate, were used (Moreno et al. 2005b). Therefore, the mobilization of Hg brought about by EDTA and the HCl amendments in our study could have increased Hg volatilization by rhizosphere bacteria which would have caused significant reductions in the total amount of Hg in the substrates after the experiment in relation to what happened with the non-treated pots.
The plants reduced the Hg available in the soil after the experiments to a high extent. As it is shown in Table 1, the addition of the amendments increased the concentration of CaCl2-extractable Hg with respect to the control series, although this increase was only statistically significant when doses of 1.0 g kg−1 of amendment were used. The concentration of CaCl2-extractable Hg found for the control pots at the end of the experiment was lower than those of the amended pots. It may be explained taking into account that, increasing metal availability with chemicals, plants could be not enough able to uptake all the mobilized Hg. Additional evidences about mercury mobilization are given from the analysis of the Hg concentration in the soil pore water (Fig. 1). Thus, the effects the amendments we are having on the concentration of Hg in the soil pore water began to be evident approximately 10 days after their application, reaching the highest values (up to three times higher than that found for the control) on day 86 (21 days after applying the treatment). Finally, thereafter and until the end of the experiment, the concentration of Hg in the soil water decreased for all the treatments. The trend observed for concentrations of Hg in the samples of soil pore water clearly shows the increase of soluble Hg caused by EDTA and HCl. The subsequent decrease observed for this parameter in the last 15 days of the experiment may be attributed in part to the significant uptake of Hg by the plants although other soil processes such as Hg volatilization caused by microbial activity cannot be ruled out.
Effect of amendments on plant growth
The values for the roots, shoots and the total dry biomass of the lupine plants harvested after 95 days of growth in the Hg-polluted substrate (30 days after adding EDTA and HCl amendments) are shown in Table 2.
When no amendments were added to the soil (control), all the plants showed normal growth without visual signs of metal toxicity. However, when EDTA solutions were added, this led to a significant decrease in plant growth (until 55 % for EDTA 1.0 treatment) when compared to that found with the control pots (Table 2). In fact, in all the pots for which EDTA was applied, visual signs of plant toxicity (strong chlorosis and stunting) were observed few days after applying the amendments; this effect was more pronounced for the highest EDTA dose. Conversely, amendment of the soils with hydrochloric acid did not significantly affect plant biomass which even showed significant improvement (48 %) with the lower HCl dose (Table 2). There were no symptoms of plant toxicity for the substrates amended with hydrochloric acid. The total biomass the lupine plants reached was in the order of HCl 0.5 > HCl 1.0 ≥ control > EDTA 1.0–EDTA 0.5.
The use of EDTA to improve metal mobility in soils in phytoextraction experiments has been reported to produce low biomass, leaf wilt, chlorosis and necrosis, abscission, shoot desiccation and reduced transpiration (Lombi et al. 2001; Römkens et al. 2002; Grčman et al. 2003; Eissa 2016). However, plant growth in EDTA-assisted phytoextraction is related to several factors, e.g. the EDTA dose applied, the time of application, the plant species and the type and concentration of metals (Lombi et al. 2001; Grčman et al. 2003; Evangelou et al. 2007). Smolińska and Cedzyńska (2007) found that application of 1.0 g of EDTA per kg of soil did not significantly decrease the biomass of garden cress (Lepidium sativum) grown in a soil which had been polluted artificially with Hg. On the other hand, EDTA and other chelating substances may also reduce plant growth by increasing the bioavailability of soil metals (Eissa 2016). According to our previous results on mercury phytoextraction with white lupine (Rodríguez et al. 2007), where no toxicity was found for concentrations of Hg in the shoots for up to 4 μg g−1 (quite higher than those found in this study, see below), it can be assumed that EDTA is the only agent responsible for reducing the growth of lupine plants in this study. The latter is additionally supported by the fact that the plants treated with HCl, with higher concentrations of Hg in the roots and shoots (see below), did not display visual signs of toxicity.
