Abstract
The use of lagooning as a complementary natural method of treating secondary effluents of wastewater treatment plants has been employed as an affordable and easy means of producing reclaimed water. However, using reclaimed water for some purposes, for example, for food irrigation, presents some risks if the effluents contain microbial pathogens. Classical bacterial indicators that are used to assess faecal contamination in water do not always properly indicate the presence of bacterial or viral pathogens. In the current study, the presence of faecal indicator bacteria (FIB), heterotrophic bacterial counts (HBC), pathogens and opportunistic pathogens, such as Legionella spp., Aeromonas spp., Arcobacter spp., free-living amoeba (FLA), several viral indicators (human adenovirus and polyomavirus JC) and viral pathogens (noroviruses and hepatitis E virus) were analysed for 1 year in inlet and outlet water to assess the removal efficiency of a lagooning system. We observed 2.58 (1.17–4.59) and 1.65 (0.15–3.14) log reductions in Escherichia coli (EC) and intestinal enterococci (IE), respectively, between the inlet and outlet samples. Genomic copies of the viruses were log reduced by 1.18 (0.24–2.93), 0.64 (0.12–1.97), 0.45 (0.04–2.54) and 0.72 (0.22–2.50) for human adenovirus (HAdV), JC polyomavirus (JCPyV) and human noroviruses (NoV GI and GII), respectively. No regrowth of opportunistic pathogens was observed within the system. FLA, detected in all samples, did not show a clear trend. The reduction of faecal pathogens was irregular with 6 out of 12 samples and 4 out of 12 samples exceeding the EC and IE values, specified in the Spanish legislation for reclaimed water (RD 1620/2007). This data evidences that there is a need for more studies to evaluate the removal mechanisms of lagooning systems in order to optimize pathogen reduction. Moreover, surveillance of water used to irrigate raw edible vegetables should be conducted to ensure the fulfilment of the microbial requirements for the production of safe reclaimed water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water scarcity is a major problem worldwide, with an estimated 2.7 million people living close to river basins that are affected by severe water need (Oki and Kanae 2006; Hoekstra et al. 2012). The United Nations estimates that the world population will reach 9 billion in approximately 2050 and that water availability will therefore decrease. Current projections indicate that there will be a 55 % increase in water demand between 2000 and 2050 (Gurría 2012). It is clear that reducing water use, recycling and reusing water resources are and will continue to be a priority in the near future.
The use of recycled treated wastewater, also called reclaimed water, for irrigation has proven to be a good strategy for reducing water scarcity. Reclaimed water is a source of water that is independent of weather conditions and includes additional nutritional input that can produce better vegetables, field crops, and fruits (Wheaton et al. 2001; Dare 2015). However, the use of improperly treated reclaimed water for food irrigation presents a risk to human health if the pathogenic microorganisms contained in the water are not inactivated (Carter 2005; Riera-Montes et al. 2011). The increase of fresh food consumption has been linked to an increase in foodborne outbreaks (Sivapalasingam et al. 2004; Kozak et al. 2013; Callejón et al. 2015). One of the sources of food viral contamination is irrigation water. Maunula et al. (2013) found that HAdV was present in 9.5 % of irrigation water used to irrigate berries proving that it was faecally contaminated. Recently, a major outbreak of norovirus-related gastroenteritis affected nearly 11,000 people in Germany and was linked to strawberries that were probably irrigated with contaminated water during fruit production (Bernard et al. 2014). Although it is not stated in the report if the water used for irrigation was reclaimed or not, this incident provides a good example of how contaminated water or improperly treated reclaimed water can result in a health risk.
The Spanish legislation on water reuse (Royal Decree 1620/2007) has represented an important advancement to standardize reuse practices differentiating 14 uses under five main areas: urban, agricultural irrigation, industrial, recreational and environmental. To produce reclaimed water with a higher quality and to minimize/prevent the risk of human infections, several processes, such as microfiltration and ultrafiltration, are being applied after the secondary treatment in wastewater treatment plants (WWTP). The technological needs and high costs of these treatments pose substantial challenges, especially in some countries, because of economic constraints. Among the different low-cost methods used to produce reclaimed water, storing treated or untreated wastewater in stabilization ponds, polishing ponds, or natural or artificial lagoons appears to be an effective solution to overcome these challenges (Campos et al. 2002; Oragui et al. 2011). These methods are affordable in developing countries and small communities, and they result in high microbial inactivation rates via the use of cheap technological approaches with low operational costs (Mara et al. 1992; Peña et al. 2000).
Traditionally, the efficiency of microbial removal for these methods has been evaluated using faecal indicator bacteria (FIB) such as faecal coliforms. However, the presence of faecal coliforms is not always correlated with the presence of viral pathogens, such as noroviruses (Gerba et al. 1979; Marzouk 1980; Pusch et al. 2005; Jiang 2006; Bofill-Mas et al. 2013) or other pathogenic bacteria, such as Legionella spp., Aeromonas spp., which are indigenous to freshwater ecosystems, and Arcobacter spp., which is considered to be an opportunistic pathogen and a signature indicator of sewage contamination (Harwood et al. 2005; Collado and Figueras 2011). The weakness of this correlation has been attributed to differences in the survival rates of these species and differences in the efficiencies of treatments used in WWTPs. This lack of correlation has also been described in water stabilization ponds and lagooning systems in previous studies (Mara and Pearson 1987; Donnison and Ross 1995). Nevertheless, new studies based on molecular techniques are needed to confirm or deny a lack of correlation between these biological indicators. For example, in a recent study published by Jurzik et al. (2015), the use of polishing ponds as a tertiary treatment resulted in a reduction of 1.84–2.65 log units of bacteria and bacteriophages without reducing/affecting the concentrations of animal viruses. However, in the later study, viruses were tested by molecular methods and infectivity data was not included. The information derived from molecular methods is useful, especially for those viral agents which are not cultivable, such as NoV or bacterial species that might enter into a viable but not cultivable (VBNC) state. However, this is a limiting factor when evaluating the removal efficiency of a specific water treatment process because non-infective viral genomic material can be detected by q(RT)-PCR methods. To overcome this limitation, cell culture methods were applied, for HAdV, to test the infectivity of viral concentrates. Human adenovirus is widely used as a human viral faecal marker showing a high occurrence during all periods of the year (Gerba et al. 1979; Lipp et al. 2001; Bofill-Mas et al. 2013). The presence of human adenovirus infectious viral particles is important when evaluating the risks derived from water reuse, for example, when testing water used for crops irrigation.
The current study describes the removal efficiency of a lagooning system by analysing the presence of faecal viral markers, including human adenoviruses (HAdVs) and JC polyomavirus (JCPyV), heterotrophic bacteria counts (HBC), and classical FIBs, (E. coli (EC) and intestinal enterococci (IE)). In addition, the presence of pathogenic noroviruses (GI and GII), the hepatitis E virus (HEV), potential bacterial pathogens, such as Arcobacter spp., Aeromonas spp., Legionella spp. and free-living amoeba were analysed to determine whether these water systems are reservoirs or niches that might promote the regrowth of pathogenic microorganisms and thereby represent a new threat with regard to further water reuse.
Materials and methods
Description of the site and sampling program
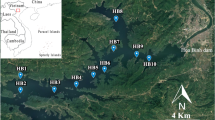
The lagooning system evaluated is situated in south Catalonia (Spain) in a zone with typical Mediterranean weather. The lagooning system has a theoretical total volume capacity of 24,087 m3 and a surface area of 16,864 m2. The system receives water from the secondary outlet of a WWTP that treats a volume of 25,000 m3/day of raw sewage from approximately 200,000 inhabitants. The flow rates were measured in continuous using a magnetic flow meter. The volume of water entering the lagoons is registered daily and was provided by the WWTP, and the data is presented in Table 1. Once the water has been treated with a conventional secondary treatment (activated sludge), its entry into the stabilization ponds of the lagooning tertiary treatment depends on water demands and is regulated by a water level control system. The system is composed of four lagoons with depths ranging from 1.95 to 3.15 m. This volume of water, which does not take evaporation into account, provides an indirect measure of reclaimed water produced and supplied to 140 users who use this water as the main irrigation source for their olive and hazelnut trees and vineyards, which cover an area of 135 ha.
One year of sampling, from September 2012 to August 2013, was completed. Two-litre water samples were collected monthly from the secondary outlet of the WWTP as it entered the lagooning system (lagooning inlet), and 2 L was collected from the tertiary effluent after it exited the stabilization ponds (lagooning outlet). A volume of 1 L was obtained from each sampling point and used for the viral analysis, 500 mL were used to analyse the samples for the presence of FIB, Aeromonas spp. and Arcobacter spp., and 500 mL were used to analyse the samples for the presence of HBC, Legionella spp. and free-living amoebas. Water temperature, pH and conductivity were measured using the corresponding probes (XS instruments device) and following the US EPA guidelines 150.1 and 120.1, respectively. Turbidity was determined by nephelometry using a hanna instrument and following the US EPA 180.1 guideline. All the variables were recorded during sampling. Precipitation and solar radiation data were collected from the Catalan Meteorological Institute (http://www.meteo.cat/servmet/index.html). All of this information is presented in Table 1.
