Abstract
Flubendiamide insecticide is widely used in Indian subtropical condition to control lepidopteron pests mainly in rice and cotton. The present study reports leaching behaviour of flubendiamide, N 2-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-3-iodo-N 1-[2-methyl-4-[1,2,2,2-tetrafluoro-1 (trifluoromethyl)ethyl] phenyl]-1,2-benzene dicarboxamide, in packed soil columns under different rainfall conditions. Flubendiamide did not leach out of the 25 cm long soil columns even after percolating water equivalent to 462.18 mm rainfall. After leaching with water equivalent to 462.18 mm rainfall, in analytical grade treatment, 68.06% of the recovered flubendiamide was the major amount present in 5–10 cm depth whereas in the formulation 67.22% of the recovered flubendiamide was confined to 0–5 cm depth. Results revealed that with percolating 160 mL of water residues of desiodo flubendiamide detected up to 20–25 cm layer along with 9.47% residues in this layer, indicating that metabolite is more mobile as compared to analytical grade flubendiamide and 39.35% SC formulation. Formulation slowed the downward mobility of flubendiamide in soil column. Flubendiamide is slightly mobile in sandy loam soil, but desiodo flubendiamide is relatively more mobile and may leach into ground water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
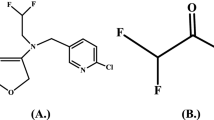
Man-made chemicals may reach soil directly via deliberate application (e.g. agrochemicals) or via indirect routes (e.g. via waste water → sewage sludge → soil or air → wet/dry deposition). For risk assessment of these chemicals, it is important to estimate their potential for transformation in soil and for movement (leaching) into deeper soil layers and eventually into groundwater. Flubendiamide, N 2-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-3-iodo-N 1-[2-methyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]-1,2-benzene dicarboxamide, belongs to phthalic acid diamide group. The structure of flubendiamide and desiodo-flubendiamide are presented in Fig. 1. Flubendiamide is mainly effective for controlling lepidopteron pests including resistant strains in rice, cotton, corn, grapes, other fruits and vegetables (Masaki et al. 2006). Flubendiamide activates ryanodine sensitive intracellular calcium release channels in insects (Tohnishi et al. 2005). It has a novel biochemical action as it affects calcium ion balance irrespective of sodium or potassium ion balance which causes contraction of insect skeletal muscle. Information on flubendiamide leaching behaviour is mainly restricted to the registration/regulatory documents mentioning that flubendiamide satisfies most of the leaching criteria of Cohen et al. (1984). The ground water ubiquity score (GUS) of flubendiamide is 0.59, indicate that it has very low potential to leach down to ground water (Gustafson 2002). Des-iodo flubendiamide (KFOC = 234–581 L/kg), a major photoproduct of flubendiamide, is more soluble in water than flubendiamide and expected to be moderately mobile (Pesticide Fact Sheet 2008).
Sandy soils with low organic matter content are most vulnerable to leaching by mass flow while well structured clay soils were most vulnerable to leaching by preferential/by-mass flow. Recent work highlighted the importance of preferential flow for the movement of sorbed chemicals through soil. Recently, flubendiamide has been registered in India for rice and cotton cultivation. Absolutely no information is available on leaching behaviour of flubendiamide or its metabolites in soils under Indian subtropical conditions. Therefore, the present investigation was designed to obtain comparative information on the mobility behaviour of flubendiamide, its formulation (39.35% SC) and metabolite desiodo-flubendiamide in packed soil columns under laboratory condition in a sandy loam soil from Northern India (subtropical condition) under different rainfall conditions by adding water which enabled the leaching behaviour of both to be studied simultaneously. The results of this study will contribute to a better understanding of the potential of this insecticide to contaminate ground water and assist regulatory agencies by reducing uncertainty when assessing their ecological risk.