Evangelou et al. (2006) reported that the addition of organic acids (citric, oxalic and tartaric acids) did not adversely affect dry matter produced by the tobacco plants when the application rate of the acid was below 62.5 mmol kg−1 (equivalent to doses in the 5–12 g kg−1 range, approximately), and there was even a slight increase in shoot yields discernible in some cases; higher doses of acids resulted in decreases in biomass, probably due to physiological changes in the root barriers which controlled the uptake of solutes. Huang et al. (1998) stated that on adding citric acid to contaminated soils, the pH was transiently reduced by 0.5–1.0 units with the plant biomass remaining unaffected. In other studies carried out with citric acid and Indian mustard, there were no signs of toxicity (Evangelou et al. 2007). Our data are in keeping with previous research, since addition of hydrochloric acid did not affect lupine growth in spite of the observed decrease in the soil pH (by 1–2 units with respect to the control soil, Table 1). Moreover, lupine growth increased significantly with the lowest HCl dose used. This could be put down to the fact that white lupine grows better in acidic soils than calcareous or limed ones (Bertoni et al. 1992; Kerley and Huyghe 2002).
Effect of amendments on Hg uptake by Lupinus albus
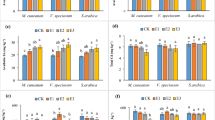
The lupine plants were capable of taking up and accumulating Hg for all the treatments applied (Fig. 2), although the concentrations reached were relatively low. Root concentrations were much higher than those found in the shoots, thereby showing how difficult Hg translocation in the plants was. Root concentrations were in the 1.39–4.60 μg g−1 range, while shoot concentrations ranged only between 0.11 and 0.41 μg g−1; thus, TF values found were low, i.e. in the 0.02–0.14 range (Table 3). Addition of the two amendments had an important influence on the Hg concentration in the lupine plant tissues. On the one hand, for the two doses used, both EDTA and HCl were able to significantly increase the Hg concentration (up to three times for the HCl 1.0 treatment) in the whole plant. More specifically, treating the soil with hydrochloric acid and EDTA with a 1.0 g kg−1 dose significantly increased concentrations of Hg in the roots in relation to what occurred with the non-treated control (Fig. 2), while adding HCl (with both doses used) and EDTA (with the 0.5 g kg−1 dose) led to significant increases in Hg shoot concentrations with respect to the control (Fig. 2).
Hg concentration in plant tissues (root, shoot and total plant) in μg g−1 at the end of the phytoextraction experiment (95 days) for the different experimental series (non-treated, EDTA 0.5, EDTA 1.0, HCl 0.5, HCl 1.0). Error bars represent the SD of three replicates. The different letters mean significant differences (p < 0.05) between treatments
Smolińska et al. (Smolińska and Cedzyńska 2007; Smolińska and Król 2012) have reported that EDTA addition increased plant uptake of Hg by garden cress plants (L. sativum) although most of the mercury was accumulated in the roots. Our results showed that concentrations of Hg in the shoots were enhanced with the lowest EDTA dose, i.e. 0.5 g kg−1, while the EDTA 1.0 treatment led to a significant increase for Hg concentration in roots. This different trend was probably due to the toxic effects produced in the lupine plants for the highest dose. The mechanism by which there is enhanced uptake of metals with EDTA is still partially unknown as it depends on both the metal and the plant used (Evangelou et al. 2007).
It may be hypothesized that the high efficiency hydrochloric acid has in enhancing mercury phytoextraction is based on two synergic effects: (i) the decrease in soil pH and (ii) the chelation between Hg and chloride anions. It is generally accepted that low pH values favour metal mobility and availability (Wang et al. 2004; Clemente et al. 2005). However, a decrease in soil pH is not enough to enhance metal uptake by plants in some cases. Huang et al. (1998), studying the assisted phytoextraction of uranium, assessed that nitric and sulphuric acids reduced soil pH by a similar amount as with citric acid; however, both soil uranium desorption and uranium accumulation in the shoots of Indian mustard were far less when inorganic acids were used. They concluded that the reduction in soil pH only partly contributed to improving the availability of U, while the chelation between citric acid and uranium could have been the most important parameter in uptake of U by the plants. Subirés-Muñoz et al. (2011) studied the effectiveness of some chelating agents, i.e. sodium thiosulphate, EDTA, sodium chloride, potassium iodide and HNO3, in remediating a soil from the Almadén mining district by means of washing; their results showed that nitric acid was not able to extract detectable concentrations of Hg, while with chloride solution, the amounts of Hg extracted corresponded to approximately 2 % of the initial mercury. Hg2+ has been reported to have a strong tendency to build complexes with Cl−, OH−, S2−, S-containing functional groups of organic ligands and NH3 because of their high abundance and stability with mercury (Schuster 1991). In general, more concentrated chloride reduces the capacity of inorganic and organic materials to adsorb Hg due to the highly stable bond between Hg and chloride ions (with HgCl2 being the most abundant mercury-chloride complex over the whole pH range); it could, in turn, potentially increase the bioavailability of mercury (Gabriel and Williamson 2004). According to these findings, it may be said that the chelation between Hg and chloride anions (released from HCl) was the main mechanism by which the enhanced mercury accumulation in the plants in our research occurred.