Viral analysis
Viral concentration and nucleic acid extraction
The viruses present in 1 L samples were concentrated using skimmed milk organic flocculation. The method has a recovery efficiency of about 50 % (20–95 %) (Calgua et al. 2008, 2013). All samples were adjusted to a conductivity of 1.5 mS/cm2 and acidified to a pH 3.5 using 1 N HCl. Briefly, a suspension of skimmed milk was prepared by adding 10 g of skimmed milk powder (Difco, Detroit, MI, USA) to 1 L of artificial seawater (Sigma-Aldrich Chemie GMBH, Steinheim, Germany), and the solution was then adjusted to pH 3.5 using 1 N HCl to obtain a pre-flocculated 1 % (w/v) skimmed milk solution (PSM). Then, 10 mL of PSM was added to previously conditioned samples to obtain a final concentration of 0.01 % of skimmed milk. Samples were kept for 8 h while stirring at room temperature, and flocks were allowed to sediment by gravity during 8 h. The supernatant was carefully removed, and the remaining 500 mL of the solution were centrifuged at 8000×g for 30 min at 4 °C. Pellets were suspended in 1 mL of phosphate buffer (pH 7.5) and stored at −80 °C until nucleic acid (NA) extractions were performed. A negative concentration control for each process sample was also included. For these, we used tap water as the matrix, and we first neutralized the free chlorine by adding 100 mL of a 10 % sodium thiosulfate solution.
To extract viral nucleic acids, 140 μL of viral concentrates were processed using a QIAamp® Viral RNA Mini Kit (Qiagen, Valencia, CA, USA) and the automated QIACube system (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. Nucleic acids were stored at −80 °C until analysed. A negative control consisting of DNAse/RNAse-free molecular water was included in each extraction batch.
Quantitative and nested PCR assays to evaluate viruses
Samples were analysed to determine the presence and concentrations of viral faecal markers and other pathogenic viruses.
Specific real-time qPCR assays were used to quantify the viral faecal markers HAdV and JCPyV (Bofill-Mas et al. 2006; Hernroth et al. 2002; Pal et al. 2006) using TaqMan® Environmental Master Mix 2.0 (Life technologies, Foster City, CA, USA). Real-time primers and probes for HAdV can amplify A, C, D, E, F and some B HAdV serotypes, so the most frequently described HAdV can be detected with this assay. Specific RT-qPCR assays were performed to quantify the levels of the human norovirus genogroups I and II (NoVGI and NoVGII) (Kageyama et al. 2003; Loisy et al. 2005) and the RNA UltraSense™ One-Step Quantitative RT-PCR System (Invitrogen, Carlsbad, CA, USA). All samples were analysed in duplicate using undiluted and 1:10 dilutions of the nucleic acids extracts. Dilutions were tested to detect the potential inhibition of amplification resulting from the presence of inhibiting substances in the undiluted samples. The analysis of direct and ten-fold dilutions of environmental samples may indicate the presence of enzymatic inhibition if a difference of Cq between direct and diluted sample is lower than 3.3. In this case, the value considered was the one obtained by testing the diluted sample and more dilutions of that sample were tested to rule out if inhibition was still occurring. Also, external amplification controls were used to evaluate the potential inhibitory capability of the studied samples by adding known amounts of standard plasmid (1 × 103 GC/reaction). A MX3000Pro qPCR sequence detector system (Stratagene, La Jolla, CA, USA) was used to quantify the levels of viral genomes in the samples.
Nested RT-PCR (nRT-PCR) assays were used to test for the presence of the hepatitis E virus (HEV), as previously described by Erker et al. (1999). Nested PCR was selected because low numbers of HEV were expected, and nested PCR facilitated sequencing analysis of the virus. Reverse transcription of the extracted RNA was performed using a one-step RT-PCR Kit (QIAGEN, Valencia, CA, USA), and semi-nested PCR was performed using AmpliTaq™ Gold DNA polymerase.
The limit of detection (LOD) in 100 mL of water of the (RT)qPCR assays that were used in this study was found to be 29 GC for HAdV, 80 GC for JCPyV, 343 GC for NoVGI and 229 GC for NoVGII following the FSA 2006 guidelines.
Controls for (RT)-qPCR assays
Plasmid DNA suspensions were used as positive controls and quantitative standards. For HAdV and JCPyV, the hexon region (8961 bp) of HAdV41 and the whole genome (5130 bp) of JCPyV Mad1 were cloned into the plasmid pBR322. The capsid protein regions of NoVGI.4 (2931 bp) and NoVGII.13 (3004 bp) were cloned into the pTrueBlue®-Pvu II vector and used as the qRT-PCR standard.
To reduce the possibility of DNA contamination in the laboratory, 10 μg of each plasmid DNA was linearized using specific restriction enzymes as follows: BamHI for the HAdV41 plasmid (Promega, Madison, WI), NruI for the JCPyV plasmid (Promega, Madison, WI), SacI for the NoVGI plasmid and XhoI for the NoVGII plasmid (Promega, Madison, WI). The reaction products were subsequently purified and quantified. Samples and standard plasmids were added in two different rooms to avoid the possibility of contamination. Serial dilutions in TE buffer were performed using linearized standards ranging from 100 to 105 molecules per 5 or 10 μL (for viral RNA or DNA, respectively). Aliquot standard dilutions were stored in individual tubes at −80 °C until use.
All qPCR assays included non-template controls (NTC), and control extractions were included to evaluate any possible contamination during the extraction and amplification process. Moreover, all qPCR, RT-qPCR, nPCR and RT-PCR mixes, sample inoculations and standard additions were performed in separated areas to avoid any potential contamination. Negative PCR controls were also included for each analysis.
Infectivity assays in HAdV using the ICC-qPCR approach
An infectivity assay was performed for HAdV using the human embryonic kidney cell line HEK 293A (Life technologies, R705-07). Cells were infected with the four inlet samples that had the highest number of HAdV viral genomic copies and the four corresponding outlet samples. Cells were used from passages 12 to 15 and cultured using Dulbecco’s modified Eagle’s medium (DMEM) containing a high concentration of glutamine (Glutamax, Life Technologies). The medium was supplemented with 10 % fetal bovine serum (Life Technologies), 1 % streptomycin-ampicillin and 1 % non-essential amino acids (Life Technologies), as previously described in the literature (Ogorzaly et al. 2013).
HEK 293A cells were seeded in 25 cm2 cell culture flasks at a density of 5 × 104 cells/mL and incubated in 5 % CO2 at 37 °C until confluence was achieved. Each environmental sample was analysed in two infected cell flasks (T0-1 h incubation and T8-8 day incubation). Cell culture flasks were infected using 100 μL of viral concentrate that was diluted in DMEM (1:1) to achieve a final infective solution of 200 μL because previous assays showed toxicity when they were infected with undiluted viral concentrates. Cells were incubated at 37 °C for 60 min. The cells were subsequently washed with PBS 1× three times to remove non-infective viral particles that were attached to cell surfaces. Finally, 5 mL of DMEM supplemented with 1 % non-essential amino acids, 2 % fetal bovine serum, 2 % streptomycin-ampicillin and 2 % kanamycin were added to the cell flasks. A negative (DMEM) and a positive control (HAdV35) were performed in parallel.
Positive samples were quantified with a most probable number (MPN) approach. Briefly, nine 25-cm2 cell culture flasks were inoculated using a ten-fold dilution series (direct to 10−2) in triplicate. All of the infected cell-cultured flasks were scratched after 3 days of incubation and analysed using 140 μL of the scratched cell suspension in a QIAamp Viral RNA mini Kit (QIAGEN). Negative samples had <8 MPN infective HAdV in 100 mL.
Cell cultures presumptively positive for adenovirus were analysed using a nested PCR (Allard et al. 2001), and the amplicon was sequenced to typify the most abundant HAdV types grown in cell cultures. The pair of primers selected were broad primers to amplify all HAdV known serotypes. The amplicons obtained after the nested-PCR were purified using a QIAquick PCR purification kit (QIAGEN, Inc.). The purified DNA was directly sequenced using an ABI PRISM™ Dye Terminator Cycle Sequencing Ready Reaction kit version 3.1 with AmpliTaq® DNA polymerase FS (Applied Biosystems) according to the manufacturer’s instructions. Conditions for the 25-cycle sequencing amplification were denaturing at 96 °C for 10 s, annealing for 5 s at 50 °C and extension at 60 °C for 4 min. Nested primers were used for the sequencing reactions at a concentration of 0.05 μM.
Sequencing results were checked using an ABI PRISM 377 automated sequencer (PerkinElmer, Applied Biosystems). Sequences were compared to the GenBank and European Molecular Biology Library (EMBL) using the basic National Center for Biotechnology Information (NCBI) BLAST programme (http://www.ncbi.nlm.nih.gov/BLAST/).
Bacteria and protozoa analysis
Heterotrophic bacteria quantification
Heterotrophic bacterial counts (HBC) were determined in accordance with International Organization for Standardization (1999) following the standards for water quality. Briefly, ten-fold dilution series were prepared in Ringer 1/4 (Scharlau Chemie; Barcelona, Spain), plated in plate count modified agar media (Scharlau Chemie; Barcelona, Spain) and incubated at 22 °C for 72 h as previously described ( Serrano-Suárez et al. 2013) .
