Abstract
A spatiotemporal evaluation of butyltin contamination was performed between 2010 and 2012 along Todos os Santos Bay (Northeast Brazil) using surface sediments, bivalve tissues (Anomalocardia brasiliana and Mytella guyanensis), and imposex occurrence (Stramonita rustica). The spatial study detected high tributyltin (TBT) levels (maximum values of 262 ng Sn g -1 - 21,833 ng Sn g−1 of total organic carbon - for surface sediments and 421 ng Sn g−1 for bivalve tissues) in the innermost part of the bay. The TBT levels detected in M. guyanensis tissues might cause human health risk since local population consumes these organisms. These high concentrations observed in the bivalves might result in ingestions higher than the safe limits established by European Food Safety Authority (250 ng TBT kg−1 day−1). Considering the temporal evaluation, no difference (p > 0.05) was observed between TBT concentrations in sediments obtained during the two sampling campaigns (2010/2011 and 2012). However, the increasing predominance of TBT metabolites (butyltin degradation index (BDI) >1) in more recent sediments indicates further degradation of old TBT inputs. In spite of that, recent inputs are still evident at this region. Nevertheless, a reduction of imposex parameters in S. rustica over the last decade suggests an overall decline in the TBT contamination, at least in the outermost and possible less impacted region of the bay. The TBT contamination is probably reducing due to the national and international legislative restrictions on the use of TBT as antifouling biocide. The contamination levels, however, are still relevant especially in the inner part of Todos os Santos Bay since they are above those that are likely to cause toxicity to the biota.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tributyltin (TBT) compounds were used as biocides in antifouling paints for more than five decades being considered a priority contaminant due to their proved high lipophilicity, persistence (in anoxic sediments), and toxicity (Hoch 2001). Since the antifouling systems are needed to protect ship hulls and offshore facilities against biofouling (Kotrikla 2009), the biocides used in these coating formulations are slowly released into the aquatic system causing deleterious effects to the biota (Yebra et al. 2004). TBT is considered an endocrine and metabolic disrupter to the biota, including humans, even at low environmental concentrations (Cao et al. 2009; Horiguchi 2009; Meador et al. 2011; Graceli et al. 2013). Consequently, the imposex incidence in prosobranch gastropods (the most well-known TBT biological effect) has been widely used as biomarker of TBT exposure, which is directly associated with coastal areas under the influence of maritime activities such a harbors, marinas, and shipyards (Matthiessen and Gibbs 1998; Paz-Villarraga et al. 2015).
Due to the deleterious effects associated to butyltin contamination, several local regulations (Gipperth 2009) and the convention on the control of harmful antifouling systems on ships (AFS Convention) of the International Maritime Organization (IMO 2008) have been banning TBT-based antifouling paints since its entry into force in September 2008. As a consequence, the environmental levels of TBT as well as imposex incidence have begun to decline in many areas worldwide (Oliveira et al. 2009; Galante-Oliveira et al. 2011; Guomundsdóttir et al. 2011). In spite of that, due to its persistence (mainly under anoxic conditions) and, in some cases, its illegal use, TBT can still be considered as a priority contaminant in some areas under the influence of harbors, marinas, and shipyards (Castro et al. 2012). Additionally, despite its banishment, the TBT levels recently detected in environmental samples all over the world are in the same or above those concentration ranges that can cause harmful effects to biota (Abidli et al. 2012; Azevedo et al. 2012; Grimón et al. 2016).
Within South America, several environmental investigations about butyltin (BT) contamination and imposex occurrence were performed during the last years. However, most of these studies have only appraised their spatial distribution and potential sources to the environment (Bigatti et al. 2009; Pinochet et al. 2009; Santos et al. 2009; Toste et al. 2011; Azevedo et al. 2012; Castro et al. 2012; Castro and Fillmann 2012), while only Castro et al. (2012) have analyzed the temporal trends of TBT contamination. Temporal studies are crucial to assess the effectiveness of national or international restrictions over time, although most of the South American countries have no regulations against TBT-based antifouling paints nor are signatories of the AFS Convention (IMO 2008). In fact, among South American countries, Brazil and Argentina are the only where legal restrictions on the use of TBT have been applied; moreover, Brazil is the only signatory of the AFS Convention in this extensive geographical area. In Brazil, threshold levels were established for dreading sediments (CONAMA 454, 2012) and water column (CONAMA 357, 2005), and the use of TBT-based antifouling paints is prohibited by 2007 by the National Maritime Authority in all vessels >5 m in length, floating platforms, and submerged structures (subject to registration with the maritime authority and likely to be transported in the water) (NORMAM 2007). Thus, the effectiveness of such measures in reducing environmental pollution by TBT, which is almost unknown, and the implementation of additional specific regulations are still needed in this region.