Our results showed that approximately 60–65 % of the mercury is accumulated in the roots of lupine plants with limited translocation to the aerial part (Table 3). Furthermore, this trend did not change after the amendment was added (see translocation factor values in Table 3). In the research regarding Hg phytoextraction, there is a general consensus that Hg mainly accumulates in plant roots (Wang and Greger 2006; Smolińska and Cedzyńska 2007; Cassina et al. 2012; Marrugo-Negrete et al. 2015; Smolińska and Rowe 2015; Smolińska and Leszczynska 2015). It has been suggested that the Hg accumulated in plant roots is linked to the root cell walls or to a sulphydryl groups of cysteine which is present in phytochelatines; in any case, this Hg becomes unavailable for transportation to the shoots (Smolińska and Cedzyńska 2007; Marrugo-Negrete et al. 2015). However, there is varying evidence as regards to how effective chelating agents are in enhancing mercury translocation in plants. Wang and Greger (2006) found that on adding iodide, translocation of Hg in willow plants was not enhanced; Smolińska and Leszczynska (2015) reported that application of potassium iodide could improve the translocation factor of Hg for L. sativum by up to 3.6 times with respect to that of the non-treated soil; lastly, it has been reported that by adding thiosulfate, there is enhanced Hg uptake and translocation to shoots for different plant species, i.e. Chenopodium glaucum, Brassica juncea and Helianthus annuus (Moreno et al. 2005b; Wang et al. 2011; Cassina et al. 2012; Smolińska and Rowe 2015), but it decreased the translocation factor for L. albus (Franchi et al. 2016). In the only research into EDTA-assisted phytoextraction of mercury, it was also found that this chelating agent was able to increase Hg translocation in L. sativum, although they did not provide any explanation for this (Smolińska and Cedzyńska 2007). Based on the results obtained in our study and those mentioned above, it can be concluded that the effectiveness of translocation is dependent on both the plant species and the mobilizing agent.
Plant mercury uptake patterns
Figure 3a shows how the Hg accumulation evolved in the plants (mg of Hg per plant) throughout the whole experiment. Figure 3b, c shows the concentration of Hg (μg of Hg per g of plant) in the roots and shoots of the lupine plants, respectively.
Evolution of the total accumulation of mercury in the plant (a, mg Hg per plant), Hg root concentration (b, μg g−1) and Hg shoot concentration (c, μg g−1) in white lupine (mg DW per plant) throughout the phytoextraction experiment for the different experimental series (non-treated, EDTA 0.5, EDTA 1.0, HCl 0.5, HCl 1.0). Amendments were added on day 65. Error bars represent the SD of three replicates
It can be seen that Hg is accumulated in the roots to a higher extent than the shoots throughout the two first months of growth (Fig. 3b, c) with there being no significant differences between treatments (as expected because the amendment had still not been added). The plants grown in the non-amended pots took up and accumulated mercury continuously throughout the 95 days of exposure, with there being a more pronounced increase from day 74 (Fig. 3a). Mercury concentrations in the roots of the non-treated plants also increased continuously throughout the experiment (Fig. 3b); however, the trend with concentrations in the shoots was rather different: They sharply increased between days 60 and 74 and thereafter decreased until the end of the experiment (Fig. 3c). Marrugo-Negrete et al. (2015) suggested that Hg may be reduced from divalent mercury to elemental mercury with the subsequent volatilization by transpiration in the plant leaves. Thus, the accumulation of Hg in the shoots would be a result of the balance between the Hg uptake and accumulation kinetics and its transportation in the transpiration flux. Although Hg volatilization was not registered in this research, the aforementioned mechanisms may be used reasonably to explain the trend in the accumulation of Hg found for lupine plants grown in the unamended pots. According to our findings, Hg would continuously be taken up and translocated in the lupine plants during the whole growth period, but from day 74 to the end of the experiment, the transpiration flux and the subsequent volatilization of Hg0 would be faster than the Hg uptake and accumulation which would lead to falling concentrations in the shoots. This would additionally be supported by the sharp decrease in concentrations of Hg found in the soil pore water between days 60 and 70 and the less pronounced variation until day 95 (Fig. 1).