FIB
The determination of E. coli (EC) and intestinal enterococci (IE) was performed using 96-well microplate MPNs according to the International Organization for Standarization (1998a, 2012) methods (Bio-Rad, France), respectively. The detection method used for EC was based on the expression of the β-d-glucuronidase enzyme, while the expression of β-glucosidase was the target used to detect IE.
Detection and quantification of Arcobacter
The quantification of Arcobacter in reclaimed water was performed according to the MPN method using five replicate tubes. Each contained 2.5 mL of Arcobacter broth supplemented with CAT (cefoperazone, amphotericin B and teicoplanin) and 0.5 mL of a ten-fold serial dilution of the water samples. The tubes were incubated at 30 °C for 48 h. For tubes that showed turbidity, 200 μL of the broth was inoculated using passive filtration (0.45-μm membrane) on blood agar plates (Trypticase Soy Agar (TSA), BD, Spain) supplemented with 5 % sheep’s blood. Eight presumptive Arcobacter colonies were selected for molecular identification from each positive sample. The 16S rDNA-RFLP method (Figueras et al. 2012) was used. The MPN values were calculated using MPN Build 23 software (Mike Curiale software), and the results were expressed as MPN/100 mL.
Detection and quantification of Aeromonas
All water samples were investigated for the presence of Aeromonas (n = 24), but quantification was performed according to the MPN method using five replicate tubes only between March 2013 and August 2013 (n = 12). Buffered peptone water (BPW, Oxoid, UK) was used to prepare ten-fold serial dilutions of the water samples; 0.5 mL of each dilution was inoculated into a tube containing 2.5 ml of alkaline peptone water (APW, Oxoid, UK) supplemented with ampicillin (APW-A, 10 mg/L, Sigma-Aldrich, Steinheim, Germany). The tubes were incubated at 30 °C for 24 h. From each dilution, 100 μL was inoculated in ampicillin dextrin agar (ADA, CRITERION, Santa Monica, California, USA) plates and incubated at 30 °C for 24 h. When the ADA plates were found to be positive for the presence of Aeromonas, eight presumptive yellow colonies were subcultured in TSA and incubated under identical conditions. For molecular identifications performed at the genus level, the PCR method described by Chacón et al. (2002) was used. The MPN was calculated as described above.
Legionella spp. culture and typification
The detection and quantification of Legionella spp. in water samples were performed by culturing samples on BCYE agar supplemented with GVPC (MAIM, Spain). Samples (100 mL each) were filtered through a 0.45-μm pore size nylon membrane (Filter HNWP Millipore; Ireland), and the retained material was then suspended in 10 mL of Ringer 1/40 using vortexing for 2 min. The concentrates were cultured either directly or after two treatments: a thermal treatment at 50 °C for 30 min or an acid treatment in which 100 μL of acid buffer was added to 900 μL of the sample concentrate, as described in International Organization for Standardization (1998b).
Presumptive Legionella colonies were tested using a Legionella latex test (Oxoid, Basingstoke, Hampshire, England) according to the manufacturer’s instructions. This kit enables the differentiation of L. pneumophila serogroup 1, L. pneumophila serogroups 2–14 and seven additional Legionella species.
Legionella spp. DNA extraction and qPCR analysis
Nucleic acids were extracted from 1 mL of Legionella sample concentrates using a Wizard genomic DNA purification kit (Promega, Madison, Wis.).
All samples were tested for the presence of Legionella spp. using a primer pair that was described in Herpers et al. (2003) and a probe described in Declerck et al. (2007). All reactions were performed in a final volume of 25 μL that contained 0.9 μM of each primer, 0.2 μM TaqMan MGB probe, 12.5 μL of 1× TaqMan Universal Master Mix and 5 μL of the extracted nucleic acids.
Legionella spp. positive samples were further tested for the presence of L. pneumophila. These assays targeted the mip gene and were based on the primers and probe described in Diederen et al. (2007). All of these reactions were performed in a final volume of 25 μL that contained 0.2 μM of the MipF primer, 0.3 μM of the MipR primer, 0.15 μM TaqMan probe Lpn-Mip, 12.5 μL of the 1× TaqMan Universal Master Mix and 5 μL of the extracted nucleic acids.
Free-living amoeba quantification
To quantify free-living amoebae (FLAs), 100 mL of each sample was concentrated to 10 mL by centrifugation at 800×g for 20 min. The concentrates were quantified by culturing them in non-nutrient agar (NNA) plates according to the MPN method described in Cervero-Aragó et al. (2013). MPN values were obtained from MPN tables (International Organization for Standardization 2005). The detection limit of the method ranged from 2 × 102 MPN of FLA/mL to 2 × 106 MPN of FLA/mL.
Statistical analyses
The data obtained for the concentrations of the microbiological parameters at the inlet and the outlet of the lagooning system were analysed using software packages developed in R (R Core Team 2013). The Wilcoxon test implemented in the R Package “exactRankTests” was used to calculate p values. This test was applied to calculate whether there were statistically significant differences between the values obtained at the inlet compared to those obtained at the outlet. The results relating to bacteria and free-living amoeba were analysed using two-sided Wilcoxon tests, while one-sided Wilcoxon tests were used to analyse results related to human viruses. The inclusion of the LOD for each microorganism and technique has been chosen to replace all non-detect. To compare the significance of the p values obtained for each microorganism, p values adjusted for multiple testing were calculated (Online resource 1) using the R package by applying the false discovery rate (FDR) test (Benjamini and Yekutieli 2001). A redefined adjusted p value of 0.05 was chosen to be the cut-off for statistical significance.
Pearson’s correlation tests were performed using R software to determine if some relation existed between pH, water temperature, atmospheric temperature, theoretical hydraulic retention time and the microbial load at the outlet. At the same time, correlations among the different microorganisms at the outlet of the lagooning were tested.
Results
Results obtained for microbiological parameters in the inlet and outlet samples for each sampling point and date are shown in Table 2. Mean values before and after the lagooning tertiary treatment and the logarithmic reductions are presented in Tables 3 and 4 and Fig. 1. Negative or non-detected samples have been replaced by the LOD for each microorganism and technic. This conservative decision might underestimate the lagooning removal efficiency.
In general, all studied microorganisms were reduced in concentration after lagooning, with reductions ranging from 1.18 to 0.45 log for some enteric viruses and up to more than 2 log for EC (Fig. 1). The observed reductions in human viral faecal markers (HAdV and JCPyV) and FIB (EC and IE) were statistically significant (Online resource 1). No significant statistical correlation was found between the measured physico-chemical water parameters and any of the microorganisms analysed at the lagooning outlet. A significative negative correlation was found between HRT and heterotrophic bacterial counts (R 2 = −0.72, p value = 0.0078). Only significative correlations between norovirus genogroups GI and GII (R 2 = 0.84, p value = 0.0006) were found.
The average water retention time in the system was estimated to be of 31 days with two main periods: a high demand period (from June to September ˃25,000 m3/month) with an average estimated retention time of 16 days and a low demand period (from October to May, ˂25,000 m3/month) with an average estimated retention time of 88 days (see Tables 3 and 4). Retention time was influenced by raining events, which decreased the water demand from farmers. Although initial analyses appear to indicate differences in microbial removal between these periods, the small number of samples tested for each period group does not enable to conclude if differences are statistically significant.
Efficiency of virus removal and inactivation
A high abundance of viruses was observed in the secondary effluents analysed over the entire year. The majority of the samples (11/12) entering into the lagooning system were positive for HAdV with a mean value of 3.71 × 103 GC/100 mL, whereas only half of the outlet samples were positive (6/12), with mean values of 2.44 × 102 GC/100 mL (Tables 2 and 3). For JCPyV, the number of positive samples was high (9/12) at the inlet, with a mean value of 6.06 × 102 GC/100 mL, while the number of positive samples decreased at the outlet (3/12), where samples showed a mean value of 1.38 × 102 GC/100 mL. Human pathogens NoV GI and GII were highly prevalent at the inlet (in 8/12 and 11/12 of the samples, respectively). The concentrations of both genogroups at the inlet of the system were 6.43 × 103 GC/100 mL and 6.67 × 103 GC/100 mL, respectively, while the prevalence of these viruses at the outlet of the system slightly decreased (7/12 of the samples at 2.27 × 103 GC/100 mL and 8/12 of the samples at 1.38 × 103 GC/100 mL, respectively). None of the inlet or outlet samples were positive for HEV.
The infectivity experiments presented one out of four samples with infectious HAdV in the inlet (233 MPN IU/100 mL). None of the four outlet samples tested showed infectious HAdV (LOD ˂ 8MPN infective virus/100 mL). The positive inlet sample for HAdV was amplified, and the amplicon obtained was sequenced and corresponded to HAdV41 (nucleotide accession number HG976918).