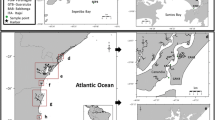
Todos os Santos Bay (TSB) (Northeastern Brazil, 12° 50′ S) is the second largest bay of the Brazilian coast (1233 km2), sheltering an important petrochemical and industrial complex, commercial and fishing harbors, shipyards, and several marinas, being surrounded by more than three million inhabitants (Hatje and Andrade 2009). Therefore, TSB is an area highly susceptible to the impact produced by the use of antifouling paints. In fact, the TBT contamination was already detected in some areas of TSB (Castro et al. 2007; Felizzola et al. 2008; Pletsch et al. 2010). Thus, the present study evaluated BT contamination spatially using two bivalve species (Mytella guyanensis and Anomalocardia brasiliana) and spatiotemporally using surface sediments (2010/2011–2012) and imposex incidence (2004–2012) in the gastropod Stramonita rustica. Additionally, the risk evaluation by consumption of these bivalves by the local population was also assessed.
Material and methods
Sampling
Surface sediments (≤2 cm) were taken between February 2010 and April 2011 (campaign 1) and June 2012 (campaign 2) (Fig. 1) using a “Van-veen” dredge, while 20 adult bivalves from the species M. guyanensis and A. brasiliana were only sampled during campaign 1. To assess imposex, up to 30 adult specimens of S. rustica were caught (either manually or by snorkeling) in the intertidal zones of the TSB during campaign 2. Gastropods were sampled in the same sites studied during 2004 by Castro et al. (2007). The sampling sites encoded by “S” were used in the spatial evaluation, while by “T” were somehow sampled for a second time to be used in the temporal study.
Sediment characterization
Total organic carbon (% TOC) was determined in sediment samples by an elementary analyzer (CHNS Perkin Elmer 2400 Series II) after decarbonation in a desiccator containing HCl (37 %) (Culmo et al. 2003). Sediment granulometry was determined as sand and fine (clay + silt) fractions by sieving dried samples as described by Gray (1981). Results were expressed as fine percentage (%).
Sample preparation and butyltin analysis
Butyltin (TBT, dibutyltin (DBT), and monobutyltin (MBT)) levels were analyzed according Castro et al. (2012). Briefly, 5 g of freeze-dried sediments or 1 g of freeze-dried bivalve integral tissues (pool of 20 organisms) were placed into 40-mL vials. These samples were spiked with 100 ng of tripropyltin (TPrT) as surrogate standard. After 30 min of equilibration, 15 mL of tropolone solution (0.05 % w/v) in methanol and 1 mL of concentrated HCl (37 %) were added. The vials were ultra-sonicated for 15 min and centrifuged at 3250 rpm for 10 min. This procedure was repeated three times. The supernatants were transferred to a 250-mL separatory funnel filled with 150 mL of a 10 % NaCl solution and extracted with 3 × 20 mL of dichloromethane. The extracts were collected through anhydrous sodium sulfate, added to 5 mL of hexane, and blown down to approximately 2 mL using a Syncore system. Extracts were then derivatized by adding 2 mL of n-pentyl magnesium bromide in diethyl ether solution (2 M), according to Morabito et al. (2000). After derivatization, the pentylatedbutyltins were extracted with 3 × 5 mL of hexane and evaporated down to 0.5 mL with N2 flow. A cleanup was then performed using a silica column (3.5 g). Analytes were eluted with 15 mL of hexane/toluene (1:1). Finally, the solution was concentrated down to 0.9 mL (N2 flow), and 100 μL of tetrabutyltin solution (1000 ng Sn mL−1) was added as internal standard. Extracts were analyzed by gas chromatography using a Perkin Elmer Clarus 500MS equipped with mass spectrometer detector, split/splitless injector, and auto sampler. An Elite-5MS (5 % diphenyl/95 % dimethyl polysiloxane) capillary column (30 m × 0.25 mm I.D., 0.25 μm film thickness) was used. The method of standard additions was used to eliminate matrix effects. The quality assurance and quality control were based on regular analyses of blanks, spiked matrices, and certified reference material for sediments (PACS-2/National Research Council of Canada, Ottawa, Canada) and mussel tissue (ERM-CE477). Results obtained for the PACS-2 (TBT - 798 ± 23, DBT - 1104 ± 12, and MBT - 670 ± 20 ng Sn g−1) and ERM-CE477 (TBT - 1858 ± 173, DBT - 1412 ± 132, and MBT - 1387 ± 193 ng Sn g−1) were in good agreement with the PACS-2 (TBT - 890 ± 105, DBT - 1047 ± 64, and MBT - 600 ng Sn g−1) and ERM-CE477 (TBT - 2200 ± 190, DBT - 1540 ± 120, and MBT - 1500 ± 280 ng Sn g−1) certified values. The sample recoveries were between 88.5 and 109 % and relative standard deviation (RSD) below 20 % (IUPAC 2002). The limits of quantification (LOQs) for sediment samples were 2, 2, and 3 ng Sn g−1 for TBT, DBT, and MBT, respectively, and 5, 5, and 7 ng Sn g−1, respectively, for biota.
Butyltin degradation index
Butyltin degradation index (BDI) was calculated based on the following equation: BDI = ([MBT] + [DBT]) / [TBT]. Recent TBT inputs are normally associated to values of BDI < 1 (Díez et al. 2002).
