Abstract
Arbuscular mycorrhizal (AM) fungi and nitrogen-fixing bacteria play important roles in plant growth and recovery in degraded ecosystems. The desertification in karst regions has become more severe in recent decades. Evaluation of the fungal and bacterial diversity of such regions during vegetation restoration is required for effective protection and restoration in these regions. Therefore, we analyzed relationships among AM fungi and nitrogen-fixing bacteria abundances, plant species diversity, and soil properties in four typical ecosystems of vegetation restoration (tussock (TK), shrub (SB), secondary forest (SF), and primary forest (PF)) in a karst region of southwest China. Abundance of AM fungi and nitrogen-fixing bacteria, plant species diversity, and soil nutrient levels increased from the tussock to the primary forest. The AM fungus, nitrogen-fixing bacterium, and plant community composition differed significantly between vegetation types (p < 0.05). Plant richness and pH were linked to the community composition of fungi and nitrogen-fixing bacteria, respectively. Available phosphorus, total nitrogen, and soil organic carbon levels and plant richness were positively correlated with the abundance of AM fungi and nitrogen-fixing bacteria (p < 0.05). The results suggested that abundance of AM fungi and nitrogen-fixing bacteria increased from the tussock to the primary forest and highlight the essentiality of these communities for vegetation restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal (AM) fungi form a symbiotic relationship with most terrestrial plant species (Smith and Read 2008). These fungi are obligate symbionts and are entirely dependent on a supply of carbohydrates from their host plants. In exchange for plant-derived carbon, AM fungi improve the supply of soil nutrients to plants and increase the tolerance of plants to nutrient-poor soil (Johnson et al. 2010). These fungi can also strengthen ecosystem’s sustainability by improving soil stability and aggregation (Wilson et al. 2009). Thus, AM fungi comprise an important part of plant growth in and restoration in degraded ecosystems.
Rhizobia play an important role in biological nitrogen fixation during nitrogen cycling in natural ecosystems. Most (~80 %) of the biological nitrogen fixation is performed by rhizobia in symbiosis with legumes (Peoples et al. 1995). However, free-living nitrogen-fixing bacteria (e.g., Pseudomonas, Azospirillum, and Azotobacter) inhabiting soils can fix significant amounts of nitrogen (Burgmann et al. 2004; Kahindi et al. 1997). This may be particularly crucially important within sustainable low-input natural systems that rely on biological processes rather than agrochemicals to maintain soil fertility and plant growth.
Karst landscapes are distributed worldwide, and cover approximately 12 % of the earth’s land surface (Yuan 2002). In China, the southwest karst region covers 550,000 km2, approximately one third of the total area of China (Yuan 1994). The karst region is characterized by a high ratio of bedrock outcrop to shallow soil and by low vegetation cover. These special topographical characteristics, together with anthropogenic activities (deforestation, over-cropping, and overgrazing), pose a major threat to the sustainability of karst ecosystems (Wang et al. 2004). Over the past few decades, these ecosystems have come under severe pressure because of land degradation and desertification. Disturbance of natural plant communities is the first visible symptom of desertification, but is often accompanied or preceded by loss of key physicochemical and biological soil properties (i.e., loss of microbial activity and communities; Fichtner et al. 2014). The loss of these properties limits the reestablishment of plants due to their largely determine the soil quality and fertility (Smith and Read 2008). Therefore, desertification reduces diversity of functional microbes, a key ecological factor governing the cycles of major plant nutrients, and limits vegetation restoration. AM fungi and nitrogen-fixing bacteria are important functional microbes for sustaining soil quality (Smith and Read 2008). They promote plant establishment and growth by increasing the supply of nutrients to the plant, particularly phosphorus and nitrogen. Reduction in the potential to form these functional microbes therefore hinders vegetation restoration (Jasper et al. 1989). Many previous studies reported that AM fungi and nitrogen-fixing bacteria had close relationship due to their complementary roles in meeting plant nutritional needs based on the pot experiment (Bauer et al. 2012; Mortimer et al. 2013). However, few field studies were conducted on the relationship between these two microorganisms. Thus, more attention should be paid to changes in AM fungi and nitrogen-fixing bacterial communities along vegetation restoration in natural ecosystems.
A previous study by our team indicates that soil fertility improves from naturally revegetated land to primary forests in the karst region of southwest China (Chen et al. 2012). Strong interaction exist between plant diversity and bacterial and fungal diversity during restoration of karst vegetation (He et al. 2008). Therefore, we hypothesized that the abundance of AM fungi and nitrogen-fixing bacteria increased from the tussock to the primary forest. Our objectives were as follows: (1) to investigate the changes of the AM fungus and nitrogen-fixing bacterial communities from the tussock to the primary forest and (2) to investigate the relationships among abundance of AM fungi and nitrogen-fixing bacteria, plant species communities, and soil properties.