Efficiency of inactivation of HBC and standard FIB
HBC
Heterotrophic bacteria were present in all of the samples analysed, with a geometric mean of 2.91 × 107 cfu/100 mL in the inlet samples and 9.43 × 106 cfu/100 mL in the outlet samples. The results showed a reduction of 0.49 logs in the HBC count, but this difference was not statistically significant (Table 4 and Online resource 1). Moreover, these differences varied slightly during the year. A peak reduction in HBC of 0.95 logs was observed during the months with lower water demand. In contrast, there was an increase in the bacterial count to 0.44 logs in the summer during the period of high water demand.
Standard FIB
All 12 inlet water samples were positive for EC and IE, with geometric means of 7.23 × 104 MPN/100 mL and 5.11 × 103 MPN/100 mL, respectively (Table 2). Only eight outlet water samples were positive for EC (1.92 × 102 MPN/100 mL), whereas 10 outlet samples were positive for IE (1.14 × 102 MPN/100 mL). The lagooning reduced 2.58 and 1.65 log EC and IE, respectively (Table 4).
During the low demand period, the mean concentration of EC in the inlet water was 4.81 × 104 MPN/100 mL, while in the outlet, the mean concentration was 8.47 × 101 MPN/100 mL. These data indicate a 2.75 log reduction. For the same period, the mean concentration of IE was 4.23 × 103 MPN/100 mL for the inlet water and 8.03 × 101 MPN/100 mL for the outlet water, with an observed reduction of 1.72 logs.
During the high demand period, the mean concentration of EC was 1.63 × 105 MPN/100 mL and 9.80 × 102 MPN/100 mL for the inlet and the outlet water, respectively, indicating a 2.22 log reduction. During this period, the concentration of IE in the inlet water was 7.46 × 103 MPN/100 mL, while the concentration at the outlet was 2.28 × 102 MPN/100 mL, indicating a 1.51 log reduction.
Evaluation of the potential regrowth of bacteria and other opportunistic pathogens
Arcobacter
All 12 inlet water samples were positive for Arcobacter, while only 5 (41.6 %) of the outlet water samples were positive (Table 2). The average concentration of Arcobacter in the inlet water samples was 7.51 × 106 MPN/100 mL, while in the outlet water samples, the average concentration was 4.59 × 102 MPN/100 mL (Table 4). During the low demand period, the mean concentration of Arcobacter in the inlet water was 7.80 × 106 MPN/100 mL, while in the outlet, it was 9.47 × 102 MPN/100 mL. This data represents a 3.92 log reduction. Notice that during the high demand period, the mean concentration of Arcobacter was 6.9 × 106 MPN/100 mL for inlet water and 1.08 × 102 MPN/100 mL for outlet water, and these data represent a 4.81 log reduction.
Aeromonas spp.
All water samples (12 inlet and 12 outlet) were positive for Aeromonas (Table 2). Over the 6 months during which quantification was performed (March 2013 and August 2013), the average concentration of Aeromonas spp. in the inlet water was 1.08 × 106 MPN/100 mL and the average concentration in the outlet was 1.94 × 105 MPN/100 mL. These data represent a 1.02 log reduction in the Aeromonas spp. load during the storage period (Table 4). During the lower demand period, the mean concentration of Aeromonas in the inlet water was 1.06 × 106 MPN/100 mL, while in the outlet water, it was 1.05 × 104 MPN/100 mL. These data indicate a 2 log reduction. In contrast, during the high demand period, the mean concentration of Aeromonas was 3.97 × 105 MPN/100 mL in the inlet water and 3.68x105 MPN/100 mL in the outlet water, representing only 0.03 log reduction.
Legionella spp.
Legionella spp. were detected using qPCR, but not culture methods, in both inlet and outlet samples. In the samples with higher concentrations, which were obtained from February to April, it was not possible to isolate any Legionella spp. because there was a high concentration of accompanying microbiota that also grew on the BCYE agar plates. Hence, it was not possible to differentiate between species or serogroups using sero-agglutination. The qPCR results showed that Legionella spp. were present in 11 out of the 12 samples obtained from the lagooning inlet and in 7 out of 12 samples obtained from the outlet (Table 2). Within these samples, L. pneumophila was detected in six inlet samples and two outlet samples. Overall, a global reduction of 1.72 log was observed in Legionella (Table 4). However, when only the positive samples obtained during the low and high water demand periods were compared, we observed a log reduction of 1.97 and 0.89, respectively, in Legionella.
FLA
All inlet and outlet water samples contained FLA, with geometric means of 1.19 × 105 MPN/100 mL and 3.04 × 104 MPN/100 mL, respectively. In general, higher concentrations of FLA were detected in the inlet samples, and a reduction of 0.59 log was observed after lagooning. Moreover, an 0.40 log reduction was observed during the low water demand period, and a 0.98 log reduction was observed during the high demand.
Discussion
In this work, a lagooning system, considered as a natural and low-cost tertiary disinfection method, is used to treat the secondary wastewater effluent produced by a wastewater treatment plant.
The stabilization pond system studied in this report decreased the concentration of all microorganisms analysed, with the exception of FLA and HBC which presented on some months similar counts at the outlet. Higher numbers of HBC in the outlet samples were observed during periods with higher water temperatures, despite increased solar radiation. These findings support the idea that temperature can positively impact the regrowth of HBC, as was reported by Niquette et al. (2001). Microorganisms that are able to grow in aquatic environments, such as some of the studied bacteria and protozoa, were analysed for either regrowth or inactivation. It is known that lagooning applied to produce reclaimed water reduces the levels of bacteria in the effluent (Jjemba et al. 2010; Derry and Attwater 2014). Nevertheless, bacteria regrowth has been observed in the mentioned studies in the reservoir and distribution systems, where there was a loss of residual disinfectant and high levels of assimilable organic carbon. Despite this fact, HBC and FLA should be considered when reclaimed water produced by lagooning is going to be used for irrigation purposes as both groups contain potentially pathogenic microorganisms and their regrowth may represent a health risk.
Viral concentrations decreased between 0.45 and 1.18 log (NoVGI and HAdV respectively) (Table 3). The obtained values were similar to those reported by Maynard et al. (1999); however, depending on the lagooning characteristics, higher reductions can be reached (up to 2 log) (Shuval 1990; Pay Drechsel et al. 2010), depending on the characteristics of the lagooning system. Jurzik et al. (2015) reported a high degree of effectiveness in the removal of FIB and bacteriophages in a lagooning system, whereas human viruses, such as HAdV and JCPyV, were not significantly decreased. The lack of infectivity assays in previous studies constitutes an important limitation as those viral particles detected could not be infectious. In the current study, higher reductions of genome copies were observed for the same faecal virus markers. This might be related to the higher water temperatures expected from a Mediterranean site and also due to differences in the retention times. Infectivity results showed that one out of four inlet samples but none of the four outlet samples contained infective HAdV particles (less than 8 MPN infectious HAdV in the 100 mL of sample analysed), showing that lagooning could achieve a reduction of 1.49 log. It is widely accepted that cell culture assays have some limitations, including the bias produced by the efficiency or inability of certain adenovirus genotypes to replicate in specific cell lines. Hence, these data should be taken only as an indication of the risk of infection.
Noroviruses are the leading cause of foodborne disease outbreaks worldwide (Koo et al. 2010), most of those sporadic cases and outbreaks being related to NoVGII (Lopman et al. 2004; Lodder and de Roda Husman 2005; Kroneman et al. 2008). This virus was prevalent in the inlet samples throughout the year and showed a seasonal peak in winter, from December to May, as previously reported (Haramoto et al. 2006; Katayama et al. 2008; Nordgren et al. 2009), with higher viral titres ranging from 104 to 105 GC/100 mL. July was the only month during which NoVGII was not detected. NoVGI was the less prevalent of the two genogroups, but when it was present, its viral titres were higher than those of NoVGII (Table 2). Higher resistance to wastewater treatment was observed for NoVGI compared to NoVGII, as previously reported (Da Silva et al. 2007; Nordgren et al. 2009). Norovirus titres should be a matter of concern, especially considering its low infectious dose of 18 particles (Teunis et al. 2008). The risk of infection through the consumption of raw edible vegetables irrigated with reclaimed water, containing NoV genomic copies, has been recently quantified (Sales-Ortells et al. 2015).
HEV was not found in either the inlet or the outlet samples of the lagooning system, even though it is widely known that this virus circulates in industrialized countries (Legrand-Abravanel et al. 2009; Masclaux et al. 2013). The low millilitre equivalents (8.75 mL) of the samples tested by molecular methods when samples are concentrated by skimmed milk flocculation (Rusiñol et al. 2015) in combination with the lower prevalence of HEV virus compared to other enteric viruses (Masclaux et al. 2013) may explain the absence of positive results.
The viral reduction values reported by Jurzik et al. (2015), in addition to those in the current study, indicate that the use of traditional FIB and bacteriophages as surrogates for predicting the presence or absence of viral pathogens in reclaimed water is not always reliable (Baggi et al. 2001; Hot et al. 2003; Ottoson et al. 2006a). The lack of correlation found between FIB and viral faecal markers at the lagooning outlet reinforces that idea, as previously reported (Mara and Pearson 1987; Donnison and Ross 1995).
