Abstract
This work provides a preliminary study of the destination, mobility, and availability of tributyltin (TBT), dibutyltin (DBT), and monobutyltin (MBT) in contaminated sediments and water column within Puerto Rosales Port, located in the middle zone of the Bahía Blanca Estuary (Argentina). Therefore, this study presents the first comprehensive results of the role of several physicochemical parameters (temperature, pH, Eh, salinity, turbidity, organic matter, chlorophyll, and macronutrients) in behavior of organotin compounds (OTCs) in a marine-coastal ecosystem. The samples were collected seasonally in May, August, and November during 2014. Levels of OTCs were determined in sediments and water column samples by means of gas chromatography–mass spectrometry analysis. Degradation index analyses suggested not recent inputs of TBT at the area of study. However, results submitted a continuous input of TBT into the column water; further, its distribution and degradation pattern were shown to be influenced by salinity, turbidity, particulate organic matter, chlorophyll, and nitrates. These last two parameters, chlorophyll and nitrates, also were very important for sediment samples. Chlorophyll together with high temperatures recorded in the surface sediments triggers biodegradation process of TBT and DBT resulting in high MBT levels while nitrates seemed to promote debutylation process. Furthermore, pH appeared to influence drastically the adsorption/desorption activity of TBT and DBT in sediment. Finally, the Eh obtained suggested a degradation of TBT thanks to the presence of Fe (III) in this compartment. In addition, in fact, the results outlined a possible MBT additional input that contributes to the pollution observed in the study area.

Organotin compounds behavior according to several physicochemical parameters
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prior to its regulation and complete prohibition in January 2008 (IMO 2005), tributyltin (TBT) was extensively used as additive in antifouling paints during the 1960s and 1970s. TBT is a highly toxic biocide that prevents the attachment and growth of algae, barnacles, tubeworms, mollusk, and other marine organisms on the hulls of large ships and boat, sewage pipe systems, docks, fishnets, and buoys (Shue et al. 2014; Ogbomida and Ezemonye 2016). In addition, TBT as well as dibutyltin (DBT) and monobutyltin (MBT) has been used in other industrial and agricultural applications mainly as insecticides, fungicides, and bactericides (Cruz et al. 2015; Fang et al. 2017). Furthermore, these compounds—mainly DBT and MBT—have also been used extensively in the PVC industry (Hoch 2001). This large-scale utilization led to the release of high amounts of organotin compounds (OTCs) into many estuarine and marine ecosystems causing lethal effects on target and non-target organisms (Sabah et al. 2016; Zhang et al. 2017).
Once in the aquatic environment, via both biotic and abiotic degradations, TBT goes under a sequential removal of organic groups from the tin atom (debutylation) to produce DBT and MBT (Furdeck et al. 2016). Despite the fact that TBT exhibits the highest toxicity to aquatic organisms, a high toxicity has also been reported for its breakdown products while no control to these compounds has been introduced up to now (Fang et al. 2017; Zhang et al. 2017). A key environmental importance of these products relies on their persistent nature and bioaccumulative potential (Hoch 2001); in consequence, since 2001, TBT has been classified as a persistent organic pollutant by the European Commission Parliament list of priority hazardous substances (Amending water policy directive 2000/60/EC) (Castro and Fillmann 2012; Ogbomida and Ezemonye 2016).
While abiotic degradation of TBT includes thermal, chemical, and photodegradation, several microorganisms (bacteria, algae, and fungi) govern biotic degradation (Hoch 2001). The latter appears to be the most important pathway for the decomposition of TBT in the environment (Ayanda et al. 2012; Filipkowska et al. 2014); therefore, microorganisms play an important role in the biodegradation of organotin compounds and their diffusion mechanism (Point et al. 2007). Macronutrients are an essential requirement of microorganisms to grow and develop (Sakultantimetha et al. 2011b). Essential elements as carbon, nitrogen, and phosphorous are required by all microorganisms, and their availability critically influences bacterial biodegradation (Jin et al. 2011). OTCs degradation also depends on several environmental conditions, such as salinity, temperature, and redox conditions (O2) (Sakultantimetha et al. 2011a, b). In regard to the matrix, OTCs degradation in the water column is considered to be faster than in sediments, with a half-life about of days to weeks and several years to decades, respectively (Furdeck et al. 2016). Consequently, sediments emerge as a secondary and persistent source of OTCs in affected coastal environments, turning in a potential threat to them (Pougnet et al. 2014). Moreover, in recent years, it has also been evidenced the occurrence of OTCs fluxes at the sediment–water interface (Point et al. 2007).