Materials and Methods
Soil required for the study was collected from the plough layer (0–15 cm soil profile) of the research farm of Indian Agricultural Research Institute, New Delhi, India, with no history of pesticide application. The soil was spread on aluminium sheet and moisture was allowed to evaporate under natural room conditions for 4–5 days for air-drying. It was then ground, sieved through a 2-mm mesh screen and stored in plastic containers. This soil was later used for leaching studies. The physicochemical characteristics of the soil, determined by standard methods, were pH 8.18 measured at 1:2.5 soil: water ratio using Control Dynamics pH meter (Model APX 175 E/C) fitted with calomel glass electrode assembly; organic carbon 0.16 by the Walkley and Black methods (1965); mechanical fraction (%) of sand 72.12, silt 19.55 and clay 8.33, employing the Bouyoucos hygrometer method (Jackson 1967). Flubendiamide (99.5% purity), metabolite des-iodo flubendiamide (99.2% purity) and 39.35% SC formulation were obtained from M/S Bayer Crop Science Limited (New Delhi, India).The stock solution of flubendiamide and its metabolite of 1,000 mg L−1 was prepared in acetonitrile and stored at 4°C, working standards were prepared by appropriate dilutions with acetonitrile. Organic solvents like hexane, acetone, dichloromethane and methanol were glass distilled before use. Sodium sulfate was washed with acetone and then activated at 110°C for 4 h before use. HPLC grade acetonitrile was procured from Merck India Ltd. Acetonitrile was filtered and de-gassed prior to use.
Leaching studies were conducted with analytical grade flubendiamide, its 39.35% SC formulation and its metabolite des-iodo flubendiamide in packed soil columns under continuous flow conditions following different leaching (rainfall) conditions in duplicate. Initially soil (2 g) was fortified at 20 μg level with analytical grade flubendiamide, metabolite desiodo flubendiamide and its formulation separately. Fortified soil was mixed with glass rod to allow complete evaporation of solvent. The experiments used columns (50 cm length × 2.1 cm ID) were constructed from polyvinyl chloride (PVC) pipes which were first cut longitudinally into two halves and then rejoined using adhesive tape to allow easy separation of the column after completion of the leaching cycle. The lower end of the column was capped with perforated polyethylene sheet and packed by adding ~220 g soil so that soil was filled up to a height of 25 cm and compacted the soil with equal force so as to give the columns of uniform packing. After packing, 1 day before flubendiamide application, the soil columns were pre-wetted with artificial rain (0.01 M CaCl2) from bottom to top in order to displace the air in the soil pores by water. Thereafter the soil columns were allowed to equilibrate and the excess water was drained off by gravity. The soil (2 g) spiked with flubendiamide (20 μg) was spread uniformly at the top surface for proper distribution of flubendiamide and leaching was started. Water (0.01 M CaCl2 as artificial rain) was allowed to leach down under natural flow conditions (0.01 mL min−1). This application dose was higher than the recommended dose (48 g a.i. ha−1) for flubendiamide. The columns were leached with 20, 40, 80 and 160 mL of water (0.01 M CaCl2 as artificial rain) simulating 57.77, 115.55, 231.09 and 462.18 mm rainfall to compare the leaching behaviour of analytical grade flubendiamide, metabolite des-iodo flubendiamide and 39.35% SC formulation.