A reduction of more than two logs was observed for EC in the lagooning system. This value is slightly superior to the average removal value reported for EC by Goyal (2013). In the case of IE, although similar removal load was observed, this indicator appears to be more resistant to outdoor storage than EC because only two samples were below the detection limit for IE, whereas four of the outlet samples were below the limit for EC. The higher survival capacity of IE compared to EC is well described (Fleischer et al. 2000; Figueras and Borrego 2010), and the results obtained in this study are in agreement with those reported (Tyagi et al. 2008). In Spain, reclaimed water is controlled under the regulation RD 1620/2007, which establishes water uses according to different EC levels. The removal efficiency achieved in the lagooning system in this study was not enough to meet the regulation requirements because on some occasions the concentration of EC was higher than 100 MPN/100 mL which is the maximum allowed by the legislation for raw edible vegetables (RD 1620/2007). Therefore, the produced reclaimed water would not be suitable for some irrigation purposes (e.g., to irrigate raw-edible vegetables). Specifically, the outlet water exceeded the 100 CFU/100 mL recommended for EC in 7 out of 12 of the samples tested. Moreover, three out of seven of the positive outlet water samples had concentration values that were higher than the 1000 CFU/100 mL faecal coliforms. These higher values were observed also in summer during the time of the year when lagooning water is mainly used for irrigation.
Arcobacter which was present at high concentrations (7.51 × 106 MPN/100 mL) at the inlet has been clearly reduced 4 log (p value 6.21 × 10−4). In fact, four out of the seven samples in which Arcobacter was not detected were also negative for the viruses tested. This indicates that lagooning is very effective in removing some potentially pathogenic bacteria. Numerous studies have shown that Arcobacter is abundant in wastewater. No other studies have systematically quantified the presence of Arcobacter in wastewater or its presence in a lagooning system as we have done in this study. In previous studies performed by our group, a correlation was demonstrated between the presence of Arcobacter and the presence of faecal pollution (Collado et al. 2008, 2010; Fisher et al. 2014). However, recently, in a study that used metagenomics, it was shown that the abundance of Arcobacter is due to its growth within the sewer environment and not due to human input. This conclusion was based on the low abundance of these microbes in the faeces of symptomatic and asymptomatic patients with diarrhoea (Figueras et al. 2014; Fisher et al. 2014). Other authors have reported the presence of Arcobacter spp. in sewage in the UK and associated its detection with the underestimation of these bacteria in the human community (Merga et al. 2014). Despite this, underestimation continues to occur, as we have suggested in previous studies (Collado and Figueras 2011; Fisher et al. 2014). These facts alone do not explain the high concentration found in sewage, from which Arcobacter can be isolated by direct plating without any enrichment. This latter finding indicates the growth and amplification capacity of these bacteria in sewage.
The low reduction (0.03 log) observed in Aeromonas during the warm season (June–September) correlated with high temperatures that ranged between 23 and 28 °C (corresponding to the optimum growth temperature for this bacteria) and also with the low retention time of the water in the lagooning system (Table 4). These results agree with those of Monfort and Baleux (1990), who studied the Aeromonas dynamics in a sewage treatment pond and reported a slightly higher reduction in winter (99.8 %) than in summer (98.3 %). These authors found a positive correlation between pond water temperature and Aeromonas concentrations. However, opposite results were reported by (Hassani et al. 1992) in a study performed in Morocco, where the removal efficacy of the stabilization pond treatment used to clear domestic wastewater was higher in the warm months (98.8 %), when temperatures were approximately 30 °C, than in the colder months (97 %), when the temperatures were lower than 21 °C.
Isolating Legionella from complex environmental samples is a well-known arduous job (Joly et al. 2006; Serrano-Suárez and Araujo 2013; Blanky et al. 2015). The difficulties involved in isolating Legionella using culture methods include the low sensitivity of the culture media wherein other bacteria with faster growth rates than Legionella spp. suppress or mask its growth, or the fact that under stressful conditions, Legionella spp. enter into a viable but non-culturable (VBNC) stage (Steinert et al. 1997; Joly et al. 2006; Rodríguez-Martínez et al. 2015). However, the use of molecular techniques, such as qPCR, has enabled the detection of similar Legionella concentrations, as have been described in the literature (Palmer et al. 1993; Medema et al. 2004; Declerck et al. 2007). Legionella was more abundant in the inlet samples than in the outlet samples (Table 2). Nevertheless, a more substantial reduction was observed in winter, when the retention time was longer and temperatures were lower (Table 4). In summer, the lower reduction might be explained by the shorter retention time and higher temperatures, which were closer to the replication temperature of the bacteria. Half of the inlet samples contain L. pneumophila, but it was detected only in two of the outlet samples (data not shown). Hence, according to Spanish legislation (RD 1620/2007), this water can be considered as safe water and used for aerosoled irrigation. However, further studies are needed to improve the methods recommended for the detection of this bacteria in complex water matrices and also its health risk associated to their presence in reclaimed irrigation water.
Free-living amoeba were detected in all of the samples analysed, with no clear trend related to water matrix, retention time, temperature or other physicochemical parameters. The FLAs are a complex and heterogeneous group of microorganisms characterized by having two live stages: trophozoites and cysts. The cysts, which are a resistant and dormant form, enable these species to survive harsh environmental conditions, including the disinfection methods used in conventional WWTP. The presence of FLA in the effluents of WWTPs has been reported in several studies (Garcia et al. 2013; Magnet et al. 2013). The high numbers of trophozoites observed in the inlet and outlet water samples suggest that FLAs play a key role in the lagooning ecosystem. These species increase the presence of some microorganisms rather than others by acting as grazers (Danes and Cerva 1981, 1984; Greub and Raoult 2004; Lorenzo-Morales et al. 2007; Declerck 2010). This fact has promoted adaptative changes in the microorganisms in which they prey on, which must survive amoebal predation. For example, the presence of FLA may explain the presence of Legionella spp. because these bacteria have the ability to replicate within FLA that play a protective role against harsh environmental conditions (Richards et al. 2013; Cervero-Aragó et al. 2015). Recently, a publication reported the internalization of HAdV by ciliates in wastewater (Battistini et al. 2013). As has been previously shown in some bacterial species, viruses may use FLA or other protozoa as a shelter to wastewater treatments (Scheid and Schwarzenberger 2012). In the current study, no significant correlation was found between HAdV and FLA in the outlet samples. Further studies of these species could improve our understanding of viral survival in the environment. Unfortunately, the methodology used in the current study did not enable the identification of the genera of the isolated FLA, but data obtained provides an overview of its presence in two different water matrixes.
No significant regrowth of opportunistic pathogens was observed throughout the lagooning system. The microorganism removal efficiency of other tertiary waste water disinfection methods, such as membrane filtration, has been shown to reduce human noroviruses by less than 1 log and to reduce EC and IE by of 3.23 and 3.17 logs, respectively (Ottoson et al. 2006b). Chlorination after secondary treatment reduced IE by up to 2.57 logs and EC 1.18 logs, whereas HAdV was reduced by 0.81 logs (Francy et al. 2012). UV irradiation (254 nm) reduced EC, IE and human adenoviruses by 3.82, 3.32 and 0.24 logs, respectively, at a genomic copy level (Francy et al. 2012). The application of a more complex wastewater treatment method that is composed of membrane ultrafiltration in combination with chlorination and UV disinfection reduced HAdV (qPCR data), EC and IE by 1.44, 2.12 and 1.84 logs, respectively (Rusiñol et al. 2015). Similar logarithmic removal values of HAdV–at a genomic copy level—EC and IE were obtained in the lagooning in comparison with conventionally tertiary water treatments. However, the high construction costs and maintenance of more complex systems in combination with the difficulty of applying them in low-income developing countries make lagooning a sustainable and effective method of producing reclaimed water for irrigation purposes. Nevertheless, a quality control system should be implemented to ensure that the reclaimed water requirements contained in the legislation are fulfilled.
Conclusions
-
1.
The results obtained in the present study demonstrate the variability of removal efficiency in lagooning systems as previously reported by other authors (Berg 1973; Maynard et al. 1999). The lagooning system evaluated in this study achieved significant logarithmic reductions in the human viral faecal markers HAdV and JCPyV ranging from 1.18 (0.24–2.93) to 0.64 (0.12–1.97), at the genomic copy level respectively. A 2.58 (1.17–4.59) and 1.65 (0.15–3.14) EC and IE log reduction was observed.
-
2.
No regrowth of FIBs was observed in the system, which obtained a reduction of nearly 2 logs between the inlet and outlet samples. However, although FIBs were reduced, in half of the samples analysed, the concentration at the outlet exceeded the recommendations of the Spanish legislation (RD/1620/2007). The absence of FIBs does not guarantee the absence of viruses because some samples that were negative for FIBs presented viral faecal markers. Therefore, the inclusion of viral faecal markers, such as HAdV and JCPyV, in reclaimed water legislation should be considered to minimize risks.
-
3.
Opportunistic pathogens, common inhabitants of water systems as Legionella spp. and Aeromonas spp., showed a pattern of reduction that was different from that of FIBs, while the pattern observed for Arcobacter was more in agreement with that of FIBs. In addition, HBC and FLA, which are microorganisms that are representative of complex heterogeneous groups, showed small reductions throughout the lagooning, and in some occasions, their counts were higher in samples collected at the outlet than in those collected at the inlet, suggesting bacterial and protozoa regrowth. This fact reinforces the existence of two different microbial communities. Both communities are differently influenced by environmental factors such as temperatures above 20 °C, but further studies are necessary to confirm these trends and to obtain a better understanding of the composition of these populations.