Imposex determination
The organisms were previously narcotized with a 3.5 % MgCl2 solution for 2 h. Shell lengths (SLs) were then measured with a digital caliper (0.01 mm) from the spiral top to lip of the siphonal canal. Posteriorly, shells were removed for the analysis of the soft tissues. Gender identification was based on the presence or absence of sexual accessory glands (albumen, capsule, and seminal receptacle). The penis length (PL) and the presence of vas deferens in females and males were also registered.
Imposex levels were assessed using the following indices: percentage of imposex-affected females (% I), female penis length index (FPL = mean penis length of all females in the population, including the zero values), and relative penis length index [RPLI = (mean penis length in females / mean penis length in males) × 100] (Castro et al. 2004). The vas deferens sequence index (VDSI), based on the development of male sexual characters (particularly the vas deferens) by females, was evaluated according to Toste et al. (2013). The VDSI stages proposed by Toste et al. (2013) are similar to the index developed by Gibbs et al. (1987) for Nucella lapillus and globally used for imposex assessments in several gastropod species. However, the applied scale allows assessments of aphallic imposex not originally foreseen by Gibbs et al. (1987). Although this VDSI scale had been originally developed for imposex assessments using Stramonita haemastoma, it can be appropriately used in the present study (with S. rustica) due to phylogenetic similarities between both species (Castro et al. 2012).
Statistical analysis
Normality and homogeneity of data (biometric parameters, imposex indices, and BT concentrations) were verified using Shapiro-Wilk and Levene tests, respectively. Spearman non-parametric correlation analysis was used to investigate the relationship between sediment parameters (% TOC vs % fine) and TBT concentrations (sediments vs mussel tissues). A paired t test was performed to evaluate statistical differences in BDI values obtained between the two sampling campaigns. The imposex parameters (RPLI, FPLI, and VDSI) reported for 2004 by Castro et al. (2007) were compared with imposex data obtained in same sites during the campaign 2 (2012) using Mann-Whitney U test. All statistical analyses were performed using Statistica® (version 12.0 (Statsoft)) with a significance level of 0.05.
Results and discussion
Total organic carbon and granulometry
The concentration of total organic carbon (% TOC) in surface sediments collected inside TSB ranged from 0.4 % (S6—Madre de Deus harbor and S14—Ribeira Bay) to 5.0 % (S21—east coast of the island Itaparica, see Fig. 1) for samples collected during the campaign 1 (2010/2011) and from 0.4 % (T8—Rio do Cunha) to 2.7 % (T13—southwest of Aratu island) during the campaign 2 (2012) (Table 1). Similarly, the granulometry has also presented a large variation among the studied sites (1.1 to 98 % of fines) showing a significant correlation with % TOC (r 2 = 0.65, p < 0.05). Similar behavior has been normally detected in sediment samples since organic matter tends to concentrate mostly in the fine fraction (Gray 1981). In addition, organic fraction of sediments is an important parameter to estimate the sorption capacity of organic contaminants due to inherent physicochemical sediment complexity, such as different composition of organic matter, relative absorbability onto inorganic particles, and the existence of biological activity in the sediment layers (Town and Filella 2002). Thus, the occurrence of large differences in % TOC from sediment samples used in environmental monitoring studies might bias the data interpretation of organic contaminants (Di Toro and Rosa 1991). Therefore, in the present study, the concentrations of TBT, DBT, and MBT were standardized by % TOC due to large variability of the organic fraction in the sediments of sites from both campaigns. This approach has been recommended when the studied contaminants present ionic character and the analyzed sediments exhibit a wide range in the organic content (Wang et al. 2015).
Spatial and temporal distribution of butyltins in surface sediments
Butyltin residues were detected in all sediment samples collected along the TSB during campaign 1 (2010/2011, S) and 2 (2012, T) (Table 1). During 2010/2011, BT concentrations ranged from <2 to 262 ± 14 ng Sn g−1 for TBT, 4 ± 1.4 to 21 ± 0.0 ng Sn g−1 for DBT, and <3 to 4.5 ± 6.4 ng Sn g−1 for MBT. TBT concentrations of 27 ng Sn g−1 (6750 ng Sn g−1 TOC), 15 ng Sn g−1 (2142 ng Sn g−1 TOC), and 262 ng Sn g−1 (21,833 ng Sn g−1 TOC) were detected at sites S6 (Madre de Deus harbor), S7 (nearby Madre de Deus harbor), and S8 (Rio do Cunha), respectively, which are located at the innermost part of TSB. S6 and S7 are characterized by intense ship traffic while S8 by boat activities, which are potential sources of TBT contamination to the environment (Castro et al. 2012). However, the organotin distribution is strongly influenced by sorption processes to the organic matter or anionic surface complexation in the clay minerals in aqueous systems (Hoch and Schwesig 2004; Burton et al. 2006). Thus, the higher the concentration of organic carbon, the greater the ability of micropollutants to adsorb to the sediments (Di Toro and Rosa 1991; Pinochet et al. 2009). Moreover, the sediments that showed TOC values above 0.5 % can be considered as enriched by organic carbon (Burton et al. 2006), as seen at sites S7/T7 (0.7 %) and S8/T8 (1.2 %). Therefore, in order to compare levels between different sampling sites and campaigns, BT concentrations were standardized by organic carbon levels as recommended by Di Toro and Rosa (1991). Thus, the reduction of TBT concentrations seen for site T8 (12 ng Sn g−1) in comparison to S8 (2010/2011) can be partially explained by the lower levels of TOC in this sediment, since standardized levels reached 3000 ng Sn g−1 TOC. A possible reduction of inputs for this area might also explain the levels seen for T8. Although S7/T7 does not show any reduction of TBT levels over time, the neighboring site T6 does. Although only a slight decrease was observed (15 ng Sn g−1), this could be again biased by the higher TOC levels of this sediment (1000 ng Sn g−1 TOC). On the other hand, an increase of MBT metabolite was detected from 2010/2011 to 2012 for sites S6/T6 [from ˂3 ng Sn g−1 (250 ng g−1 Sn TOC) to 20 ng Sn g−1 (1333 ng Sn g−1 TOC)], S7/T7 [from ˂3 ng Sn g−1 (142 ng g−1 Sn TOC) to 35 ng Sn g−1 (3182 ng Sn g−1 TOC)], and S8/T8 [from ˂3 ng Sn g−1 (83 ng g−1 Sn TOC) to 48 ng Sn g−1 (12,000 ng Sn g−1 TOC)]. Even though indicating a decrease in TBT contamination due to the possible reduction of recent inputs, these data suggest that a significant TBT degradation is also taking place in these sediments.