In comparison with the northern hemisphere, information concerning the dynamic of OCTs in South American coasts is lacking. Highly fragmented data has been reported for OTCs in selected Argentinean marine environments (Delucchi et al. 2011; de Castro et al. 2012; Commendatore et al. 2015; Laitano et al. 2015; Quintas et al. 2016, 2017; Del Brio et al. 2016; Martínez et al. 2017). In Argentina, there are no precedents of assessing the distribution of OTCs between sediment and water (column and porewater) but there are only a few in other regions of the world (Furdeck et al. 2016; Briant et al. 2016; Cole et al. 2018). Considering the hypothesis that the OTCs concentration dynamics is independent of the variation of physicochemical parameters at different matrices, the main aim of this study was to investigate for the first time the role of physicochemical parameters on OTCs distribution/degradation in water column and sediments in field samples of an impacted South American coastal environment. Then, efforts are oriented to assess the relationships among the environmental temperature, pH, Eh, salinity, turbidity, organic matter, chlorophyll, macronutrients, and the OTCs distribution in coastal sediments and water. Results will fill a gap in the knowledge of OTCs dynamics in the South American environments and set the basis for future studies.
Material and methods
Study area and sampling site
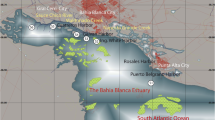
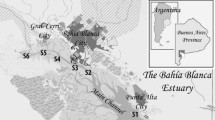
Bahía Blanca estuary (BBE) (Fig. 1) is the second largest estuary of Argentina, South America, located in the southeast coast of the country (38° 45′–35° 10′ S and 61° 45′–62° 30′ W). This estuary extends over about 2300 km2 and could be described as a series of major NW-SE tidal channels separated by extensive tidal flats (1150 km2) with patches of low salt marshes, and islands (410 km2) (Piccolo et al. 2008). The principal energy input is produced by a quasi-stationary, semidiurnal tidal wave (Perillo et al. 2001).
Two freshwater tributaries enter the estuary from the northern shore: Sauce Chico River (drainage area of 1600 km2 with a mean flow of 1.9 m3 s−1) and Napostá Grande Creek (drainage area of 1240 km2 with a mean flow of 0.8 m3 s−1) (Carbone et al. 2016). However, the largest input nutrients and contaminants are provided by the sewage discharges from Bahía Blanca, General Cerri, Punta Alta, and Ingeniero White cities. Furthermore, in the northern coast, the Canal Principal houses the largest and deeper port system in South America, which discharges processing residues into the streams or directly into the estuary without pre-treatment (Severini et al. 2009; La Colla et al. 2017). This Channel requires regular dredging because of the frequent shipping of cargo and transport vessels. This practice favors the mobilization of tons of sediment. The tidal oscillations of 4 m and the prevailing northwest winds create a strong tidal current, which facilitates the mixing of water resulting in a vertically uniform distribution of oceanographic parameters (Ferrer et al. 2000). BBE shows an important anthropogenic impact, including eutrophication, chemical pollution, riverine habitat loss, hypoxia, and introduction of invasive species (López Abbate et al. 2017). Moreover, BBE is characterized by high levels of dissolved inorganic nutrients especially ammonium nitrogen species, high levels of organic matter, a winter phytoplankton bloom, and an important pelagic-benthonic coupling (Guinder et al. 2015; Spetter et al. 2015).
