Abstract
In this paper, the removal of three common dyes (orange I, orange II, and methylorange) and of the anticonvulsant drug carbamazepine from aqueous solutions by means of enzymatic and photocatalytic treatment was studied. Soybean peroxidase (SBP) was used as biocatalyst, both free in solution and immobilized on silica monoliths, and titanium dioxide as photocatalyst. The combination of the two catalysts led to a faster (about two to four times) removal of all the orange dyes compared to the single systems. All the dyes were completely removed within 2 h, also in the presence of immobilized SBP. As for carbamazepine, photocatalytic treatment prevails on the enzymatic degradation, but the synergistic effect of two catalysts led to a more efficient degradation; carbamazepine’s complete disappearance was achieved within 60 min with combined system, while up to 2 h is required with TiO2 only.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Removal of organic pollutants from urban and industrial wastewaters is one of the main topics in modern research, and different methods have been proposed to efficiently and inexpensively solve the problem. Indeed, several chemical–physical methods, such as coagulation, flocculation, adsorption, electrochemical, and advanced oxidation processes (AOP), were studied and commonly applied to remove water pollutants (Martínez-Huitle and Brillas 2009; Gupta and Suhas 2009; Khin et al. 2012; Kalsoom et al. 2012; Oturan and Aaron 2014; Mehrjouei et al. 2015). Moreover, in modern plants, these methods are usually coupled with biological treatments by means of active sludge which allow to obtain the almost complete transformation of the organic matter in CO2 (Saratale et al. 2011; Sarayu and Sandhya 2012; Ozgun et al. 2013). Sequential chemical–biological treatments are generally effective in the degradation of organic pollutants but, as recently reviewed by Guieysse and Norvill (2014), the whole process has to be evaluated and adapted to specific situations, in order to obtain the complete removal of pollutants.
In the last decades, new chemicals were detected in wastewaters as a result of new industrial processes and increased consumption of pharmaceuticals and personal care products (PPCPs) (Stuart et al. 2012; Santos et al. 2013; Richardson and Ternes 2014; Arpin-Pont et al. 2016). Some of these emerging contaminants are very stable in water and recalcitrant to degradation with the common treatments, so exercising for long periods an adverse effect on the environment. Therefore, new strategies are needed to assess the quality of water treatments, and new methods are required to unravel the problem of recalcitrant pollutants (Prasse et al. 2015).
In addition to chemical and biological methods (Chengalroyen and Dabbs 2013), the use of isolated enzymes has been proposed for the degradation of pollutants, in particular in the case of bleaching of azo dye wastes (Singh et al. 2015). Laccases and peroxidases, both from microorganism or plant, have been successfully employed in the oxidative decolorization of several dyes and other pollutants (Husain 2010; Strong and Claus 2011; Ramirez-Montoya et al. 2015; Kalsoom et al. 2015).
Enzymatic processes allow to exploit the potential of enzymes as biological catalyst avoiding the problems arising from the need to keep alive the microorganisms from which they derive. On the other hand, in the use of isolated enzymes, other problems arise, such as the need to prevent their inactivation, their selective activity, and their higher cost.
In order to enhance enzyme stability and reduce the operating costs, several methods of immobilization have been proposed and applied in many different conditions (Garcia-Galan et al. 2011; DiCosimo et al. 2013; Sheldon and Van Pelt 2013). However, immobilization methods generally induce conformational changes in the enzymatic structure that can result in a lower catalytic efficiency. In the case of peroxidases, the balance between the gain in stability and reuse and the loss of activity is generally positive and allowed to use these systems in different biotechnological applications (Longoria et al. 2010; Husain 2010).