However, this trend was significantly affected by the addition of amendments (day 65, Fig. 3). Well in keeping with other previously reported results (Smolińska and Cedzyńska 2007), on adding EDTA, Hg availability and, consequently, Hg uptake was enhanced, and the translocation process increased from the first days after adding the amendment. Due to this, there were visible toxicity effects with the plants treated with the high EDTA dose, and as a result, translocation of Hg to the shoots was hindered (Greger et al. 2005). Conversely, translocation of mercury sharply increased after day 80 with the lowest EDTA dose (Fig. 3c), showing that EDTA, with certain doses, is able to enhance Hg translocation in lupine plants to some extent. The effects of adding HCl to the uptake in Hg and translocation by plants were particularly clear from day 74 when increasing values for both the total Hg content in the plants, and the concentrations of it in the shoots and roots were found. The observed slowdown in both plant biomass (data not shown) and mercury uptake registered in the first days after the treatment seemed to be due to plant stress due to the initial sharp decrease in the soil pH triggered by adding an acid. This is supported by the abovementioned trend in pH found for the soil pore water (Fig. 1).
Effectiveness of Hg phytoextraction
The amount of Hg phytoextracted was calculated as being the product of biomass yield and Hg concentration in plant tissues; the results of which are shown in Table 3. Only with hydrochloric acid was the phytoextraction yields of lupine plants significantly increased when compared to those found with the non-treated soils. Thus, on adding HCl, the total amount of Hg uptake in the roots, shoots and plant as a whole increased by 322–369, 253–314 and 294–347 %, respectively, with the best results found for the highest HCl dose, i.e. 1.0 g kg−1. Adding EDTA did not significantly increase the mercury phytoextraction yields reached for the control series due to its toxic effect in lupine (Table 3).
According to Wang et al. (2011), mercury bioaccumulation factor values were calculated taking into account the total amount of Hg concentrated in the soil (BAFTotal) and the CaCl2-extractable Hg (BAFAvail) at the end of the phytoremediation experiment (Table 3). With the exception of EDTA 1.0, all the treatments significantly increased the BAFTotal value corresponding to the control series, although all of them were very low. Regarding the BAFAvail values, as a consequence of the low concentrations of available Hg in the growth substrates, they were four orders of magnitude higher than the BAFTotal ones. With both EDTA 0.5 and HCl 1.0 treatments, the bioaccumulation factor values with respect to those of the non-treated pots increased significantly. The ranges for both parameters (BAFTotal and BAFAvail) were in the same order of magnitude as those reported by Wang et al. (2011) for the assisted phytoextraction of mercury by C. glaucum L. by using ammonium thiosulphate.
The potential heavy metals have for leaching below the root zone of the plants should be taken into account when considering chemically assisted phytoextraction (Smolińska and Król 2012; Wang et al. 2012). Our results showed that the initial CaCl2-extractable Hg in the original substrate greatly decreased after the phytoextraction experiment (treated and non-treated pots, Table 1) and there was a strong significant correlation (p < 0.05) between this parameter and both the Hg in the roots and the total amount of Hg in the plants at the end of the experiment. Moreover, the final CaCl2-extractable Hg in the amended substrates only significantly increased with the highest doses of EDTA and HCl (Table 1), and the concentration of Hg in the soil pore water was very low and similar for all the treatments and also for the non-treated pots (Fig. 1). These results show that the risks of metal leaching with the use of chelating agents may be partially prevented by using doses and chemicals suitable to the concentration of soil metal and plant performance.
Conclusions
The data presented in this paper show that hydrochloric acid was able to significantly enhance the uptake and accumulation of mercury by white lupine. In fact, addition of HCl to the polluted soil increased both plant biomass production and Hg concentration in roots and shoots. As a consequence, the use of HCl increased Hg uptake in the roots, shoot and plant as a whole by 322–369, 253–314 and 294–347 %, respectively, with the best results found with the highest HCl dose (1.0 g kg−1). Addition of EDTA led to significant increases in the concentration of Hg in the plant tissues, but, due to the decrease in plant biomass caused by EDTA toxicity, the Hg phytoextraction yields were not significantly different to those from the non-treated plants. This means that with both amendments, Hg availability was enhanced to some extent. It has been hypothesized that the formation of corresponding Hg complexes with EDTA and chloride ions was the driving factor behind this Hg mobilization. Considering our results, it seems that the HCl-assisted mercury phytoextraction with white lupine could potentially be used to reduce the potentially available mercury in soils polluted by mining activities.