-
4.
Data obtained from this study reinforces the idea that more studies on lagooning systems are required for improving its design and management in order to fulfil the safety requirements established in the RD 1620/2007 and ensure the production of safe reclaimed water to irrigate raw edible vegetables.
References
Allard A, Albinsson B, Wadell G (2001) Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J Clin Microbiol 39(2):498–505, Available from: http://jcm.asm.org/content/39/2/498
Baggi F, Demarta A, Peduzzi R (2001) Persistence of viral pathogens and bacteriophages during sewage treatment: lack of correlation with indicator bacteria. Res Microbiol 152(8):743–51, Available from: http://www.ncbi.nlm.nih.gov/pubmed/11686388
Battistini R, Marcucci E, Verani M, Di Giuseppe G, Dini F, Carducci A (2013) Ciliate-adenovirus interactions in experimental co-cultures of Euplotes octocarinatus and in wastewater environment. Eur J Protistol 49(3):381–8, Available from: http://www.sciencedirect.com/science/article/pii/S0932473912000946
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29(4):1165–88
Berg G (1973) Removal of viruses from sewage, effluents, and waters. I. A review. Bull World Health Organ 49(5):451–60, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2480995&tool=pmcentrez&rendertype=abstract
Bernard H, Faber M, Wilking H, Haller S, Höhle M, Schielke A et al (2014) Large multistate outbreak of norovirus gastroenteritis associated with frozen strawberries, Germany, 2012. Euro Surveil 19(8):20719, Available from: http://www.ncbi.nlm.nih.gov/pubmed/24602278
Blanky M, Rodríguez-Martínez S, Halpern M, Friedler E (2015) Legionella pneumophila: from potable water to treated greywater; quantification and removal during treatment. Sci Total Environ 533:557–65, Available from: http://www.ncbi.nlm.nih.gov/pubmed/26188406
Bofill-Mas S, Albinana-Gimenez N, Clemente-Casares P, Hundesa A, Rodriguez-Manzano J, Allard A et al (2006) Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol 72(12):7894–6, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1694247&tool=pmcentrez&rendertype=abstract
Bofill-Mas S, Rusiñol M, Fernandez-Cassi X, Carratalà A, Hundesa A, Girones R (2013) Quantification of human and animal viruses to differentiate the origin of the fecal contamination present in environmental samples. Biomed Res Int 2013:192089, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3671278&tool=pmcentrez&rendertype=abstract
Calgua B, Mengewein A, Grunert A, Bofill-Mas S, Clemente-Casares P, Hundesa A et al (2008) Development and application of a one-step low cost procedure to concentrate viruses from seawater samples. J Virol Methods 153(2):79–83, Available from: http://www.ncbi.nlm.nih.gov/pubmed/18765255
Calgua B, Rodriguez-Manzano J, Hundesa A, Suñen E, Calvo M, Bofill-Mas S et al (2013) New methods for the concentration of viruses from urban sewage using quantitative PCR. J Virol Methods 187(2):215–21, Available from: http://www.sciencedirect.com/science/article/pii/S0166093412003710
Callejón RM, Rodríguez-Naranjo MI, Ubeda C, Hornedo-Ortega R, Garcia-Parrilla MC, Troncoso AM (2015) Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathog Dis 12(1):32–8, Available from: http://www.ncbi.nlm.nih.gov/pubmed/25587926
Campos C, Guerrero A, Cárdenas M (2002) Removal of bacterial and viral faecal indicator organisms in a waste stabilization pond system in Choconta, Cundinamarca (Colombia). Water Sci Technol 45(1):61–6, Available from: http://www.ncbi.nlm.nih.gov/pubmed/11833732
Carter MJ (2005) Enterically infecting viruses: pathogenicity, transmission and significance for food and waterborne infection. J Appl Microbiol 98(6):1354–80, Available from: http://www.ncbi.nlm.nih.gov/pubmed/15916649
Cervero-Aragó S, Rodríguez-Martínez S, Canals O, Salvadó H, Araujo RM (2013) Effect of thermal treatment on free-living amoeba inactivation. J Appl Microbiol. Oct 30 [cited 2014 Dec 28]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24251398
Cervero-Aragó S, Rodríguez-Martínez S, Puertas-Bennasar A, Araujo RM (2015) Effect of common drinking water disinfectants, chlorine and heat, on free legionella and Amoebae-associated Legionella. PLoS One 10(8):e0134726, Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0134726
Chacón MR, Castro-Escarpulli G, Soler L, Guarro J, Figueras MJ (2002) A DNA probe specific for Aeromonas colonies. Diagn Microbiol Infect Dis 44(3):221–5, Available from: http://www.ncbi.nlm.nih.gov/pubmed/12493167
Collado L, Figueras MJ (2011) Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 24(1):174–92, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3021208&tool=pmcentrez&rendertype=abstract
Collado L, Inza I, Guarro J, Figueras MJ (2008) Presence of Arcobacter spp. in environmental waters correlates with high levels of fecal pollution. Environ Microbiol 10(6):1635–40, Available from: http://www.ncbi.nlm.nih.gov/pubmed/18215159
Collado L, Kasimir G, Perez U, Bosch A, Pinto R, Saucedo G et al (2010) Occurrence and diversity of Arcobacter spp. along the Llobregat River catchment, at sewage effluents and in a drinking water treatment plant. Water Res 44(12):3696–702, Available from: http://www.ncbi.nlm.nih.gov/pubmed/20427071
R Core Team (2013) R: A language and environment for statistical computing. [Internet]. Available from: http://www.r-project.org/
Da Silva AK, Le Saux JC, Parnaudeau S, Pommepuy M, Elimelech M, Le Guyader FS (2007) Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl Environ Microbiol 73(24):7891–7
Danes L, Cerva L (1981) Survival of polioviruses and echoviruses in Acanthamoeba castellanii cultivated in vitro. J Hyg Epidemiol Microbiol Immunol 25(2):169–74, Available from: http://www.ncbi.nlm.nih.gov/pubmed/6265550
Danes L, Cerva L (1984) Poliovirus and echovirus survival in Tetrahymena pyriformis culture in vivo. J Hyg Epidemiol Microbiol Immunol 28(2):193–200, Available from: http://www.ncbi.nlm.nih.gov/pubmed/6088623
Dare A (2015) Irrigation with treated municipal wastewater in Indiana, United States. J Soil Water Conserv 70(4):89A–94A, Available from: http://www.jswconline.org/content/70/4/89A.extract
Declerck P (2010) Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol 12(3):557–66, Available from: http://www.ncbi.nlm.nih.gov/pubmed/19678829
Declerck P, Behets J, van Hoef V, Ollevier F (2007) Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res 41(14):3159–67, Available from: http://www.ncbi.nlm.nih.gov/pubmed/17544473
Derry C, Attwater R (2014) Regrowth of enterococci indicator in an open recycled-water impoundment. Sci Total Environ 468-469:63–7, Available from: http://www.ncbi.nlm.nih.gov/pubmed/24008073
Diederen BMW, de Jong CMA, Marmouk F, Kluytmans JAJW, Peeters MF, Van der Zee A (2007) Evaluation of real-time PCR for the early detection of Legionella pneumophila DNA in serum samples. J Med Microbiol 56(Pt 1):94–101, Available from: http://www.ncbi.nlm.nih.gov/pubmed/17172523
Donnison AM, Ross CM (1995) Somatic and F-specific coliphages in New Zealand waste treatment lagoons. Water Res 29(4):1105–10, Available from: http://www.sciencedirect.com/science/article/pii/004313549400227X
Erker JC, Desai SM, Schlauder GG, Dawson GJ, Mushahwar IK (1999) A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J Gen Virol 80(3):681–90, Available from: http://www.ncbi.nlm.nih.gov/pubmed/10092008
Figueras MJ, Borrego JJ (2010) New perspectives in monitoring drinking water microbial quality. Int J Environ Res Public Health 7(12):4179–202, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3037048&tool=pmcentrez&rendertype=abstract
Figueras MJ, Levican A, Collado L (2012) Updated 16S rRNA-RFLP method for the identification of all currently characterised Arcobacter spp. BMC Microbiol 12:292, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3548738&tool=pmcentrez&rendertype=abstract
Figueras MJ, Levican A, Pujol I, Ballester F, Rabada Quilez MJ, Gomez-Bertomeu F (2014) A severe case of persistent diarrhoea associated with Arcobacter cryaerophilus but attributed to Campylobacter sp. and a review of the clinical incidence of Arcobacter spp. New Microbes New Infect 2(2):31–7, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4184587&tool=pmcentrez&rendertype=abstract
Fisher JC, Levican A, Figueras MJ, McLellan SL (2014) Population dynamics and ecology of Arcobacter in sewage. Front Microbiol 5:525, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4224126&tool=pmcentrez&rendertype=abstract
Fleischer J, Schlafmann K, Otchwemah R, Botzenhart K (2000) Elimination of enteroviruses, other enteric viruses, F-specific coliphages, somatic coliphages and E. coli in four sewage treatment plants of southern Germany. J Water Supply Res Technol AQUA 49(3):127–38, Available from: http://aqua.iwaponline.com/content/49/3/127.abstract