Materials and methods
Study area
The study site is located in Huanjiang County, in the Guangxi autonomous region of southwest China (107° 51′ to 108° 43’ E, 24° 44′ to 25° 33′ N). This region is dominated by a subtropical mountainous monsoon climate, with a mean annual rainfall of 1389 mm and a mean annual air temperature of 18.5 °C. The wet season, during which 70 % of the annual precipitation occurs, lasts from April to August (He et al. 2008).
Three typical types of vegetation restoration, namely, tussock, shrub, and secondary forest, in Tongjin Village, Huanjiang County, Guangxi Autonomous Region, Southwest China, were selected. The space-for-time substitution method was used for studying natural vegetation restoration. In order to make the comparability between ecosystems, ecosystems across which anthropogenic disturbance and the background of land use history before vegetation recovery were consistent were selected. The selected tussock, shrub, and secondary forest were autogenically recovered from farmlands that were abandoned 8, 30, and 50 years, respectively. Moreover, the selected sites had only experienced minimal anthropogenic disturbance because they were far away from residential areas. A neighboring primary forest in the Mulun National Nature Reserve (25° 07′ N, 108° 00′ E), which had grown over 200 years, was selected as a reference vegetation type. In total, 12 plots (i.e., three plots × four vegetation types = 12 plots) were established, and the size of each plot was 20 × 30 m2. The plots of four vegetation types were set up as close to each other as possible to reduce the effect of geographical distance on AM fungal and nitrogen-fixing bacterial diversity, and those microbes were well represented in the experiment. In addition, all the plots were located in the mid-slope position and the soil type was dolomite.
Plant survey and soil sampling
All soil samplings and plant survey were conducted in 2012 in June when plant biomass peaks in this region. Each plot (20 m × 30 m) was divided into six subplots (10 m × 10 m). In the secondary forest and primary forest, each subplot was divided into three layers (arboreal, shrubby, and herbaceous), whereas the shrub subplots were divided into two layers (shrubby and herbaceous). For the arboreal layers, all woody stems with diameter at breast height (DBH) ≥ 2.5 cm were tallied, identified, and measured to the nearest 0.1 cm. For the shrubby (stem diameter < 2.5 cm) and herbaceous (herbaceous climbing plants and ferns) layers, the fascicles and height of each plant species were tallied. All plant species were identified, and the cover, height, and density of each species were measured.
From each plot, 15 soil cores (5 cm diameter, 0–15 cm depth) were collected and then mixed to form one composite soil sample. This sampling strategy ensured that samples were representative of each sampling site. Twelve soil samples were collected from the four vegetation types (i.e., three plots × four vegetation types = 12 soil samples). Each soil sample was divided into two subsamples. One subsample (approximately 50 g) was immediately frozen in liquid nitrogen and transported to the laboratory for molecular analysis. Another subsample was air-dried for analysis of soil physicochemical properties.
DNA extraction from soil
Microbial DNA was extracted, in triplicate, from 500 mg of freeze-dried soil using the sodium dodecyl sulfate-guanidine isothiocyanate-polyethylene glycol (SDS-GITC-PEG) method described by Chen et al. (2012). The DNA extracted was dissolved in 50 μL water, quantified by spectrophotometry, and stored at −20 °C until further use.
Polymerase chain reaction amplification and terminal restriction fragment length polymorphism analyses
The composition of the AM fungal and nitrogen-fixing bacterial communities was estimated by terminal restriction fragment length polymorphism (T-RFLP) analysis. The extracted DNA was subjected to nested polymerase chain reaction (PCR) with the primer sets Geo11F/GeoA2R (first-round primers) and NS31/AM1 (second-round primers) (Table 1) for amplification of an 18S ribosomal RNA (rRNA) gene fragment of AM fungi. The forward primer of second-round primers was labeled at the 5′ end with 6-carboxy-fluorescein (FAM; Invitrogen, China). The reaction mixture for the first round of PCR (50-μL volume) contained 25 μL 2× PCR Premix (0.1 U Prime STAR HS DNA polymerase, 0.5 mM dNTPs; Tiangen, China), 10 pM each primer, 20 ng genomic DNA, and 19 μL H2O. The cycling parameters were as follows: 95 °C for 2 min; 35 cycles each of 94 °C for 60 s, 60 °C for 60 s, and 72 °C for 60 s; and 72 °C for 10 min. The first amplification product was diluted with double-distilled water (1:10) and a 1-μL aliquot of the diluted product was used as a template for the second round of PCR amplification under the same conditions, except that 30, instead of 35, PCR cycles were performed and the annealing temperature was 64 °C, instead of 60 °C.