Nevertheless, it should be taken into account that we carried out a laboratory experiment with a limited duration and the results corresponded to a single growing cycle. Therefore, the conclusions of this study should be validated on a broader scale by means of field tests consisting of several harvests and several amendment additions. Those experiments could contribute to determine the fate of Hg in subsequent cycles both in plant tissues and in soil fractions. Lastly, the potential risk derived from Hg volatilization, brought about by rhizosphere microorganisms and/or plant transpiration, together with Hg leaching should carefully be considered before applying this technique in the field.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881. doi:10.1016/j.chemosphere.2013.01.075
Bertoni GM, Pissaloux A, Morard P, Sayag DR (1992) Bicarbonate-pH relationship with iron chlorosis in white lupine. J Plant Nutr 15:1509–1518. doi:10.1080/01904169209364417
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351. doi:10.1016/S0045-6535(99)00283-0
Cassina L, Tassi E, Pedron F, Petruzzelli G, Ambrosini P, Barbafieri M (2012) Using a plant hormone and a thioligand to improve phytoremediation of Hg-contaminated soil from a petrochemical plant. J Hazard Mater 231-232:36–42. doi:10.1016/j.jhazmat.2012.06.031
Clemente R, Walker DJ, Bernal MP (2005) Uptake of heavy metals and as by Brassica juncea grown in a contaminated soil in Aznalcóllar (Spain): the effect of soil amendments. Environ Pollut 138:46–58. doi:10.1016/j.envpol.2005.02.019
Eissa MA (2016) Effect of sugarcane vinasse and EDTA on cadmium phytoextraction by two saltbush plants. Environ Sci Pollut R 23:10247–10254. doi:10.1007/s11356-016-6261-9
Evangelou MWH, Ebel M, Schaeffer A (2006) Evaluation of the effect of small organic acids on phytoextraction of Cu and Pb from soil with tobacco Nicotiana tabacum. Chemosphere 63:996–1004. doi:10.1016/j.chemosphere.2005.08.042
Evangelou MWH, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68:989–1003. doi:10.1016/j.chemosphere.2007.01.062
Franchi E, Rolli E, Marasco R, Agazzi G, Borin S, Cosmina P, Petruzzelli G (2016) Phytoremediation of a multi contaminated soil: mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J Soils Sediments 1–13. doi:10.1007/s11368-015-1346-5
Gabriel MC, Williamson DG (2004) Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ Geochem Hlth 26:421–434. doi:10.1007/s10653-004-1308-0
Gray JE, Hines ME, Higueras PL, Adatto I, Lasorsa BK (2004) Mercury speciation and microbial transformations in mine wastes, stream sediments, and surface waters at the Almadén mining district. Spain. Environ Sci Technol 38:4285–4292. doi:10.1021/es040359d
Grčman H, Vodnik D, Velikonja-Bolta S, Lestan D (2003) Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. J Environ Qual 32:500–506
Greger M, Wang Y, Neuschütz C (2005) Absence of Hg transpiration by shoot after Hg uptake by roots of six terrestrial plant species. Environ Pollut 134:201–208. doi:10.1016/j.envpol.2004.08.007
Huang JW, Blaylock MJ, Kapulnik Y, Ensley BD (1998) Phytoremediation of uranium-contaminated soils: role of organic acids in triggering uranium hyperaccumulation in plants. Environ Sci Technol 32:2004–2008. doi:10.1021/es971027u
Kerley SJ, Huyghe C (2002) Stress-induced changes in the root architecture of white lupin (Lupinus albus) in response to pH, bicarbonate, and calcium in liquid culture. Ann Appl Bio 141:171–181
Lombi E, Zhao FJ, Dunhamm SJ, McGrath SP (2001) Phytoremediation of heavy metal-contaminated soils: natural hyperaccumulation versus chemically enhanced Phytoextraction. J Environ Qual 30:1919–1926
MAPA (Ministerio de Agricultura, Pesca y Alimentación de España) (1994) Métodos oficiales de análisis de suelos y aguas. Madrid (Spain)
Marrugo-Negrete J, Durango-Hernández J, Pinedo-Hernández J, Olivero-Verbel J, Díez S (2015) Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 127:58–63. doi:10.1016/j.chemosphere.2014.12.073