Furthermore, the combination of different treatments, such as AOP and biological treatments, has been proven effective in complete removal of various type of pollutants (Oller et al. 2011). In a previous work, the synergistic effect of TiO2 and commercial soybean peroxidase (SBP) in the degradation of 2,4,6-trichlorophenol was observed (Calza et al. 2014). SBP is a monomeric Fe(III)-heme peroxidase (Henriksen et al. 2001) that catalyzes the oxidation of inorganic and organic substrates by hydrogen peroxide and has high resistance to thermic and chemical denaturation (Kamal and Behere 2002; Boscolo et al. 2006). These properties make SBP particularly suitable for industrial applications, and particularly useful in the degradation of dyes (Marchis et al. 2011; Kalsoom et al. 2013; Silva et al. 2013).
In this work, we extend the studies on the TiO2/SBP system to the degradation of three common orange dyes and a recalcitrant pollutant, carbamazepine, by means of SBP extracted by soybean hulls in our lab and immobilized on silica monoliths. Single pieces of porous silica were previously prepared with different techniques and used as support for enzymes to obtain the solid phase of separative columns (Magner 2013). For our purposes, monoliths were obtained by stable aggregation of silica particles in the presence of bio-based soluble organic substances extracted by composted urban waste and successively functionalized with SBP by using a previously reported method (Magnacca et al. 2012).
Materials and methods
Materials
HPLC grade water was obtained from MilliQ System Academic (Waters, Millipore). HPLC grade acetonitrile (BDH) was filtered through a 0.45-μm filter before use. Experiments were carried out using TiO2 P25 Evonik as the photocatalyst. Orange I (4-(4-hydroxy-1-naphthylazo)benzenesulfonic acid sodium salt), orange II (4-(2-hydroxy-1-naphthylazo)benzenesulfonic acid sodium salt), methylorange (4-(4-dimethylaminophenylazo)benzenesulfonic acid sodium salt), and carbamazepine were purchased by Aldrich.
SBP purification
Soybean peroxidase was extracted from hulls of fresh soybean (Glycine max) seeds and successively purified by means of the following procedure:
-
a.
One hundred grams of seed hulls was added to 400 mL of phosphate buffer 0.025 M at pH 7, left under stirring for 30 min at room temperature, and then separated from the solution by filtration with gauze. The treatment was repeated until the resulting solution gave a negative response to peroxidase activity test with the H2O2/DMAB-MBTH system (Ngo and Lenhoff 1980).
-
b.
The SBP containing solutions were combined and concentrated by a Vivaflow 50 (Sartorius, 30000 MWCO) tangential filter. The proteins were then precipitated by addition of ammonium sulfate until saturation (53 g/100 mL), and the mixture was left under stirring for one night at 4 °C.
-
c.
The precipitate was centrifuged for 20 min at 4000 rpm and dissolved in 250 mL of phosphate buffer 0.025 M pH 7. The resulting solution was then dialyzed for 24 h at 4 °C against the same buffer.
-
d.
The dialyzed fraction was loaded onto a column (4 cm × 20 cm) containing DEAE-Sepharose CL-6B (Sigma-Aldrich) ionic exchange resin, washed with three volumes of phosphate buffer 0.025 M pH 7, and eluted with a KCl gradient 0–0.5 M in the same buffer. The fractions were collected, analyzed by means of UV–visible spectroscopy, and selected on the basis of their RZ values (Reinheitszahl, RZ = Abs403nm/Abs280nm).
-
e.
The selected fractions were pooled and concentrated by ultrafiltration on Vivaspin 20 (Sartorius, 10000 MWCO) until their concentration was approximately 0.100 mM. The final SBP sample was then stored frozen at −12 °C until use.
Monolith synthesis and functionalization
Silica monoliths were synthesized and functionalized with SBP by means of a previously reported procedure (Magnacca et al. 2012).
A mixture of FK320 silica powder (Degussa) dispersed in deionized water and bio-based soluble organic substances obtained by composted urban vegetable residues (Montoneri et al. 2013) was first dried at room temperature for 24 h and then heated at 500 °C to yield spherical mesoporous monoliths with a 3–4-mm diameter.