The PolF/PolR primer set was used to amplify the nitrogen-fixing bacterial nifH gene (Table 1). The reaction was performed in a 50-μL volume with 25 μL 2 × PCR Premix (0.1 U Prime STAR HS DNA polymerase, 0.5 mM dNTPs), 10 pM each primer, 20 ng genomic DNA, and 19 μL H2O. The cycling parameters were as follows: 95 °C for 2 min; 35 cycles each of 94 °C for 30 s, 55 °C for 60 s, and 72 °C for 60 s; and a final 10-min extension at 72 °C.
The labeled PCR product was purified using the QIAquick PCR purification kit (Tiangen Biotech Ltd., China), and quantified using NanoDrop ND-1000 (Thermo Scientific, USA). The fluorescent labeled products (approximately 200 ng) for the fungal 18S rRNA gene and the bacterial nifH gene were digested with HinfI and HaeIII enzymes, respectively. The digestion products were then analyzed using an automated sequencer (model 373A; Applied Biosystems, Weiterstadt, Germany) by Sunny Company (Shanghai, China).
Data comprising T-RFLP profiles were processed using the Gene Scan analysis software package (version 2.1; Applied Biosystems). Peak areas of terminal restriction fragments (T-RFs) that differed by ±2 bp were summed and considered as one fragment. The relative abundance (RA) of each T-RF was calculated as described by Lukow et al. (2000) with the following formula: RA = (ni/N) × 100, where ni represents the peak area of one distinct T-RF and N is the sum of all peak areas in one sample. Minor peaks, where the relative abundance was ˂1 %, were regarded as background noise (Lukow et al. 2000) and were not used in the statistical analysis. Peaks with RA >5 % were regarded as dominant T-RFs.
As Aldrich-Wolfe (2007) and Lekberg et al. (2007) described, using database T-RFLP identified AM fungal and nitrogen-fixing bacterial species: (i) T-RFLP profiles of AM fungi were determined for our 454 pyrosequencing sequences submitted to the MG-RAST public database (http://metagenomics.anl.gov/) under ID 4540338.3. (ii) T-RFLP profiles of nitrogen-fixing bacteria were determined for 40 nifH sequences in the same region, which have been published in GenBank under accession numbers KF859859 to KF859898.
Quantitative analysis of the 18S rRNA and nifH genes
The abundance of the 18S rRNA gene of AM fungi and the nifH gene of nitrogen-fixing bacteria was determined by real-time quantitative PCR (qPCR; ABI 7900, Foster City, CA) with the primer sets AMV4.5NF/AMDGR and PolF/PolR, respectively. Although AMV4.5NF/AMDGR can amplify non-AM fungal sequences, we had previously found that most sequences obtained from 454 pyrosequencing with these primers belonged to AM fungi in our study region.
The qPCR assay was carried out in a volume of 10 μL containing 5 μL 1× SYBR Premix, 0.2 μM each primer (Invitrogen, China), ExTaq, 0.2 μL Rox (Takara Bio, Shiga, Japan), 1 μL DNA template (the DNA had been diluted to 5 ng DNA μL−1 using sterile water), and 3.4 μL sterilized water. The cycling conditions for the fungal gene were as follows: 20 s at 95 °C; 30 cycles each of 95 °C for 10 s, 62 °C for 15 s, and 72 °C for 15 s. The cycling conditions for the nifH gene was as follows: 20 s at 95 °C; 5 cycles each of 15 s at 95 °C, 20 s at 64 °C, and 15 s at 72 °C; and 35 cycles each of 15 s at 95 °C, 25 s at 60 °C, and 15 s at 72 °C. The qPCR was performed using an ABI Prism 9700 Real-Time PCR System (PerkinElmer, Applied Biosystems, USA). Four technical replicates were used for each sample.
A standard curve ranging from 102 to 108 fungal or bacterial copies per microliter was generated using 10-fold serial dilutions of a plasmid (1010 copies μl−1). The plasmid contained a partial fragment of fungal 18S rRNA gene from Glomus sp. M20 (EU332717) and bacterial nifH gene from Bradyrhizobium sp. ISA1601 (KF859886), respectively. The reactions for standard curve samples, negative controls without template DNA, and soil DNA samples were performed in a single 384-well plate. The efficiency of the reaction was 98 and 103 % for 18S rRNA and the nifH gene, respectively. The R 2 value for the two standard curves was 0.99. One sharp peak was observed for the two standard curves. Data analysis was performed automatically using the SDS 2.3 software included with the real-time PCR system.