Conversely, all TBT (and almost all DBT and MBT) levels showed a slight increase (even the TOC standardized levels) at the northeastern part of the TSB, especially at sites S10/T10 (Caboto), S11/T11 (entrance of Aratu Bay), and S12/T12 (innermost part of Aratu Bay) (Table 1). However, an increase in BDI values, with BDI > 1 for the 2012 campaign with a predominance of metabolites (MBT > DBT > TBT) for T10 (3.6), T11 (4.8), and T12 (4.7), indicates an historical release of TBT. In this case, although the BDI values indicate old inputs, small TBT contributions at this region might be coming either from recent releases (from ship and/or boat activities) or sediment remobilization. Site T9, located just outside Aratu Bay, showed a similar pattern of predominance of metabolites. Similar situations were observed at some sites studied in Babitonga Bay, Southern Brazil (TBT - 125, DBT - 394, and MBT 312 ng Sn g−1) (Oliveira et al. 2010), and San Vicente Bay (TBT - 135, DBT - 766, and MBT - 470 ng Sn g−1) (Pinochet et al. 2009), and Concepcion (TBT - 35, DBT - 47, and MBT - 118 ng Sn g−1) (unpublished data) in Chile. Similarity, an increase in TBT and MBT concentrations were also seen for site S14/T14 (Ribeira Bay), located at the east entrance of the TSB. Other sites at the same region [T15 41 ng Sn g−1 (5125 ng Sn g−1 TOC) and T16 77 ng Sn g−1 (6417 ng Sn g−1 TOC)] also showed relatively high values of TBT and BDI > 1.
Considering all sites sampled during the both sampling campaigns, a significant reduction for BDI (paired t test, p = 0.004) values was observed. These observations might indicate a relative effectiveness of recent national (NORMAM 2007) and global (IMO 2008) restrictions toward the use of TBT-based antifouling paints.
High level of TBT (138 ng Sn g-1/6272 ng Sn g−1 TOC) was also found at the west coast of Itaparica Island (site S20). The maximum turbidity zone located at the outlet of the Paraguaçu River estuary might contribute to the accumulation of contaminants through flocculation processes at this area (Genz et al. 2006). In addition, a marina located nearby this sampling site may also contribute to the input of TBT to the region. On the contrary, a much lower level of TBT was detected in site S21 (12 ng Sn g-1/240 ng Sn g−1 TOC) located less than 10 km south of S20 and inside the Itaparica channel. In this case, the low concentrations can be attributed to the distance from potential TBT sources in the region. Reasonable levels of TBT were also seen at the east coast of Itaparica (T5 - 26 ng Sn g-1/1529 ng Sn g−1 TOC) during the 2012 campaign. Low concentration of TBT (7 ng Sn g-1/259 ng Sn g−1 TOC) was also seen at the mouth of Subaé River (S22) in the northwest part of the TSB, where no shipping or boating activities take place. These findings are in accordance with Felizzola et al. (2008), where the lower TBT levels were detected in areas far away from harbors, shipyards, and marinas. Although more contaminated areas had been located nearby marinas and commercial harbors, the sources of BTs within the bay could not be accurately identified but seem to become from different areas as pointed out above. In addition, the spatial distribution of BTs within the TSB is not only associated to those sources but also to its capacity to disperse the contaminants (Cirano and Lessa 2007), as well as to the sediment composition, which influence their capacity to adsorb onto sediments.
According to Waite et al. (1991), concentrations (not TOC standardized) between 4–18, 22–73, and 109–365 ng Sn g−1 indicate sites under low, moderate, and high contamination of TBT, respectively. Thus, the sites S8 and S20 may be classified as highly contaminated, while the other areas along TSB as low to moderately contaminated. Although this classification might be useful to differentiate between contaminated and uncontaminated sites did not take into consideration relevant differences among geographical areas. In addition, the low TBT concentration detected in surface sediments from TSB are in the same order of magnitude of those capable of causing biological effects. For instance, Castro et al. (2012) have reported imposex in various species of mollusk gastropods from Ecuador coastal areas where sediment TBT contamination was as low as 12 ng Sn g−1.