The monolith surface was activated by reaction for 3 h at 80 °C with a 10 % solution of 3-aminopropyltriethoxysilane in water. After filtration in a Buchner funnel and washing with water, 1 g of activated monoliths was suspended in a glutaraldehyde 2.5 % (v/v) solution in phosphate buffer 0.1 M pH 7 and left under stirring for 1 h in the dark at room temperature. After the reaction, monoliths were washed with deionized water, added to 20 mL of SBP 0.010 mM in phosphate buffer at pH 7.5, and left to react at 4 °C for 20 h. The final product, obtained by filtration, was washed three times with deionized water and stored at 4 °C. The amount of immobilized enzyme was obtained spectrophotometrically as the difference between the initial amount of enzyme and that recovered in the washing liquids.
Irradiation procedures
The irradiations were performed by using a TL K05 UV/A lamp 25 mW/m2 centered at 365 nm, and the experiments were carried out in stirred Pyrex glass cells filled with 5 mL of sample for experiments in the presence of TiO2 suspension/SBP or 30 mL when using monoliths.
Orange dyes or carbamazepine were irradiated in the presence of: (1) SBP (1 × 10−8 M) and H2O2 (1 × 10−4 M); (2) dispersed TiO2 (100 mg L−1); and (3) dispersed TiO2 (100 mg L−1) and SBP (1 × 10−8 M). The temperature reached during the irradiation was 38 ± 2 °C. The entire content of each cell was filtered through a 0.45-μm filter and then analyzed by the appropriate technique.
Analytical techniques
The disappearance of analytes as a function of the irradiation time was followed using an HPLC system (Merck-Hitachi L-6200 pumps), equipped with a Rheodyne injector, a RP C18 column (Lichrochart, Merck, 12.5 cm × 0.4 cm, 5 μm packing), and a UV–Vis detector (Merck-Hitachi L-4200). Elution was carried out with acetonitrile and phosphate buffer (1 × 10−2 M) at pH 2.8 (40:60 % v/v) at a flow rate of 1 mL/min. The analytical detector wavelength was 220 nm.
Results and discussion
Soybean extraction and purification
Several methods are reported in the literature for the extraction and purification of soybean peroxidase from seed hulls (Gillikin and Graham 1991; Gacche et al. 2003; Bassi et al. 2004; Ghaemmaghami et al. 2010; Silva et al. 2013; Steevensz et al. 2013). We take inspiration from the literature to establish a fast and efficient purification procedure as reported in detail in the SBP purification section.
The protein was extracted from the seed hulls by several washing cycles with phosphate buffer 0.025 M at pH 7. After each wash, the supernatant was tested for peroxidase activity by the DMAB-MBTH method (Ngo and Lenhoff 1980) and the washing cycles continued until the resulting activity was negligible. At the end of the washings, the fractions were pooled, and the proteins precipitated with ammonium sulfate and then dialyzed against phosphate buffer. The final purification step involved an ion exchange chromatography by a 0–0.5 M KCl gradient. Figure 1 shows the results of the protein elution: a total of 21 mg of SBP with RZ >1.2 (see inset of Fig. 1) was obtained starting from 100 g of seed hulls, in agreement with literature data (Gillikin and Graham 1991). Although the RZ value is not as high as the commercial SBP, the purification process was limited to these steps to achieve a reasonable compromise between the enzyme purity and the total yield.
Soybean immobilization
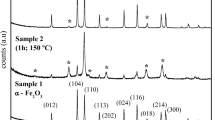
A portion of SBP was immobilized on silica monoliths (Fig. 2) obtained by aggregations of silica micro particles in the presence of bio-based soluble organic substances extracted by composted urban vegetable residues as templating agent. With this procedure, we were able to obtain stable monoliths with a surface area of about 120 m2/g (Magnacca et al. 2012), suitable for the functionalization with SBP. Furthermore, the presence of mesopores with a pore size distribution centered to 35 nM (Magnacca et al. 2012) allows to functionalize all the surface of the monoliths with the protein and to facilitate an high exchange rate of substrates and products between the solution and the inner part of the monoliths.