Soil physicochemical properties
Total nitrogen (TN), available phosphorus (P), soil organic carbon (SOC), and pH were measured. Soil pH was determined with a soil/water ratio of 1:2.5 (w/v) using a pH meter (Delta 320; Mettler-Toledo Instruments Ltd., China). Available P was extracted with 0.5 M sodium bicarbonate and analyzed using the Mo–Sb colorimetric method Colwell (1963). SOC was measured using K2Cr2O7–H2SO4 oxidation–reduction titration, and TN was determined by the Kjeldahl method (Bremner, 1965).
Statistical analyses
Statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL). Differences at p < 0.05 were considered statistically significant based on the least significant difference (LSD) test. Data that were not normally distributed were log(x+1)-transformed. Pearson correlation analysis was used to assess the relationship between microbial abundance and environmental factors. Bray-Curtis distance matrices were used to calculate the similarity in the plant communities between any two ecosystems by using the R software (Version 2.11.1). Redundancy analysis (RDA) was applied to visualize the effects of soil physicochemical characteristics and plant diversity on microbial community composition and was conducted with CANOCO 4.5 (Microcomputer Power, Inc., Ithaca, NY). Before the RDA, detrended correspondence analysis (DCA; gradient length < 3) was performed to confirm that the linear ordination method was appropriate for analyzing the T-RFLP data. Monte Carlo permutation tests were also used to compute statistical significance.
Results
Plant diversity and soil properties
Bray-Curtis cluster analysis indicated that the plant community compositions clearly differed between the four vegetation types (Fig. 3a). The dominant species in the tussock were Miscanthus floridulus, Humata henryana, and Microstegium nodosum. The dominant species in the shrubby layer of the shrub were Loropetalum chinense, Pittosporum tonkinense, and Vitex negundo. The dominant species in the tree layer of the secondary forest were Cyclobalanopsis glauca, Beilschmiedia fordii, and Litsea coreana. The dominant species in the tree layer of the primary forest were Cleidion bracteosum, Cryptocarya chingii, and Miliusa chunii (Table 2). The plant richness increased from the tussock and shrub to the forest (secondary and primary forests; Table 2).
The soil organic carbon, available phosphorus, and total nitrogen levels increased from the tussock to the primary forest (Table 2). Soil pH was highest in the shrub and lowest in the tussock (Table 2). The clay content was lower and the silt and sand content was higher in the primary forest than in the other three vegetation types (Table 2).
Abundance of AM fungi and nitrogen-fixing bacteria
The abundance of AM fungi and nitrogen-fixing bacteria (number of copies) in four vegetation types ranged from 3.33E + 6 to 6.55 E + 6 copies per gram dry soil and 6.29E + 5 to 8.00 E + 6 copies per gram dry soil, respectively (Fig. 1). These microbial abundances were significantly different among vegetation types (p < 0.05) and increased from the tussock to the primary forest.
Composition and structure of AM fungal and nitrogen-fixing bacterial communities
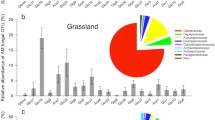
A total of seven T-RFs in the T-RFLP profiles were used to analyze the AM fungal community composition and structure in four vegetation types (Fig. 2a); four, six, four and four T-RFs were found for AM fungi from the tussock, shrub, secondary forest, and primary forest, respectively. Three T-RFs (138, 189, and 300 bp in length) were predominant in four vegetation types and accounted for 80 % of all T-RFs. There was significant variation among the plant species in seven AM fungal T-RFs (Fig. 2a). Although there are limitations to extrapolating species identities from T-RFs, the dominant 138-, 189-, and 300-bp T-RFs were most closely related to Glomus sp. M20, Glomus sp. MUCL, and uncultured Glomus, respectively.
Average relative abundance of AM fungi (a) and nitrogen-fixing bacteria (b). Terminal restriction fragments (T-RFs) among four vegetation types, as determined by endonuclease digestion with HinfI (fungi) and HaeIII (bacteria). The relative abundance of T-RFs is given as a percentage of the total peak area. Fragment sizes in the graph indicate the size of the experimental T-RFs. Bars indicate SE (n = 3). A total of six T-RFs (94, 105, 134, 158, 189, and 300) of AM fungi varied significantly among the plant species. A total of 10 T-RFs (54, 62, 72, 80, 93, 97, 110, 184, 312, and 315 bp) of nitrogen-fixing bacteria significantly differed among the four vegetation types
We obtained 14 T-RFs for nitrogen-fixing bacteria (Fig. 2b); of these, 9, 11, 11, and 7 were found for the tussock, shrub, secondary forest, and primary forest, respectively. Three predominant T-RFs (66, 157, and 180 bp) accounted for 55 % of the nitrogen-fixing bacterial T-RFs in the four vegetation types. A total of 11 T-RFs of nitrogen-fixing bacteria differed among these soils. Our analyses indicated that the dominant 75-, 157-, and 180-bp T-RFs were most closely related to Bradyrhizobium sp. CCBAU 101065, Bradyrhizobium sp. ISA1601, and Bradyrhizobium japonicum, respectively.