After leaching, columns were left for 24 h for drainage and then dissected into 5 cm sections. Experiments were carried out in duplicate and the average soil weight in each 5 cm soil column section was ~44 g. Soil was allowed to air dry for 72 h. Soil samples were transferred to 250 mL stoppered conical flasks, and 100 mL acetone and 10 g anhydrous sodium sulfate were added to each flask. The samples were equilibrated on a rotary shaker for 30 min. The acetone fraction was transferred to a 250 mL beaker, and the soil was extracted again in similar manner. A total of three extractions were performed, the acetone fractions were pooled and evaporated to dryness at room temperature and the residue was redissolved in 5 mL acetonitrile. Residues of flubendiamide in water sample were extracted using dichloromethane. The leachate fractions from each column were collected and filtered. The collected sample in a 1,000 mL separating funnel was extracted with 30 mL of dichloromethane after dilution with 100 mL of saturated sodium chloride solution (10%). The dichloromethane fraction was transferred to a 250 mL beaker, and the water fraction was extracted again in a similar manner. A total of three extractions were performed, and the dichloromethane fractions were pooled, dried over anhydrous sodium sulfate and evaporated to dryness at room temperature. The dichloromethane layers were combined, dried over anhydrous sodium sulfate and concentrated. Flubendiamide residues were redissolved in 5 mL of acetonitrile and quantified by HPLC. Residues of flubendiamide were estimated by HPLC system (Merck-Hitachi)—Consisting of a L-7100 (computer operated dual pump), a L-7400 (UV detector) and a L-7200 (Auto sampler), HPLC column (30 cm)—Lichrospher, RP-18 (5 μm). A mixture of acetonitrile—water (70: 30, v/v) was used as the mobile phase, with a flow rate of 0.5 mL min−1. The injection volume was 10 μL and the wavelength was set at 210 nm (λmax, determined by using spectrophotometer. Flubendiamide eluted at 10.1 min under these conditions. A concentration versus peak area obtained on analyzing standard solutions was plotted for the determination of flubendiamide residues present in test samples. The instrument Limit of Detection (LOD) of flubendiamide and its metabolite were found to be 0.05 and 0.01 μg mL−1, respectively. A representative HPLC chromatogram showing well resolved peak of the flubendiamide and its metabolite in leachate and wet soil is presented in Fig. 2.
Results and Discussion
On leaching the columns with water equivalent to 462.18 mm rainfall, residues of flubendiamide and its formulation were found to be distributed throughout the column. Under these conditions, flubendiamide did not leach out of the 25 cm long soil column and was not detected in the leachate fraction. Average recovery, out of 20 μg added in each soil column were 63.90% (12.78 μg) from 39.35% SC formulation and 80.93% (16.18 μg) from analytical grade flubendiamide (Table 1). Low recovery in case of 39.95% SC formulation could be due to strongly sorption to the soil in column. Percentage distribution of analytical grade flubendiamide in different soil cores (0–5, 5–10, 10–15, 15–20 and 20–25 cm) were 19.51, 68.06, 9.14, 1.95 and 0, respectively and in 39.35% SC formulation were 67.22, 28.62, 2.31, 1.85 and 0, respectively.
Comparison of the mobility behavior of flubendiamide and its formulations also revealed that in analytical grade treatment major amount of 68.06% of the recovered was present in 5-10 cm soil core whereas in 39.35% SC formulation major amount of 67.22% of the recovered was confined to 0–5 cm soil core. The results (Table 1) indicated that analytical grade flubendiamide is more mobile than its formulation in sandy loam soil.
Leaching behaviour of metabolite desiodo-flubendiamide was studied and compared with analytical grade flubendiamide in packed columns under differential leaching (rainfall) conditions. Table 2 represents downward movement and recovery of flubendiamide in packed soil columns after percolating 20, 40, 80 and 160 mL water equivalent to 57.77, 115.55, 231.09 and 462.18 mm rainfall. The results revealed that flubendiamide was fairly immobile in sandy loam soils. When leached with 20 mL of water all the residues were found to be confined within 0–5 cm of soil column. With 40–80 mL of water the residues were detected up to 10–15 cm depth but more than 85% of the recovered flubendiamide was confined in the 0–5 cm layer. Leaching with 160 mL water transferred the major fraction (~68%) to the 5–10 cm core and the residues were detected up to 20 cm soil depth.