SBP was immobilized by means of the classic APTES/glutaraldehyde method initially proposed by Weetall (Weetall 1993) and successively applied to many systems at different scale (Hartmann and Kostrov 2013; Laurenti and dos Santos Vianna Jr. 2016). The method consists of the activation of the silica surface with aminopropyl groups and the subsequent coupling with the protein by formation of imine bonds in the presence of the bi-functional reagent glutaraldehyde. The final product is active and stable and contains about 11 mg of SBP/g of monoliths, in agreement with previous results (Magnacca et al. 2012).
SBP, both in solution and immobilized, was successively used to catalyze the degradation of four different pollutants: three common azo dyes (orange I and II, methylorange) and the anticonvulsant drug carbamazepine (Fig. 3).
Degradation of dyes
The degradation of three orange dyes was initially performed using SBP free in solution and spectrophotometrically followed by recording dye solution spectra at different reaction times. The typical dye absorption band rapidly decreases as arose from Fig. 4. The presence of isosbestic points could indicate the transformation of orange II in a single reaction product with the rupture of the azo bond, as already reported in the case of the horseradish peroxidase-catalyzed reaction (Zhang et al. 2013); the same trend can be observed for the degradation of methylorange and orange I.
The enzymatic oxidation was then coupled with a photocatalytic treatment in the presence of titanium dioxide with a double purpose: to obtain a better removal of parent molecules and to produce hydrogen peroxide through TiO2 irradiation, in a adequate amount to supply the enzymatic reaction (Calza et al. 2014).
Figure 5 collects the disappearance profiles followed via HPLC for the three orange dyes as a function of the reaction time. Figure 5 (top) shows the degradation profiles obtained with SBP free in solution. The combined TiO2/SBP system allows to achieve half-life times of few minutes for all dyes and to obtain their complete degradation within 90 min. The improvement achieved with the combined system is particularly marked for orange II degradation.
Figure 5 (bottom) reports the degradation of dyes in aqueous solution using monoliths containing the immobilized SBP. The adsorption in the dark on monoliths (without SBP) was preliminary assessed and, for all dyes, it was negligible in the investigated time window (2 h). When using immobilized SBP, all dyes are nearly completely abated within 150 min, with the exception of orange II, where almost 20 % persists. The employment of immobilized SBP only for the degradation of the three dyes led, as expected, to a decrease in the abatement efficiency, with a rate ratio lowered from two (for orange II) to four times (for orange I) as assessed by the k obs values in Table 1. Lastly, we test the performance of the system using both monoliths and titanium dioxide. Again, the use of a combined SBP/TiO2 system led to an increase in the degradation rate, in this case orange II was completely removed from the solution as well. The SBP immobilization led to a very slight decrease; in combined system, the constant rate ratio between SBP free and immobilized became close to 1.3 (for methylorange and orange II) and 2 (for orange I); and within 2 h of irradiation, all dyes are completely degraded.
Degradation of carbamazepine
We test the system on a more refractory molecule, namely carbamazepine, and the degradation profiles over time are shown in Fig. 6, while the calculated rate constant are collected in Table 1. The SBP action is limited to a 25 % degradation after 2 h of reaction when free in solution (Fig. 6, top), confirming the difficulties in the carbamazepine removal by enzymatic treatments (Pearce et al. 2002; Lu and Uetrecht 2008). The monoliths with immobilized SBP very slowly degrade carbamazepine, with a percentage of abatement below 10 % after 120 min (see Fig. 6, bottom).
However, the coupling of SBP with TiO2 revealed very promising. Although peroxidase alone is not effective in the degradation of carbamazepine, the use of SBP/TiO2 leads to a sharp increase and permits to achieve the complete degradation in 60 min with both SBP free and immobilized on monoliths.