Relationships among plant diversity, soil properties, and diversity of AM fungi and nitrogen-fixing bacteria
Bray-Curtis cluster analysis indicated that the composition of the communities of AM fungi and the nitrogen-fixing bacteria significantly differed between vegetation types (Fig. 3b, c). The AM fungal community composition in the tussock, shrub, and secondary forest differed from those in primary forest (Fig. 3b). The nitrogen-fixing bacterial community composition in the shrub and secondary forest differed from those in the tussock and primary (Fig. 3c).
RDA of the T-RFLP data showed that plant richness and pH were significantly correlated with AM fungal (F = 4.901, p = 0.004) and nitrogen-fixing bacterial (F = 2.08, p = 0.048) community composition, respectively. The available phosphorus, soil organic carbon, and total nitrogen content and plant richness were positively correlated with the abundance of AM fungi and nitrogen-fixing bacteria (p < 0.05, Table 3). The abundance of AM fungi correlated well with that of nitrogen-fixing bacteria (p < 0.001, Table 3).
Discussion
Changes in the abundance of AM fungal and nitrogen-fixing bacterial communities along vegetation restoration
In this study, we found a concurrent increase in the abundance of AM fungi and nitrogen-fixing bacteria (Fig. 1), supporting the close relationship of AM fungi and nitrogen-fixing bacteria (Smith et al. 2003).Their combined effects on plant growth may explain the concurrent increase. Thus, a concurrent increase in the abundance of these two microorganisms is important to vegetation restoration in karst regions. Additionally, compared with results from other ecosystems (Reardon et al. 2014), nifH gene abundance (Fig. 1) was slightly higher in our study site, lending credence to the suggestion that nitrogen-fixing bacteria contribute more to nitrogen input in karst ecosystems. Therefore, reduction in the potential to form AM fungi and nitrogen-fixing bacteria would hinder vegetation restoration in karst regions.
Effects of plant communities and soil properties on AM fungal communities at different stages of vegetation restoration
AM fungi can improve soil nutrient content, thus promoting plant growth, and their colonization often decreases with increase in plant available nutrient content (Bhadalung et al. 2005; Zangaro et al. 2013). However, a positive correlation was observed between soil nutrient content and soil AM fungal abundance from the tussock to the primary forest in our study (Table 3). Several explanations are possible for this positive correlation. First, the soil nutrient content in the karst region may not have been lower than the threshold that leads to reduced AM fungal abundance (Smith and Read 2008). Second, other characteristics, such as soil structure, may alter how soil nutrients affect AM fungal abundance (Caravaca et al. 2005). Moreover, plant richness is also an important factor influencing AM fungal abundance (Antoninka et al. 2011; Hiiesalu et al. 2014). The observed increasing plant richness could have increased AM fungal abundance in our case (Table 2; Fig. 1), associating with the previous report by Landis et al. (2004). The increased plant richness increases microclimatic variability and habitat complexity such as soil structure and root architecture (Waldrop et al. 2006), thus influencing fungal abundance.
Glomerales dominated in all four vegetation types. This order is also dominant in many forest ecosystems from other regions (Aguilar-Fernández et al. 2009; Leal et al. 2013). Glomerales can colonize from fragments of mycelium or mycorrhizal roots (Daniell et al. 2001) and can better adapt to a range of environments (Avio et al., 2006; Lumini et al. 2010), including those in karst regions. These characteristics may partially explain the high abundance of these species relative to that of species from other orders in the ecosystems.
The AM fungal and plant community composition significantly differed between vegetation types (Fig. 3a, b). These differences might be related to the AM fungi with host-species specificity (Davison et al. 2011; Pagano et al. 2013). Plant species have selective and stable cooperative relationships with AM fungal species (Kiers et al. 2011). Specificity of AM fungus–plant interactions may occur more often in relation to ecological group plants than in relation to individual plant species (Öpik et al. 2009; Scheublin et al. 2004). Old forest plants have infrequent AM fungi, leading to different composition with young forest plants (Davison et al. 2011). This finding was further supported by our result, which showed that the community composition of AM fungi in primary forest sites was significantly different from those in other vegetation sites (tussock, shrub, and secondary forest; Fig. 3a). This indicated a synergy between the aboveground plant community and the belowground AM fungal community during vegetation restoration. In addition, significant differences of the soil clay and sand percentage between the primary forest and other three vegetation types could have an important impact on AM fungal communities (Table 2; Fig. 3b). This result was in agreement with the previous study by Xiang et al. (2014). Similarly, plant richness also had an excellent effect on the AM fungal community composition. The higher AM fungal richness observed in sites with higher plant richness suggested that decrease in plant richness could decrease AM fungal richness (Pagano et al. 2011, 2013; Vogelsang et al. 2006). Thus, AM fungi play a vital role in vegetation restoration in the karst region.