Francy DS, Stelzer EA, Bushon RN, Brady AMG, Williston AG, Riddell KR et al (2012) Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res 46(13):4164–78, Available from: http://www.ncbi.nlm.nih.gov/pubmed/22682268
FSA (2006) Review of the analytical terminology for codex use in the procedural manual. FOOD Stand AGENCY Inf Bull METHODS Anal Sampl Foodst 68(March 2006):1–15
Garcia A, Goñi P, Cieloszyk J, Fernandez MT, Calvo-Beguería L, Rubio E et al (2013) Identification of free-living amoebae and amoeba-associated bacteria from reservoirs and water treatment plants by molecular techniques. Environ Sci Technol 47(7):3132–40
Gerba CP, Goyal SM, LaBelle RL, Cech I, Bodgan GF (1979) Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am J Public Health 69(11):1116–9, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1619291&tool=pmcentrez&rendertype=abstract
Goyal B (2013) Case study on evaluation of the performance of waste stabilization pond system of Jodhpur, Rajasthan, India. Water Pract Technol 8(1):95–104, Available from: http://wpt.iwaponline.com/content/8/1/wpt.2013011.abstract
Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17(2):413–33
Gurría A (2012) OECD environmental outlook to 2050: the consequences of inaction - key facts and figures [Internet]. Eur. World. [cited 2015 Nov 20]. Available from: http://www.oecd.org/env/indicators-modelling-outlooks/oecdenvironmentaloutlookto2050theconsequencesofinaction-keyfactsandfigures.htm
Haramoto E, Katayama H, Oguma K, Yamashita H, Tajima A, Nakajima H et al (2006) Seasonal profiles of human noroviruses and indicator bacteria in a wastewater treatment plant in Tokyo, Japan. Water Sci Technol 54(11-12):301–8, Available from: http://www.ncbi.nlm.nih.gov/pubmed/17302333
Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR et al (2005) Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl Environ Microbiol 71(6):3163–70, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1151840&tool=pmcentrez&rendertype=abstract
Hassani L, Imziln B, Boussaid A, Gauthier MJ (1992) Seasonal incidence of and antibiotic resistance among Aeromonas species isolated from domestic wastewater before and after treatment in stabilization ponds. Microb Ecol 23(3):227–37, Available from: http://www.ncbi.nlm.nih.gov/pubmed/24192933
Hernroth BE, Conden-Hansson A-C, Rehnstam-Holm A-S, et al (2002) Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: the first Scandinavian report. Appl Environ Microbiol 68:4523–4533
Herpers BL, de Jongh BM, van der Zwaluw K, van Hannen EJ (2003) Real-time PCR assay targets the 23S-5S spacer for direct detection and differentiation of Legionella spp. and Legionella pneumophila. J Clin Microbiol 41(10):4815–6, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=254366&tool=pmcentrez&rendertype=abstract
Hoekstra AY, Mekonnen MM, Chapagain AK, Mathews RE, Richter BD (2012) Global monthly water scarcity: blue water footprints versus blue water availability. Añel JA, editor. PLoS One 7(2):e32688, Available from: http://dx.plos.org/10.1371/journal.pone.0032688
Hot D, Legeay O, Jacques J, Gantzer C, Caudrelier Y, Guyard K et al (2003) Detection of somatic phages, infectious enteroviruses and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water Res 37(19):4703–10, Available from: http://www.ncbi.nlm.nih.gov/pubmed/14568057
International Organization for Standardization (1998a) ISO 7899-1:1998 water quality—detection and enumeration of intestinal enterococci—part 1: miniaturized method (Most Probable Number) for surface and waste water
International Organization for Standardization (1998b) ISO 11731:1998. Water quality -- detection and enumeration of Legionella
International Organization for Standardization (1999) ISO 6222:1999 water quality -- enumeration of culturable micro-organisms -- colony count by inoculation in a nutrient agar culture medium
International Organization for Standardization (2005) ISO 8199:2005. Water quality—general guidance on the enumeration of micro-organisms by culture
International Organization for Standardization (2012) ISO 9308-2:2012 water quality—enumeration of Escherichia coli and coliform bacteria—part 2: most probable number method
Jiang SC (2006) Human adenoviruses in water: occurrence and health implications: a critical review †. Environ Sci Technol 40(23):7132–40, Available from: http://dx.doi.org/10.1021/es060892o
Jjemba PK, Weinrich LA, Cheng W, Giraldo E, Lechevallier MW (2010) Regrowth of potential opportunistic pathogens and algae in reclaimed-water distribution systems. Appl Environ Microbiol 76(13):4169–78, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2897422&tool=pmcentrez&rendertype=abstract
Joly P, Falconnet P-A, André J, Weill N, Reyrolle M, Vandenesch F et al (2006) Quantitative real-time Legionella PCR for environmental water samples: data interpretation. Appl Environ Microbiol 72(4):2801–8, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1449029&tool=pmcentrez&rendertype=abstract
Jurzik L, Hamza IA, Wilhelm M (2015) Investigating the reduction of human adenovirus (HAdV) and human polyomavirus (HPyV) in a sewage treatment plant with a polishing pond as a tertiary treatment. Water Air Soil Pollut 226(9):284, Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11270-015-2545-9
Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB et al (2003) Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol 41(4):1548–57, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=153860&tool=pmcentrez&rendertype=abstract
Katayama H, Haramoto E, Oguma K, Yamashita H, Tajima A, Nakajima H et al (2008) One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res 42(6-7):1441–8, Available from: http://www.ncbi.nlm.nih.gov/pubmed/17996920
Koo HL, Ajami N, Atmar RL, DuPont HL (2010) Noroviruses: the leading cause of gastroenteritis worldwide. Discov Med 10(50):61–70, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3150746&tool=pmcentrez&rendertype=abstract
Kozak GK, MacDonald D, Landry L, Farber JM (2013) Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J Food Prot 76(1):173–83, Available from: http://www.ncbi.nlm.nih.gov/pubmed/23317873
Kroneman A, Verhoef L, Harris J, Vennema H, Duizer E, van Duynhoven Y et al (2008) Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J Clin Microbiol 46(9):2959–65, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2546741&tool=pmcentrez&rendertype=abstract
Legrand-Abravanel F, Mansuy J-M, Dubois M, Kamar N, Peron J-M, Rostaing L et al (2009) Hepatitis E virus genotype 3 diversity, France. Emerg Infect Dis 15(1):110–4, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2660688&tool=pmcentrez&rendertype=abstract
Lipp EK, Farrah SA, Rose JB (2001) Assessment and impact of microbial fecal pollution and human enteric pathogens in a coastal community. Mar Pollut Bull 42(4):286–93, Available from: http://www.ncbi.nlm.nih.gov/pubmed/11381749
Lodder WJ, de Roda Husman AM (2005) Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl Environ Microbiol 71(3):1453–61, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1065170&tool=pmcentrez&rendertype=abstract
Loisy F, Atmar RL, Guillon P, Le Cann P, Pommepuy M, Le Guyader FS (2005) Real-time RT-PCR for norovirus screening in shellfish. J Virol Methods 123(1):1–7, Available from: http://www.ncbi.nlm.nih.gov/pubmed/15582692
Lopman B, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A et al (2004) Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet (Lond Engl) 363(9410):682–8, Available from: http://www.ncbi.nlm.nih.gov/pubmed/15001325
Lorenzo-Morales J, Coronado-Alvarez N, Martínez-Carretero E, Maciver SK, Valladares B (2007) Detection of four adenovirus serotypes within water-isolated strains of Acanthamoeba in the Canary Islands, Spain. Am J Trop Med Hyg 77(4):753–6, Available from: http://www.ajtmh.org/content/77/4/753.abstract
Magnet a, Fenoy S, Galván a L, Izquierdo F, Rueda C, Fernandez Vadillo C et al (2013) A year long study of the presence of free living amoeba in Spain. Water Res 47(19):6966–72
Mara DD, Pearson HW (1987) Waste stabilization ponds: design manual for mediterranean Europe [Internet]. [cited 2015 Jul 19]. Available from: https://books.google.es/books/about/Waste_Stabilization_Ponds.html?id=D_8nAQAAMAAJ&pgis=1
MARA DD, MILLS SW, PEARSON HW, ALABASTER GP (1992) Waste stabilization ponds: a viable alternative for small community treatment systems. Water Environ J 6(3):72–8, Available from: http://doi.wiley.com/10.1111/j.1747-6593.1992.tb00740.x
Marzouk Y (1980) Relationship of viruses and indicator bacteria in water and wastewater of Israel. Water Res 14(11):1585–90, Available from: http://www.sciencedirect.com/science/article/pii/0043135480900627