Conclusions
As previously reported, the combined effect of photocatalysis in the presence of titanium dioxide and SBP enzymatic action, is particularly efficient in the removal of water pollutants such as 2,4,6-trichlorophenol (Calza et al. 2014). Furthermore, the continuous production of H2O2 by TiO2 irradiation endorses the enzymatic action without the external addition of hydrogen peroxide and avoids the problem of inhibition by substrate, typical of a discontinuous supply.
In this paper, we applied the same approach to the degradation of four different recalcitrant molecules. The synergistic effect of the TiO2/SBP system was confirmed; indeed, first-order kinetic constants are higher for all the substrates compared to the systems with TiO2 or SBP alone; and the complete degradation is obtained in shorter times for all substrates.
The same results were obtained by using free or immobilized SBP. In general, reactions catalyzed by immobilized SBP are slower than the corresponding degradation by SBP in solution, but the possibility of reusing these catalysts makes this system more favorable for applications to real wastewaters.
References
Arpin-Pont L, Bueno MJM, Gomez E, Fenet H (2016) Occurrence of PPCPs in the marine environment: a review. Environ Sci Pollut Res 23:4978–4991. doi:10.1007/s11356-014-3617-x
Bassi A, Geng Z, Gijzen M (2004) Enzymatic removal of phenol and chlorophenols using soybean seed hulls. Eng Life Sci 4:125–130. doi:10.1002/elsc.200420021
Boscolo B, Laurenti E, Ghibaudi E (2006) ESR spectroscopy investigation of the denaturation process of soybean peroxidase induced by guanidine hydrochloride, DMSO or heat. Protein J 25:379–390. doi:10.1007/s10930-006-9024-5
Calza P, Avetta P, Rubulotta G, Sangermano M, Laurenti E (2014) TiO2-soybean peroxidase composite materials as a new photocatalytic system. Chem Eng J 239:87–92. doi:10.1016/j.cej.2013.10.098
Chengalroyen MD, Dabbs ER (2013) The microbial degradation of azo dyes: minireview. World J Microbiol Biotechnol 29:389–399. doi:10.1007/s11274-012-1198-8
DiCosimo R, McAuliffe J, Poulose AJ, Bohlmann G (2013) Industrial use of immobilized enzymes. Chem Soc Rev 42:6437–6474. doi:10.1039/c3cs35506c
Gacche RN, Firdaus Q, Sagar AD (2003) Soybean (Glycine max L.) seed coat peroxidase immobilized on fibrous aromatic polyamide: a strategy for decreasing phenols from industrial wastewater. J Sci Ind Res (India) 62:1090–1093
Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904. doi:10.1002/adsc.201100534
Ghaemmaghami F, Alemzadeh I, Motamed S (2010) Seed coat soybean peroxidase: extraction and biocatalytic properties determination. Iran J Chem Eng 7:28–38
Gillikin JW, Graham JS (1991) Purification and developmental analysis of the major anionic peroxidase from the seed coat of Glycine max. Plant Physiol 96:214–220
Guieysse B, Norvill ZN (2014) Sequential chemical–biological processes for the treatment of industrial wastewaters: review of recent progresses and critical assessment. J Hazard Mater 267:142–152. doi:10.1016/j.jhazmat.2013.12.016
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90:2313–2342. doi:10.1016/j.jenvman.2008.11.017
Hartmann M, Kostrov X (2013) Immobilization of enzymes on porous silicas—benefits and challenges. Chem Soc Rev 42:6277–6289. doi:10.1039/c3cs60021a
Henriksen A, Mirza O, Indiani C, Teilum K, Smulevich G, Welinder KG, Gajhede M (2001) Structure of soybean seed coat peroxidase: a plant peroxidase with unusual stability and haem-apoprotein interactions. Protein Sci 10:108–115. doi:10.1110/ps.37301