Effects of plant communities and soil properties on nitrogen-fixing bacterial communities at different stages of vegetation restoration
The increasing abundance of nitrogen-fixing bacteria paralleled an increase in soil nutrient content from the tussock to the primary forest. Soil nutrients are known to be a key parameter influencing nitrogen-fixing bacterial communities (van der Heijden et al. 2006). As nitrogen fixation is an energy-consuming process, it may tend to increase with increase in energy inputs, supporting the trend of higher abundance in the later vegetation restoration stages. Soil nutrient status (mineral nitrogen) also changes with plant litter inputs, with higher amounts of soil nutrients seen in later vegetation restoration stages. This enhanced soil nutrient content also leads to a high increase in nifH gene abundance (Mergel et al. 2001).
Bradyrhizobium was found to be dominant across four vegetation types in the present study, as previously reported in terrestrial (Mirza et al. 2014), and marine environments (Gobet et al. 2012). Bradyrhizobium is known as a symbiotic N fixer. However, there is an increasing understanding that this organism may also play a key metabolic role as a soil saprophyte, which can colonize soil and the rhizosphere in the presence or absence of plants (Chatel et al. 1968). The fields we investigated from the tussock to the primary forest included leguminous and non-leguminous plants (Table S1). It is highly likely that the Bradyrhizobium types found have wide niches and are excellent survivors across diverse conditions.
The variation in the composition of the nitrogen-fixing bacterial community paralleled the changes in soil properties from the tussock to the primary forest in the present study. Thus, our results indicated an interaction between plant species and soil properties with respect to effects on the community. Among the soil properties, soil pH was identified as the main driver of the composition of this community, as previously reported in a grassland ecosystem (Aliasgharzad et al. 2010). Soil pH affects the microbial community mainly by influencing soil nutrient availability (Zhalnina et al. 2015). Some taxa were susceptible to soil pH and the composition of the nitrogen-fixing bacterial community shifted in a narrow range of soil pH in our present study. In addition, plant species affect the composition of the soil microbial community mainly through root exudation (Dilworth et al. 2008). Thus, differences among plant species, such as the physiological status, which influences soil properties (Ehrenfeld et al. 2005; Lugo et al. 2015), might lead to differences in nitrogen-fixing bacteria associated with those species.
In conclusion, we demonstrated that the AM fungal and nitrogen-fixing bacterial communities had high resilience when experiencing a change from the tussock to the primary forest in the karst region. The abundance of AM fungi and nitrogen-fixing bacteria increased from the tussock to the primary forest. The abundance of both communities was positively correlated with available phosphorus, total nitrogen, and soil organic carbon levels and plant richness (p < 0.05). Therefore, AM fungi and nitrogen-fixing bacteria can be an indicator of vegetation restoration processes in the karst region. It is necessary to focus on these communities if protection and restoration of the karst region is to be effective. A high abundance of AM fungi was found to be associated with a high abundance of nitrogen-fixing bacteria under natural conditions. Therefore, in the further studies, more attention should be paid to links between AM fungi and nitrogen-fixing bacteria under natural conditions.