Butyltin spatial distribution using bivalve tissues
All samples of bivalve tissues (A. brasiliana and M. guyanensis) presented detectable levels of BTs. Concentrations ranged from <5 (LOQ) to 421, 11 to 65, and <7 to 126 ng Sn g−1 for TBT, DBT, and MBT, respectively (Table 2). Due to different ecological distributions along TSB, it was not possible to collect those two species in all sampled sites. It was assumed, however, that those two species have similar bioaccumulation factors since both live in similar substrates (soft bottoms) and are filter-feeder organisms. In fact, the use of different bivalve species with similar ecological behaviors has been frequently employed in environmental monitoring studies (Sericano et al. 1995; Otchere 2005).
The TBT concentrations quantified in bivalve tissues were higher than in surface sediments reflecting their high capacity as filter feeders to bioaccumulate organic compounds from the environment (Rittschof and McClellan-Green 2005). As seen for sediments, TBT levels in bivalve tissues were higher in sites S6, S7, and S8. In fact, a significant correlation was observed (r = 0.87 and p = 0.0007) between TBT levels found in sites where sediment and bivalve mollusk samples were simultaneously collected (S6, S7, S8, S11, S14, S20, S21, and S22). This suggests similar sources of contamination, which is often observed (Oehlmann and Schulte-Oehlmann 2003).
The highest TBT concentration found in the study area was 421 ng Sn g−1 in M. guyanensis at S8 (Rio Cunha), level that represents a risk to humans since this species is regularly consumed by fishing communities and sold as a typical dish at local restaurants (Bezerra 2008). Based on the European Food Safety Authority that recommends the maximum tolerable of daily TBT intake of 250 ng kg−1 (as TBT) body weight (EFSA 2004) and considering an adult with average weight of 70 kg, the daily safe limit of TBT intake would be up to 17,500 ng (as TBT). Thus, considering the conversion factor of 2.74 from tin, the concentration of TBT in M. guyanensis from S8 was 1153 ng g−1 (as TBT—dry weight). Assuming that M. guyanensis has a percentage of approximately 90 % water in body composition, a daily intake of 152 g (wet weight) of this bivalve collected at station S8 would be enough to extrapolate the limit established by EFSA. Furthermore, M. guyanensis can be also eaten raw, with no degradation of BTs by cooking. Similar trends were also found by Louppis et al. (2010) investigating TBT concentrations in edible species obtained on the coast of Greece and by Fernandez et al. (2005b) investigating Perna perna mussels from Guanabara Bay, showing risks derived from organotins in seafood. These findings point out for the urgent need of increasing knowledge on ingestion rates and contamination levels of seafood.
Temporal evaluation of imposex occurrence
The biometric and imposex parameters assessed for S. rustica from TSB are summarized in Table 3. Although significant levels of imposex have been previously detected in 2004 along TSB (Castro et al. (2007), no evidences of imposex in S. rustica populations were seen in the present study during 2012. Thus, the sampled sites T1, T2, T3, T5, T11, T13, T15, T16, and T17 (which were also analyzed in 2004) showed a significant reduction in imposex levels [RPLI (Mann-Whitney U test, p = 0.0002), FPLI (Mann-Whitney U test, p = 0.0002), and VDSI (Mann-Whitney U test, p = 0.0002)] along this 8-year period (Table 3). Recoveries in imposex-affected populations had been reported in several studies worldwide. For example, a recovery of N. lapillus populations from coastal areas of England (Birchenough et al. 2002), Portugal (Galante-Oliveira et al. 2009), and Iceland (Jorundsdottir et al. 2005; Guomundsdóttir et al. 2011) was reported after restrictions on the use TBT-based antifouling systems implemented in 2003. Moreover, a reduction of imposex incidences was registered in different areas of South America under the influence of harbors (Castro et al. 2012; Grimón et al. 2016). Therefore, the imposex recovery observed in S. rustica from TSB is in agreement with the implementation of restrictions on the use of TBT in antifouling paints at both national (NORMAM 2007) and global scale (Birchenough et al. 2002; Sousa et al. 2009; Galante-Oliveira et al. 2011). Despite the observed reduction, it is important to observe that the higher RPLI (14.8) and VDSI (1.5) values reported in 2004 for S. rustica from the TSB-affected areas (Castro et al. 2007) were lower than imposex levels detected for other muricid species (i.e., S. haemastoma) at the same period and several harbor areas from Brazil (Fernandez et al. 2005a; Castro et al. 2007). In fact, in 2004, there were no local restrictions to the use of TBT-based antifouling paints in Brazil, and not even the AFS Convention was fully into force (Castro et al. 2012). Therefore, it is very likely that environmental levels of TBT were much higher at that time (Fernandez et al. 2005b). In addition, previous studies have reported a low sensitivity of S. rustica to TBT contamination (Castro et al. 2012), which could not exempt other gastropod species from TSB of being affected and presenting imposex. Therefore, considering the current TBT levels detected in sediment samples, further studies should be performed in TSB to evaluate imposex occurrence in other gastropod species.