Husain Q (2010) Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: a review. Rev Environ Sci Bio/Technology 9:117–140. doi:10.1007/s11157-009-9184-9
Kalsoom U, Ashraf SS, Meetani MA, Rauf MA, Bhatti HN (2012) Degradation and kinetics of H2O2 assisted photochemical oxidation of Remazol turquoise blue. Chem Eng J 200-202:373–379. doi:10.1016/j.cej.2012.06.058
Kalsoom U, Ashraf SS, Meetani MA, Rauf MA, Bhatti HN (2013) Mechanistic study of a diazo dye degradation by soybean peroxidase. Chem Cent J 7:93. doi:10.1186/1752-153X-7-93
Kalsoom U, Bhatti HN, Asgher M (2015) Characterization of plant peroxidases and their potential for degradation of dyes: a review. Appl Biochem Biotechnol 176:1529–1550. doi:10.1007/s12010-015-1674-3
Kamal JKA, Behere DV (2002) Thermal and conformational stability of seed coat soybean peroxidase. Biochemistry 41:9034–9042. doi:10.1021/bi025621e
Khin MM, Nair AS, Babu VJ, Murugan R, Ramakrishna S (2012) A review on nanomaterials for environmental remediation. Energy Environ Sci 5:8075–8109. doi:10.1039/c2ee21818f
Laurenti E, dos Santos Vianna Jr A (2016) Enzymatic microreactors in biocatalysis: history, features, and future perspectives. Biocatalysis 1:148–165. doi:10.1515/boca-2015-0008
Longoria A, Tinoco R, Torres E (2010) Enzyme technology of peroxidases: immobilization, chemical and genetic modification. In: Torres E, Ayala M (eds) Biocatalysis based on heme peroxidases. Springer, Berlin, Heidelberg, pp. 209–243. doi:10.1007/978-3-642-12627-7_9
Lu W, Uetrecht JP (2008) Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab Dispos 36:1624–1636. doi:10.1124/dmd.107.019554
Magnacca G, Laurenti E, Vigna E, Franzoso F, Tomasso L, Montoneri E, Boffa V (2012) Refuse derived bio-organics and immobilized soybean peroxidase for green chemical technology. Process Biochem 47:2025–2031. doi:10.1016/j.procbio.2012.07.021
Magner E (2013) Immobilisation of enzymes on mesoporous silicate materials. Chem Soc Rev 42:6213–6222. doi:10.1039/c2cs35450k
Marchis T, Avetta P, Bianco-Prevot A, Fabbri D, Viscardi G, Laurenti E (2011) Oxidative degradation of Remazol turquoise blue G 133 by soybean peroxidase. J Inorg Biochem 105:321–327. doi:10.1016/j.jinorgbio.2010.11.009
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87:105–145. doi:10.1016/j.apcatb.2008.09.017
Mehrjouei M, Müller S, Möller D (2015) A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem Eng J 263:209–219. doi:10.1016/j.cej.2014.10.112
Montoneri E, Bianco Prevot A, Avetta P, Arques A, Carlos L, Magnacca G, Laurenti E, Tabasso S (2013) Food wastes conversion to products for use in chemical and environmental technology, material science and agriculture. In: Kazmi A, Shuttleworth P (eds) The economic utilisation of food co-products. RSC Green Chemistry, pp 64–109
Ngo TT, Lenhoff HM (1980) A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem 105:389–397. doi:10.1016/0003-2697(80)90475-3
Oller I, Malato S, Sánchez-Pérez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166. doi:10.1016/j.scitotenv.2010.08.061
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641. doi:10.1080/10643389.2013.829765
Ozgun H, Dereli RK, Ersahin ME, Kinaci C, Spanjers H, van Lier JB (2013) A review of anaerobic membrane bioreactors for municipal wastewater treatment: integration options, limitations and expectations. Sep Purif Technol 118:89–104. doi:10.1016/j.seppur.2013.06.036
Pearce RE, Vakkalagadda GR, Steven Leeder J (2002) Pathways of carbamazepine bioactivation in vitro I. Characterization of human cytochromes P450 responsible for the formation of 2- and 3-hydroxylated metabolites. Drug Metab Dispos 30:1170–1179. doi:10.1124/dmd.30.11.1170