References
Aguilar-Fernández M, Jaramillo VJ, Varela-Fregoso L, Gavito ME (2009) Short-term consequences of slash-and-burn practices on the arbuscular mycorrhizal fungi of a tropical dry forest. Mycorrhiza 19:179–186
Aldrich-Wolfe L (2007) Distinct mycorrhizal communities on new and established hosts in a transitional tropical plant community. Ecology 88:559–566
Aliasgharzad N, Mårtensson L, Olsson PA (2010) Acidification of a sandy grassland favours bacteria and disfavours fungal saprotrophs as estimated by fatty acid profiling. Soil Biol Biochem 42:1058–1064
Antoninka A, Reich PB, Johnson NC (2011) Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol 192:200–214
Avio L, Pellegrino E, Bonari E, Giovannetti M (2006) Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytol 172:347–357
Bauer JT, Kleczewski NM, Bever JD, Clay K, Reyolds HL (2012) Nitrogen-fixing bacteria, arbuscular mycorrhizal fungi, and the productivity and structure of prairie grassland communities. Oecologia 170:1089–1098
Bhadalung NN, Suwanarit A, Dell B, Nopamornbodi O, Thamchaipenet A, Rungchuang J (2005) Effects of long-term NP-fertilization on abundance and diversity of arbuscular mycorrhizal fungi under a maize cropping system. Plant Soil 270:371–382
Bremner JM (1965) Total nitrogen. In: Black CA (ed) Methods of soil analysis, vol 2. American Society of Agricultural, USA, pp. 1149–1178
Burgmann H, Widmer F, Von Sigler W, Zeyer J (2004) New molecular screening tools for analysis of free-living diazotrophs in soil. Appl Environ Microbiol 70:240–247
Caravaca F, Alguacil MM, Barea JM, Roldán A (2005) Survival of inocula and native AM fungi species associated with shrubs in a degraded Mediterranean ecosystem. Soil Biol Biochem 37:227–233
Chatel DL, Greenwood RM, Parker CA (1968) Saprophytic competence as an important character in the selection of Rhizobium for inoculation. In Trans. 9th Int. Cong. Soil Sci Adelaide: 11–65. Int. Soc. Soil Sci. Publisher, Sydney
Chen XB, Su YR, He XY, Wei YW, Wei WX, Wu JS (2012) Soil bacterial community composition and diversity respondto cultivation in karst ecosystems. World J Microbiol Biotechnol 28:205–213
Colwell JD (1963) The estimation of phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust J Exp Agric Anim Husb 3:190–197
Daniell TJ, Husband R, Fitter AH, Young JPW (2001) Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol Ecol 36:203–209
Davison J, Öpik M, Daniell TJ, Moora M, Zobel M (2011) Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol Ecol 78:103–115
Dilworth MJ, James EK, Sprent JIWE (2008) Newton nitrogen-fixing leguminous symbioses, library of congress, Dordrecht, the Netherlands, pp. 420
Fichtner A, Von Oheimb G, Härdtle W, Wilken C, Gutknecht JLM (2014) Effect of anthropogenic disturbances on soil microbial communities in oak forest persist for more than 100 year. Soil Biol Biochem 70:79–87
Gobet A, Böer SI, Huse SM, van Beusekom JEE, Quince C, Sogin M (2012) Diversity and dynamics of rare and of resident bacterial populations in coastal sands. ISME J 6:542–553
He XY, Wang KL, Zhang W, Chen ZH, Zhu YG, Chen HS (2008) Positive correlation between soil bacterial metabolic and plant species diversity and bacterial and fungal diversity in a vegetation succession on karst. Plant Soil 307:123–134
Hiiesalu I, Pärtel M, Davison J, Gerhold P, Metsis M, Moora M, Öpik M, Vasar M, Zobel M, Wilson SD (2014) Species richness of arbuscular mycorrhizal fungi: associations with grassland plant richness and biomass. New Phytol 203:233–244
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant–soil system. Annu. Rev Environ Resour 30:75–115
Jasper DA, Abbott LK, Robson AD (1989) Soil disturbance reduces the infectivity of external hyphae of vesicular arbuscular mycorrhizal fungi. New Phytol 112:93–99
Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. PNAS 107:2093–2098
Kahindi JHP, Woomer P, George T, de Souza Moreira FM, Karanja NK, Giller K (1997) Agricultural intensification, soil biodiversity and ecosystem function in the tropics: the role of nitrogen-fixing bacteria. Appl Soil Ecol 6:55–76
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Landis FC, Gargas A, Givnish TJ (2004) Relationships among arbuscular mycorrhizal fungi, vascular plants and environmental conditions in oak savannas. New Phytol 164:493–504
Leal PL, Siqueiraa OJ, Stürmerc SL (2013) Switch of tropical Amazon forest to pasture affects taxonomic composition but not species abundance and diversity of arbuscular mycorrhizal fungal community. Appl Soil Ecol 71:72–80
Lekberg Y, Koide RT, Rohr JR, Aldrich-Wolfe L, Morton JB (2007) Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95:95–105
Lugo MA, Reinhart KO, Menoyo E, Crespo EM, Urcelay C (2015) Plant functional traits and phylogenetic relatedness explain variation in associations with root fungal endophytes in an extreme arid environment. Mycorrhiza 25:85–95
Lukow T, Dunfield PF, Liesack W (2000) Use of the T-RFLP technique to assess spatial and temporal changes in the bacterial community structure with in an agricultural soil planted with transgenic and no transgenic potato plants. FEMS Microbiol Ecol 32:241–247
Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V (2010) Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol 12:2165–2179
Mergel A, Kloos K, Bothe H (2001) Seasonal fluctuations in the population of denitrifying and N2-fixing bacteria in an acid soil of a Norway spruce forest. Plant Soil 230:145–160
Mirza BS, Potisap C, Nüsslein K, Bohannan BJ, Rodrigues JL (2014) Response of free-living nitrogen-fixing microorganisms to land use change in the Amazon rainforest. Appl Environ Microbiol 80:281–288
Mortimer PE, Le Roux MR, Pérez-Fernández MA, Benedito VA, Kleinert A, Kleinert A, Xu JC, Valentine AJ (2013) The dual symbiosis between arbuscular mycorrhiza and nitrogen fixing bacteria benefits the growth and nutrition of the woody invasive legume Acacia cyclops under nutrient limiting conditions. Plant Soil 366:229–241
Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M (2009) Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol 184:424–437
Pagano MC, Utida MK, Gomes EA, Marriel IE, Cabello MN, Scotti MR (2011) Plant-type dependent changes in arbuscular mycorrhizal communities as soil quality indicator in semi-arid Brazil. Ecol Indic 11:643–650
Pagano MC, Zandavalli RB, Araújo FS (2013) Biodiversity of arbuscular mycorrhizas in three vegetational types from the semiarid of Ceará State, Brazil. Appl Soil Ecol 67:37–46
Peoples MB, Herridge DF, Ladha JK (1995) Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production? Plant Soil 174:3–28
Poly F, Ranjard L, Nazaret S, Gourbiere F, Monrozier LJ (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262
Reardon CL, Gollany HT, Wuest SB (2014) Diazotroph community structure and abundance in wheat–fallow and wheat–pea crop rotations. Soil Biol Biochem 69:406–412
Santos-González JC, Finlay RD, Tehlers A (2007) Seasonal dynamics of arbuscular mycorrhizal fungal communities in roots in a seminatural grassland. Appl Environ Microbiol 73:5613–5623
Sato K, Suyama Y, Saito M, Sugawara K (2005) A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl Sci 51:179–181
Scheublin TR, Ridgway KP, Young JPW, van der Heijden MGA (2004) Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70:6240–6246
Schwarzott D, Schüßler A (2001) A simple and reliable method for SSU rRNA gene DNA extraction, amplification, and cloning from single AM fungal spores. Mycorrhiza 10:203–207
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, third edn. Academic Press Inc, London, p. 815
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphorus supply to plant irrespective of growth response. Plant Physiol 133:16–20
van der Heijden MGA, Bakker R, Verwaal J, Scheublin TR, Rutten M, Logtestijin R, Staehelin C (2006) Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol Ecol 56:178–187
Vogelsang KM, Reynolds HL, Bever JD (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol 172:554–562
Waldrop MP, Zak DR, Blackwood CB, Curtis CD, Tilman D (2006) Resource availability controls fungal diversity across a plant diversity gradient. Ecol Lett 9:1127–1135
Wang SJ, Liu QM, Zhang DF (2004) Karst rocky desertification in southwestern China: geomorphology, landuse, impact and rehabilitation. Land Degrad Dev 15:115–121
Wilson GWT, Rice CW, Rillig MC, Springe A, Hartnett DC (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461
Xiang D, Verbruggen E, Hu YJ, Veresoglou SD, Rillig MC, Zhou WP, Xu TL, Li H, Hao ZP, Chen YL, Chen BD (2014) Land use influences arbuscular mycorrhizal fungal communities in the farming–pastoral ecotone of northern China. New Phytol 204:968–978
Yuan DX (1994) Karstology of China. Geological Publishing House, Beijing
Yuan DX (2002) Geology and geohydrology of karst and its relevance to society, Paris, UNESCO pp 15–18
Zangaro W, Rostirola LV, de Souza PBDB, Alves RDA, Lescano LEAM, Rondina ABL, Nogueira MA, Carrenho R (2013) Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil. Mycorrhiza 23:221–233
Zhalnina K, Dias R, Dörr de Quadros P, Davis-Richardson A, Camargo FAO, Clark IM, McGrath SP, Hirsch PR, Triplett EW (2015) Soil pH determines microbial diversity and composition in the park grass experiment. Microb Ecol 69:395–406
Acknowledgments
This project was supported by the National Science-technology Support Plan Projects (2012BAD05B03-6), and the National Natural Science Foundation of China (41171246, 31270551 and 41301273).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Yueming Liang and Fujing Pan are the first co-author. These authors contributed equally to this work.
Electronic supplementary material
Table S1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Liang, Y., Pan, F., He, X. et al. Effect of vegetation types on soil arbuscular mycorrhizal fungi and nitrogen-fixing bacterial communities in a karst region. Environ Sci Pollut Res 23, 18482–18491 (2016). https://doi.org/10.1007/s11356-016-7022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7022-5