Conclusion
Based on the amount of TBT detected in the bivalve M. guyanensis collected at S8, there is potential health risks associated with the consumption of these mussels, since the maximum tolerable TBT daily intake here estimated is over the limit established by the European Food Safety Authority.
Based on sediment evaluation, there are multiple potential sources of this contaminant within Todos os Santos Bay. Moreover, the TBT contamination does not seem to be affected only by direct sources but also by the local environmental characteristics such as strong hydrodynamics and the effect of anthropic activities (for example dredging). The temporal analysis of the data (2010/2011 and 2012) showed no significant reduction in the TBT levels in sediments from Todos os Santos Bay. However, the metabolite predominance (DBT and MBT) in 2012, associated with the observed TBT levels (resulting in BDI values >1), indicates simultaneous occurrence of recent inputs and metabolization process of TBT historically released. Based on the levels of TBT in some of the sediment samples analyzed, a slight increase in S10/T10, S11/T11, and S12/T12 was observed, despite imposex incidence have shown a clear reduction in a broader timescale.
Nevertheless, the imposex recovery observed in S. rustica populations between 2004 and 2012 indicates a decrease related to the TBT biological effects. Thus, considering the temporal evaluation of imposex performed in the present study, the results suggest that recent TBT inputs (2012) are lower than they were in the past. However, the TBT contamination levels are still relevant, especially in areas under the direct influence of Salvador harbor (T14, T15, and T16), since they are above those that cause toxicity to biota.
References
Abidli S, Santos MM, Lahbib Y, Castro LFC, Reis-Henriques MA, Trigui El Menif N (2012) Tributyltin (TBT) effects on Hexaplex trunculus and Bolinus brandaris (Gastropoda: Muricidae): imposex induction and sex hormone levels insights. Ecol Indic 13:13–21
Azevedo D, Rocha-Barreira C, Matthews-Cascon H, Castro Í (2012) Pugilina morio L., a new imposex exhibitor from South American estuarine environments: approach for a non-lethal method to evaluate imposex. Bull Environ Contamination Toxicol 89:786–792
Bezerra FJ (2008) O bosque de mangues e a pesca artesanal no Distrito de Acupe (Santo Amaro, Bahia): uma abordagem etnoecológica. Acta Scentarium 30:267–273
Bigatti G, Primost MA, Cledón M, Averbuj A, Theobald N, Gerwinski W, Arntz W, Morriconi E, Penchaszadeh PE (2009) Biomonitoring of TBT contamination and imposex incidence along 4700 km of Argentinean shoreline (SW Atlantic: From 38S to 54S). Mar Pollut Bull 58:695–701
Birchenough AC, Evans SM, Moss C, Welch R (2002) Re-colonisation and recovery of populations of dogwhelks Nucella lapillus (L.) on shores formerly subject to severe TBT contamination. Mar Pollut Bull 44:652–659
Burton ED, Phillips IR, Hawker DW (2006) Tributyltin partitioning in sediments: Effect of aging. Chemosphere 63:73–81
Cao D, Jiang G, Zhou Q, Yang R (2009) Organotin pollution in China: an overview of the current state and potential health risk. J Environ Manag 90:S16–S24
Castro ÍB, Fillmann G (2012) High tributyltin and imposex levels in the commercial muricid Thais chocolata from two Peruvian harbor areas. Environ Toxicol Chem 31:955–960
Castro IB, Meirelles CAO, Matthews-Cascon H, Fernandez MAS (2004) Thais (Stramonita) rustica (Lamarck, 1822) (Mollusca: Gastropoda: Thaididae), a potential bioindicator of contamination by organotin Northeast Brazil. Braz J Oceanogr 52:135–139
Castro IB, Lima AFA, Braga ARC, Rocha-Barreira CA (2007) Imposex in two muricid species (Mollusca: Gastropoda) from the Northeastern Brazilian coast. J Bra Soc Ecotoxicol 2:81–91
Castro IB, Perina F, Fillmann G (2012a) Organotin contamination in South American coastal areas. Environ Monit Assess 184:1781–1799
Castro ÍB, Rossato M, Fillmann G (2012b) Imposex reduction and residual butyltin contamination in southern Brazilian harbors. Environ Toxicol Chem 31:947–954
Castro IB, Arroyo M, Costa P, Fillmann G (2012c) Butyltin compounds and imposex levels in Ecuador. Arch Environ Contam Toxicol 62:68–77
Castro IB, Rocha-Barreira C de A, Fernandez MA, Bigatti G (2012d) Transplant bioassay induces different imposex responses in two species of the genus Stramonita. Mar Biol Res 8:397–404
Cirano M, Lessa GC (2007) Oceanographic characteristics of Baía de Todos os Santos, Brazil. Revista Brasileira de Geofísica 25:363–387
Culmo RF, Swanson KJ, Brennan WP (2003). Application of the PE2400 CHN elemental analyzer for organic particulate matter in water. Elemental Analysis Newsletter [S.1.], v.31.