Prasse C, Stalter D, Schulte-Oehlmann U, Oehlmann J, Ternes TA (2015) Spoilt for choice: a critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Res 87:237–270. doi:10.1016/j.watres.2015.09.023
Ramirez-Montoya LA, Hernandez-Montoya V, Montes-Moran MA, Jauregui-Rincon J, Cervantes FJ (2015) Decolorization of dyes with different molecular properties using free and immobilized laccases from Trametes versicolor. J Mol Liq 212:30–37. doi:10.1016/j.molliq.2015.08.040
Richardson SD, Ternes TA (2014) Water analysis: emerging contaminants and current issues. Anal Chem 86:2813–2848. doi:10.1021/ac500508t
Santos LHMLM, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barcelò D, Montenegro MCBSM (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ 461-462:302–316. doi:10.1016/j.scitotenv.2013.04.077
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157. doi:10.1016/j.jtice.2010.06.006
Sarayu K, Sandhya S (2012) Current technologies for biological treatment of textile wastewater – a review. Appl Biochem Biotechnol 167:645–661. doi:10.1007/s12010-012-9716-6
Sheldon RA, Van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235. doi:10.1039/c3cs60075k
Silva MC, Torres JA, de Sa LRV, Chagas PMB, Ferreira-Leitao VS, Correa AD (2013) The use of soybean peroxidase in the decolourization of Remazol brilliant blue R and toxicological evaluation of its degradation products. J Mol Catal B-Enzymatic 89:122–129. doi:10.1016/j.molcatb.2013.01.004
Singh RL, Singh PK, Singh RP (2015) Enzymatic decolorization and degradation of azo dyes—a review. Int Biodeterior Biodegradation 104:21–31. doi:10.1016/j.ibiod.2015.04.027
Steevensz A, Madur S, Al-Ansari MM, Taylor KE, Bewtra JK, Biswas N (2013) A simple lab-scale extraction of soybean hull peroxidase shows wide variation among cultivars. Ind Crop Prod 48:13–18. doi:10.1016/j.indcrop.2013.03.030
Strong PJ, Claus H (2011) Laccase: a review of its past and its future in bioremediation. Crit Rev Environ Sci Technol 41:373–434. doi:10.1080/10643380902945706
Stuart M, Lapworth D, Crane E, Hart A (2012) Review of risk from potential emerging contaminants in UK groundwater. Sci Total Environ 416:1–21. doi:10.1016/j.scitotenv.2011.11.072
Weetall HH (1993) Preparation of immobilized proteins covalently coupled through silane coupling agents to inorganic supports. Appl Biochem Biotechnol 41:157–188. doi:10.1007/BF02916421
Zhang A, Fang L, Wang J, Liu W (2013) Enzymatic decolorization of Orange II: optimization by response surface methodology and pathway. Environ Prog Sustain Energy 32:294–301. doi:10.1002/ep.11628
Acknowledgments
The authors would like to thank Prof. D. Sacco (Department of Agricultural, Forest and Food Sciences, University of Turin) for the kind supply of fresh soybean seeds.
The Marie Sklodowska-Curie Research and Innovation Staff Exchange project funded by the European Commission H2020-MSCA-RISE-2014 within the framework of the research project Mat4treaT (project number 645551) is acknowledged. Compagnia di San Paolo and University of Torino are gratefully acknowledged for funding Project Torino_call2014_L2_126 through “Bando per il finanziamento di progetti di ricerca di Ateneo – anno 2014” (Project acronym: Microbusters).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Calza, P., Zacchigna, D. & Laurenti, E. Degradation of orange dyes and carbamazepine by soybean peroxidase immobilized on silica monoliths and titanium dioxide. Environ Sci Pollut Res 23, 23742–23749 (2016). https://doi.org/10.1007/s11356-016-7399-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7399-1