Moreno FN, Anderson CWN, Stewart RB, Robinson BH (2005a) Mercury volatilisation and phytoextraction from base-metal mine tailings. Environ Pollut 136:341–352. doi:10.1016/j.envpol.2004.11.020
Moreno FN, Anderson CWN, Stewart RB, Robinson BH, Nomura R, Ghomsher M, Meech JA (2005b) Effect of thioligands on plant-Hg accumulation and volatilisation from mercury-contaminated mine tailings. Plant Soil 275:233–246. doi:10.1007/s11104-005-1755-0
Novozamsky U, Lexmond TM, Houba VJG (1993) A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. Int J Environ An Ch 51:47–58
Rodríguez L, Rincón J, Asencio I, Rodríguez-Castellanos L (2007) Capability of selected crop plants for shoot mercury accumulation from polluted soils: phytoremediation perspectives. Int J Phytoremediat 9:1–13. doi:10.1080/15226510601139359
Römkens P, Bouwman L, Japenga J, Draaisma C (2002) Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ Pollut 116:109–121
Schuster E (1991) The behavior of mercury in the soil with special emphasis on complexation and adsorption processes—a review of the literature. Water Air Soil Poll 56:667–680
Smolińska B (2015) Green waste compost as an amendment during induced phytoextraction of mercury-contaminated soil. Environ Sci Pollut Res 22:3528–3537. doi:10.1007/s11356-014-3601-5
Smolińska B, Cedzyńska K (2007) EDTA and urease effects on Hg accumulation by Lepidium sativum. Chemosphere 69:1388–1395. doi:10.1016/j.chemosphere.2007.05.003
Smolińska B, Król K (2012) Leaching of mercury during phytoextraction assisted by EDTA, KI and citric acid. J Chem Technol Biot 87:1360–1365. doi:10.1002/jctb.3826
Smolińska B, Leszczynska J (2015) Influence of combined use of iodide and compost on Hg accumulation by Lepidium sativum L. J Environ Manag 150:499–507. doi:10.1016/j.jenvman.2014.12.043
Smolińska B, Rowe S (2015) The potential of Lepidium sativum L. For phytoextraction of Hg-contaminated soil assisted by thiosulphate. J Soils Sediments 15:393–400. doi:10.1007/s11368-014-0997-y
Subirés-Muñoz JD, García-Rubio A, Vereda-Alonso C, Gómez-Lahoz C, Rodríguez-Maroto JM, García-Herruzo F, Paz-García JM (2011) Feasibility study of the use of different extractant agents in the remediation of a mercury contaminated soil from Almadén. Sep Purif Technol 79:151–156. doi:10.1016/j.seppur.2011.01.032
Wang Y, Greger M (2006) Use of iodide to enhance the phytoextraction of mercury-contaminated soil. Sci Total Environ 368:30–39. doi:10.1016/j.scitotenv.2005.09.034
Wang X, Shan X, Zhang S, Wen B (2004) A model for evaluation of the phytoavailability of trace elements to vegetables under the field conditions. Chemosphere 55:811–822. doi:10.1016/j.chemosphere.2003.12.003
Wang J, Feng X, Anderson CWN, Qiu G, Ping L, Bao Z (2011) Ammonium thiosulphate enhanced phytoextraction from mercury contaminated soil—results from a greenhouse study. J Hazard Mater 186:119–127. doi:10.1016/j.jhazmat.2010.10.097
Wang J, Feng X, Anderson CWN, Xing Y, Shang L (2012) Remediation of mercury contaminated sites—a review. J Hazard Mater 221-222:1–18. doi:10.1016/j.jhazmat.2012.04.035
Xu J, Bravo AG, Lagerkvist A, Bertilsson S, Sjöblom R, Kumpiene J (2015) Sources and remediation techniques for mercury contaminated soil. Environ Int 74:42–53. doi:10.1016/j.envint.2014.09.007
Zornoza P, Millán R, Sierra MJ, Seco A, Esteban E (2010) Efficiency of white lupin in the removal of mercury from contaminated soils: soil and hydroponic experiments. J Environ Sci 22:421–427. doi:10.1016/S1001-0742(09)60124-8
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Rodríguez, L., Alonso-Azcárate, J., Villaseñor, J. et al. EDTA and hydrochloric acid effects on mercury accumulation by Lupinus albus . Environ Sci Pollut Res 23, 24739–24748 (2016). https://doi.org/10.1007/s11356-016-7680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7680-3