Di Toro DM, Rosa LD (1991) Equilibrium partitioning and organic carbon normalization. National Sediment Bioaccumulation Conference, New Jersey
Díez S, Abalos M, Bayona JM (2002) Organotin contamination in sediments from the Western Mediterranean enclosures following 10 years of TBT regulation. Water Res 36:905–918
EFSA (2004) Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission to assess the health risks to consumers associated with exposure to organotins in foodstuffs. EFSA J 102:1–119
Felizzola JF, de Luca Rebello Wagener A, Almeida AC, Lin WO (2008) Butyltin speciation in sediments from Todos os Santos Bay (Bahia, Brazil) by GC-PFPD. Quim Nov. 31:89–93
Fernandez MAS, De Luca Rebello Wagener A, Limaverde AM, Scofield AL, Pinheiro FM, Rodrigues EF (2005a) Imposex and surface sediment speciation: a combined approach to evaluate organotin contamination in Guanabara Bay, Rio de Janeiro, Brazil. Mar Environ Res 59:435–452
Fernandez MA, Limaverde AM, Scofield AL, de Luca Rebello Wagener A (2005b) Preliminary evaluation of human health risks from ingestion of organotin contamined seafood in Brazil. Braz J Oceanogr 53:75–77
Galante-Oliveira S, Oliveira I, Jonkers N, et al (2009) Imposex levels and tributyltin pollution in Ria de Aveiro (NW Portugal) between 1997 and 2007: evaluation of legislation effectiveness. J Environ Monit 11:1405–1411
Galante-Oliveira S, Oliveira I, Ferreira N, et al (2011) Nucella lapillus L. imposex levels after legislation prohibiting TBT antifoulants: temporal trends from 2003 to 2008 along the Portuguese coast. J Environ Monit 13:304–312
Genz F, Lessa GC, Cirano M (2006) The impact of an extreme flood upon the mixing zone of the Todos os Santos Bay, Northeastern Brazil. J Coast Res 39:707–712
Gibbs PE, Bryan GM, Pascoe PL, Burt GR (1987) The use of dog-whelk Nucella lapillus, as an indicator of tributyltin (TBT) contamination. J Mar Biol Assoc U K 67:507–523
Gipperth L (2009) The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. J Environ Manage 90:S86–S95
Graceli JB, Cena GC, Lopes PF, Zamprogno GC, da Costa MB, Godoi AF, Dos Santos DM, de Marchi MR, dos Santos Fernandez MA (2013) Organotins: a review of their reproductive toxicity, biochemistry, and environmental fate. Reprod Toxicol 36:40–52
Gray JS (1981) The ecology of marine sediments. Cambridge University Press, New York
Grimón ROR, Arroyo MF, Freitas DM, Castro ÍB (2016) Tributyltin impacts in Galapagos Islands and Ecuadorian shore: marine protected areas under threat. Mar Policy 69:24–31. doi:10.1016/j.marpol.2016.03.017
Guomundsdóttir LÓ, Ho KKY, Lam JCW, Svavarsson J, Leung KMY (2011) Long-term temporal trends (1992-2008) of imposex status associated with organotin contamination in the dogwhelk Nucella lapillus along the Icelandic coast. Marine Pollution Bulletin. In Press.
Hatje V, Andrade JB (2009) Baía de Todos os Santos: Aspectos Oceanográficos. EDUFBA, Savaldor, p 303
Hoch M (2001) Organotin compounds in the environment—an overview. Appl Geochem 16:719–743
Hoch M, Schwesig D (2004) Parameters controlling the partitioning of tributyltin (TBT) in aquatic systems. Appl Geochem 19:323–334
Horiguchi T (2009) The endocrine-disrupting effect of organotin compounds for aquatic organisms. In: Arai T, Harino H, Ohji M, Langston WJ (eds) Ecotoxicology of antifouling biocides. Springer, Tokyo, pp 125–146
IMO (2008) Summary of the status of conventions as at 31 May 2007. International Maritime Organization, United Kingdom, http://www.imo.org/
IUPAC (International Union of Pure and Applied Chemistry) (2002) Analytical, applied, clinical, inorganic, and physical chemistry divisions interdivisional working party for harmonization of quality assurance schemes for analytical laboratories. Pure Appl Chem 74:835–855
Jorundsdottir K, Svavarsson K, Leung KMY (2005) Imposex levels in the dogwhelk Nucella lapillus (L.)—continuing improvement at high latitudes. Mar Pollut Bull 51:744–749
Kotrikla A (2009) Environmental management aspects for TBT antifouling wastes from the shipyards. J Environ Manag 90:S77–S85
Louppis AP, Georgantelis D, Paleologos EK, Kontominas MG (2010) Determination of tributyltin through ultrasonic assisted micelle mediated extraction and GFAAS: application to the monitoring of tributyltin levels in greek marine species. Food Chemistry. In Press, Accepted Manuscript
Matthiessen P, Gibbs PE (1998) Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ Toxicol Chem 17:37–43
Meador JP, Sommers FC, Cooper KA, Yanagida G (2011) Tributyltin and the obesogen metabolic syndrome in a salmonid. Environ Res 111:50–56