Masclaux FG, Hotz P, Friedli D, Savova-Bianchi D, Oppliger A (2013) High occurrence of hepatitis E virus in samples from wastewater treatment plants in Switzerland and comparison with other enteric viruses. Water Res 47(14):5101–9, Available from: http://www.sciencedirect.com/science/article/pii/S0043135413004806
Maunula L, Kaupke A, Vasickova P, Söderberg K, Kozyra I, Lazic S et al (2013) Tracing enteric viruses in the European berry fruit supply chain. Int J Food Microbiol 167(2):177–85, Available from: http://www.ncbi.nlm.nih.gov/pubmed/24135674
Maynard HE, Ouki SK, Williams SC (1999) Tertiary lagoons: a review of removal mecnisms and performance. Water Res 33(1):1–13, Available from: http://www.ingentaconnect.com/content/els/00431354/1999/00000033/00000001/art00198
Medema G, Wullings B, Roeleveld P, van der Kooij D (2004) Risk assessment of Legionella and enteric pathogens in sewage treatment works. Water Sci Technol Water Supply [Internet]. [cited 2015 Nov 18];125–32. Available from: http://bvsalud.org/portal/resource/esSiqueira/rep-18894
Merga JY, Royden A, Pandey AK, Williams NJ (2014) Arcobacter spp. isolated from untreated domestic effluent. Lett Appl Microbiol 59(1):122–6, Available from: http://www.ncbi.nlm.nih.gov/pubmed/24666283
Monfort P, Baleux B (1990) Dynamics of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae in a sewage treatment pond. Appl Environ Microbiol 56(7):1999–2006, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=184551&tool=pmcentrez&rendertype=abstract
Niquette P, Servais P, Savoir R (2001) Bacterial Dynamics in the drinking water distribution system of Brussels. Water Res 35(3):675–82, Available from: http://www.sciencedirect.com/science/article/pii/S0043135400003031
Nordgren J, Matussek A, Mattsson A, Svensson L, Lindgren P-E (2009) Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res 43(4):1117–25, Available from: http://www.sciencedirect.com/science/article/pii/S0043135408005940
Ogorzaly L, Cauchie H-M, Penny C, Perrin A, Gantzer C, Bertrand I (2013) Two-day detection of infectious enteric and non-enteric adenoviruses by improved ICC-qPCR. Appl Microbiol Biotechnol 97(9):4159–66, Available from: http://www.ncbi.nlm.nih.gov/pubmed/23558583
Oki T, Kanae S (2006) Global hydrological cycles and world water resources. Science 313(5790):1068–72, Available from: http://www.sciencemag.org/content/313/5790/1068
Oragui JI, Curtis TP, Silva SA, Mara DD (2011) The removal of excreted bacteria and viruses in deep waste stabilization ponds in Northeast Brazil. IWA Publishing. Feb 16 [cited 2014 Dec 30]; Available from: http://www.iwaponline.com/wst/01810/wst018100031.htm
Ottoson J, Hansen A, Björlenius B, Norder H, Stenström TA (2006a) Removal of viruses, parasitic protozoa and microbial indicators in conventional and membrane processes in a wastewater pilot plant. Water Res 40(7):1449–57, Available from: http://www.sciencedirect.com/science/article/pii/S0043135406000789
Ottoson J, Hansen A, Björlenius B, Norder H, Stenström TA (2006b) Removal of viruses, parasitic protozoa and microbial indicators in conventional and membrane processes in a wastewater pilot plant. Water Res 40(7):1449–57, Available from: http://www.ncbi.nlm.nih.gov/pubmed/16533517
Pal A, Sirota L, Maudru T, et al (2006) Real-time, quantitative PCR assays for the detection of virus-specific DNA in samples with mixed populations of polyomaviruses. J Virol Methods 135:32–42. doi:10.1016/j.jviromet.2006.01.018
Palmer CJ, Tsai YL, Paszko-Kolva C, Mayer C, Sangermano LR (1993) Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent-antibody, and plate culture methods. Appl Environ Microbiol 59(11):3618–24, Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=182507&tool=pmcentrez&rendertype=abstract
Pay Drechsel CAS, Liqa Raschid-Sally MR, Bahri A, Wastewater Irrigation and Health (2010) Assessing and mitigating risk in low-income countries. Dunstan house, London
Peña M, Rodriguéz J, Mara DD, Sepulveda M (2000) UASBs or anaerobic ponds in warm climates? a preliminary answer from Colombia. Water Sci Technol [Internet]. [cited 2015 Nov 18];59–65. Available from: http://www.bvsde.paho.org/bvsacd/leeds/uasb.pdf
Pusch D, Oh D-Y, Wolf S, Dumke R, Schröter-Bobsin U, Höhne M et al (2005) Detection of enteric viruses and bacterial indicators in German environmental waters. Arch Virol 150(5):929–47, Available from: http://www.ncbi.nlm.nih.gov/pubmed/15645371
Richards AM, Von Dwingelo JE, Price CT, Abu Kwaik Y (2013) Cellular microbiology and molecular ecology of Legionella-amoeba interaction. Virulence 4(4):307–14, Available from: http://www.tandfonline.com/doi/full/10.4161/viru.24290
Riera-Montes M, Brus Sjölander K, Allestam G, Hallin E, Hedlund K-O, Löfdahl M (2011) Waterborne norovirus outbreak in a municipal drinking-water supply in Sweden. Epidemiol Infect 139(12):1928–35, Available from: http://www.ncbi.nlm.nih.gov/pubmed/21251349
Rodríguez-Martínez S, Blanky M, Friedler E, Halpern M (2015) Legionella spp. isolation and quantification from greywater. MethodsX [Internet]. Nov [cited 2015 Nov 21]; Available from: http://www.sciencedirect.com/science/article/pii/S221501611500059X
Rusiñol M, Fernandez-Cassi X, Timoneda N, Carratalà A, Abril JF, Silvera C et al (2015) Evidence of viral dissemination and seasonality in a Mediterranean river catchment: implications for water pollution management. J Environ Manage 159:58–67, Available from: http://www.ncbi.nlm.nih.gov/pubmed/26046988
Sales-Ortells H, Fernandez-Cassi X, Timoneda N, Dürig W, Girones R, Medema G (2015) Health risks derived from consumption of lettuces irrigated with tertiary effluent containing norovirus. Food Res Int 68:70–7, Available from: http://www.sciencedirect.com/science/article/pii/S0963996914005560
Scheid P, Schwarzenberger R (2012) Acanthamoeba spp. as vehicle and reservoir of adenoviruses. Parasitol Res 111(1):479–85, Available from: http://www.ncbi.nlm.nih.gov/pubmed/22290448
Serrano-Suárez A, Araujo R (2013) Evaluation of Legionella presence in the water system of a public building by semi-nested polymerase chain reaction as a rapid screening method complementary to plate count. Water Sci Technol Water Supply 13(6):1560, Available from: http://www.iwaponline.com/ws/01306/ws013061560.htm
Serrano-Suárez A, Dellundé J, Salvadó H, Cervero-Aragó S, Méndez J, Canals O et al (2013) Microbial and physicochemical parameters associated with Legionella contamination in hot water recirculation systems. Environ Sci Pollut Res Int 20(8):5534–44, Available from: http://www.ncbi.nlm.nih.gov/pubmed/23436060
Shuval HI (1990) Summary—wastewater irrigation in developing countries—health effects and technical solutions. UNDP World Bank Water Sanit Progr 51:74
Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV (2004) Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J Food Prot 67(10):2342–53, Available from: http://www.ncbi.nlm.nih.gov/pubmed/15508656
Steinert M, Emödy L, Amann R, Hacker J (1997) Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol 63(5):2047–53
Teunis PFM, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS et al (2008) Norwalk virus: how infectious is it? J Med Virol 80(8):1468–76, Available from: http://www.ncbi.nlm.nih.gov/pubmed/18551613
Tyagi VK, Kazmi AA, Chopra AK (2008) Removal of fecal indicators and pathogens in a waste stabilization pond system treating municipal wastewater in India. Water Environ Res 80(11):2111–7, Available from: http://www.ingentaconnect.com/content/wef/wer/2008/00000080/00000011/art00004
Wheaton TA, Castle WS (2001) High application rates of reclaimed water benefit citrus tree growth and fruit production. HortSci 36:1273–7, Available from: http://www.mendeley.com/catalog/high-application-rates-reclaimed-water-benefit-citrus-tree-growth-fruit-production/
Acknowledgments
The study reported here was partially founded by programa Recercaixa 2012 ACUP-00300 and AGL2011-30461-C02-01/ALI of the Spanish Ministry of Science and Innovation and Consolidated Research Groups Program of the Catalan Government (2014SGR529 and VirBaP 2014SRG914). Xavier Fernandez-Cassi and Fadua Latif-Eugenín acknowledge the AGAUR Agency of the Catalan Government and the Universitat Rovira i Virgili, respectively, which supported their PhD grants. The authors would like to thank the lagooning system manager, Conrad Martorell, for his collaboration during this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 6 kb)
Rights and permissions
About this article
Cite this article
Fernandez-Cassi, X., Silvera, C., Cervero-Aragó, S. et al. Evaluation of the microbiological quality of reclaimed water produced from a lagooning system. Environ Sci Pollut Res 23, 16816–16833 (2016). https://doi.org/10.1007/s11356-016-6812-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6812-0