Similarly, columns treated with desiodo-flubendiamide were leached with 20, 40, 80 and 160 mL of water equivalent to 57.77, 115.55, 231.09 and 462.18 mm rainfall. However, under different rainfall conditions, des-iodo flubendiamide did not leach out of the column and was not detected in the leachate fraction. In general, analysis of soil cores for the different treatments revealed that as the volume of water added increased movement increased slowly and residues moved to lower depth. The results indicated that des-iodo flubendiamide was more mobile than flubendiamide. The results of downward movement and recovery from packed columns obtained for desiodo-flubendiamide are shown in Table 3. When leached with 20 mL of water all the residues of des-iodo flubendiamide were found to be confined within 0–5 cm of soil column. With 40–80 mL of water the residues were detected up to 10–15 cm depth but more than 90% of the recovered flubendiamide was confined in the 0–5 cm layer. Total amount of des–iodo flubendiamide recovered from soil cores varied from 80 to 85%. Results revealed that with 160 mL of water residues of desiodo flubendiamide got distributed and was detected in the 20–25 cm layer along with ~14.8% of residues in the 0–5 cm layer, indicating that the metabolite is more mobile as compared to flubendiamide. Higher mobility of des-iodo flubendiamide could be due to their lower sorption in soil and more solubility in water as compared to flubendiamide.
The study indicated that formulations slowed down the downward mobility of flubendiamide in packed soil columns. Flubendiamide is practically immobile or slightly mobile in sandy loam soil under subtropical condition of India. However, des-iodo flubendiamide is more mobile than parent molecule. Based on the present study it may be conclude that des-iodo flubendiamide may pose a threat to contaminate lower soil profiles and groundwater resources under high rainfall condition in sandy loam soil. Flubendiamide (39.35% SC formulation) is registered in India for rice and cotton cultivation for controlling lepidopteron pests. Owing to the long period cultivation of rice and cotton in field, there is a possibility of photodegradation under field condition where flubendiamide may convert into des-iodo flubendiamide which is an important photo product of flubendiamide obtained by removal one iodine molecule (Pest Management Regulatory Agency, 2008). Thus, there is a possibility that des-iodo flubendiamide may move down to lower soil profile having highly porus sandy soil under high rainfall conditions and may contaminate to ground water which will cause a threat to ground water.
References
Black CA (1965) Method of soil analysis (parts 1 and 2). American Society of Agronomy, Madison
Cohen SZ, Creeger SM, Carsel RF, Enfield CG (1984) Potential pesticide contamination of groundwater from agricultural uses, in treatment and disposal of pesticide wastes. ACS Symposium Series No.259, ed. American Chemical Society, Washington, DC, pp 297–325
Gustafson DI (2002) Groundwater ubiquity score: a simple method for assessing pesticide leachability. Environ Toxicol Chem 8:339–357
Jackson ML (1967) Soil chemical analysis. Prentice Hall, New Delhi
Masaki T, Yasokawa N, Tohnishi M, Motoba K, Hirooka T (2006) Flubendiamide: a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mole Pharmacol 69:1733–1739
Pesticide Fact Sheet on Flubendiamide (2008) Us Environmental Protection Agency. (http://www.epa.gov/opprd001/factsheet/flubendiamide.pdf)
Regulatory Note RG 2008-03 (2008) Publication coordinator, Pest Management Regulatory Agency, Health Canada, Ottawa, Canada (200). (http://www.hc-sc.gc.ca/pmra-arla)
Tohnishi M, Nakao H, Furuya T, Seo A, Kodama H, Tsubata K, Fujioka S, Kodama H, Hirooka T, Nishimatsu T (2005) Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J Pestic Sci 30:354–360
Acknowledgment
The first author is thankful to Indian Council of Agricultural Research, New Delhi for financial assistance. Contribution No. 1035, Division of Agricultural Chemicals, IARI, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, S.K., Mukherjee, I. Flubendiamide Transport Through Packed Soil Columns. Bull Environ Contam Toxicol 88, 229–233 (2012). https://doi.org/10.1007/s00128-011-0429-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0429-2