Morabito R, Massanisso P, Quevauviller P (2000) Derivatization methods for the determination of organotin compounds in environmental samples. TrAC Trends Anal Chem 19:113–119
NORMAM (2007) Normas da Autoridade Marítima para o Controle de Sistemas Anti-incrustantes Danosos em Embarcações—NORMAM/23. Marinha do Brasil, Rio de Janeiro
Oehlmann J, Schulte-Oehlmann U (2003) Molluscs as bioindicators. In: Markert BA (ed) Trace metals and other contaminants in the environment bioindicators & biomonitors principles, concepts and applications. Elsevier, Philadelphia, pp 577–635
Oliveira IB, Richardson CA, Sousa AC, et al (2009) Spatial and temporal evolution of imposex in dogwhelk Nucella lapillus (L.) populations from North Wales, UK. J Environ Monit 11:1462–1468
Oliveira CR, Santos DM, Santos Madureira LA, Marchi MR (2010) Speciation of butyltin derivatives in surface sediments of three southern Brazilian harbors. J Hazard Mater 181:851–856
Otchere FA (2005) Organochlorines (PCBs and pesticides) in the bivalves Anadara (Senilis) senilis, Crassostrea tulipa and Perna perna from the lagoons of Ghana. Sci Total Environ 348:102–114
Paz-Villarraga CA, Castro IB, Miloslavich P, Fillmann G (2015) Venezuelan Caribbean Sea under the threat of TBT. Chemosphere 119:704–710
Pinochet H, Tessini C, Bravo M, Quiroz W, De Gregori I (2009) Butyltin compounds and their relation with organic matter in marine sediments from San Vicente Bay, Chile. Environ Monit Assess 155:341–353
Pletsch LA, Beretta M, Tania Mascarenhas Tavares MT (2010) Distribuição espacial de compostos orgânicos de estanho em sedimentos costeiros e em Phallusia nigra da baía de Todos os Santos e litoral norte da Bahia - Brasil. Quim Nov. 33(2):451–457
Rittschof D, McClellan-Green P (2005) Molluscs as multidisciplinary models in environment toxicology. Mar Pollut Bull 50:369–373
Santos DM, Araújo IP, Machado EC, Carvalho-Filho MAS, Fernandez MA, Marchi MRR, Godoi AF (2009) Organotin compounds in the Paranaguá Estuarine Complex, Paraná, Brazil: evaluation of biological effects, surface sediment, and suspended particulate matter. Mar Pollut Bull 58:1926–1931
Sericano JL, Wade TL, Jackson TJ, Brooks JM, Tripp BW, Farrington JW, Mee LD, Readmann JW, Villeneuve JP, Goldberg ED (1995) Trace organic contamination in the Americas: an overview of the US National Status and Trends and the International Mussel Watch programmes. Mar Pollut Bull 31:214–225
Sousa A, Laranjeiro F, Takahashi S, Tanabe S, Barroso CM (2009) Imposex and organotin prevalence in a European post-legislative scenario: temporal trends from 2003 to 2008. Chemosphere 77:566–573
Toste R, Fernandez MA, Pessoa I de A, Parahyba MA, Dore MP (2011) Organotin pollution at Arraial do Cabo, Rio de Janeiro State, Brazil: increasing levels after the TBT ban. Braz J Oceanogr 59:111–117
Toste R, Pessoas IA, Dore DP, Parayba MA, Ferandez MA (2013) Aphallic vas deferens development in females related to the distance from organotin sources? A study with Stramonita haemastoma. Ecotoxicol Environ Saf 91:162–170
Town RM, Filella M (2002) Implications of natural organic matter binding heterogeneity on understanding lead(II) complexation in aquatic systems. Sci Total Environ 300:143–154
Waite ME, Waldock MJ, Thain JE, Smith DJ, Milton SM (1991) Reductions in TBT concentrations in UK estuaries following legislation in 1986 and 1987. Mar Environ Res 32:89–111
Wang J, Yao P, Bianchi TS, Li D, Zhao B, Xingqian C, Pan H, Zhang T, Yu Z (2015) The effect of particle density on the sources, distribution, and degradation of sedimentary organic carbon in the Changjiang Estuary and adjacent shelf. Chem Geol 402:52–67
Yebra DM, Kiil S, Dam-Johansen K (2004) Antifouling technology: past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog Org Coat 50:75–104
Acknowledgments
We thank FINEP for the financial support (project no. 01.11.0038.00). V. Artifon was sponsored by CAPES, Í.B. Castro by CAPES (802-16/2012) and FAPERGS (0885/12-1), and G. Fillmann by CNPq (PQ 312341/2013-0). We also thank Vanessa Hatje and Gilmara Eça from the Universidade Federal da Bahia (UFBA) for supplying the samples of campaign 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Rights and permissions
About this article
Cite this article
Artifon, V., Castro, Í.B. & Fillmann, G. Spatiotemporal appraisal of TBT contamination and imposex along a tropical bay (Todos os Santos Bay, Brazil). Environ Sci Pollut Res 23, 16047–16055 (2016). https://doi.org/10.1007/s11356-016-6745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6745-7