In the current study, the sampling site is located at the middle zone of this estuary, in Rosales Port (RP) (38° 55′ S; 62° 03′ W), in the northeast coast of BBE. It is a system conformed by extensive tidal flats (~ 1000 m wide) with a very soft slope (~ 0.4°) and composed of sandy to muddy siliciclastic sediments, which vary between fine sand and silty clay (Gelós et al. 2004; Fernández et al. 2016). The intertidal and supratidal area are vegetated by Spartina alterniflora and Sarcocornia perennis, patchily distributed along the tidal flat. The studied area is characterized by a dry temperate climate with a mean annual air temperature of 15.6 °C, a low mean rainfall (460.5 mm), high rate of evaporation, and a salinity about 30 to 35 (Perillo et al. 2001; Piccolo and Diez 2004). Extensive areas of the tidal flats (supra- and upper intertidal zone) are colonized by biofilms and microbial mats (Cuadrado et al. 2011; Spetter et al. 2015), as well as by the burrowing crab Neohelice granulata (Iribarne et al. 2003). It is also important to emphasize that the sampling site is in the vicinity of dry docks located in Belgrano Port, the most important military harbor of Argentina. Currently, in RP, the Free Trade Zone–South Zone operates, which aims to promote trade and industrial export activity that originates continuously a significant number of vessels transport in the study area (ZS-ZF 2007. http://www.zfzonasur.com.ar/).
Sampling methodology
Sampling was done in RP during low tide, in the daytime hours, every 3 months during May, August, and November 2014. Sediment and seawater samples were immediately transported to the laboratory in iceboxes and stored at − 20 °C prior to analysis. All the glassware used for OTCs was suitably conditioned, both for the collection and storage of the samples and as for the analysis of these. First, it was washed with non-ionic detergent and rinsed carefully with tap water and then double-distilled water. Next, it was submerged in 5% nitric acid (v/v) for 24 h. Finally, the material was rinsed again with distilled water and brought to dryness, as indicated by international protocols to avoid contamination of the sample (Michel and Averty 1999; Bancon-Montigny et al. 2000). In the case of the material used for the determination of particulate organic matter (MOP), the washing was performed with sulfochromic solution instead of nitric acid (also for 24 h). For macronutrient determination, all materials used were washed with non-ionic detergent (1% v/v), rinsed with main water, and then were submerged in 10% HCl (v/v) for 24 h. Next, at least three rinses with distilled water were made. For Chl-a, material washing was done with non-ionic detergent, magnesium carbonate (1%), and finally distilled water.
Sediment samples
Surface sediment layer (approximately 1 cm) was collected at RP intertidal flats. For OTCs determination, composite samples of sediments were collected using a stainless steel sampler and stored on solvent-cleaned amber glass containers avoiding exposure to light.
For determination of moisture content and organic matter, sediment samples were collected in duplicate with small cores of PVC (35 mm i.d.; 120-mm length) (Simpson et al. 2005).
Triplicate sediment samples for chlorophyll a (Chl-a) analysis were collected using mini corers (11 mm i.d.; 40-mm length) avoiding exposure to light (Gómez et al. 2009).
Simultaneously, temperature, pH, and Eh in surface sediment were in situ measured by means of a probe (Hanna Instruments, model HI991003).
Water samples
Seawater samples for OTCs were obtained from a dock, using 1.5-L amber glass bottles, previously washed and conditioned. They were submerged 0.50 m down, rinsed three times before filling, double bagged, and transported to the laboratory in refrigerated boxes.
For the analysis of dissolved inorganic nutrients, particulate organic matter (POM) and Chl-a, seawater samples were collected near the surface (approximately 0.50 m depth) with a 2.5-L Van Dorn bottle. Porewater samples (~ 30-mm depth) were in situ extracted employing a manual vacuum pump (Adams 1994; Di Bonito et al. 2008) coupled to a PVC pipe (35 mm i.d.; 150-mm length) with a pore size of 500 μm (Martin et al. 2003). In addition, physicochemical parameters (salinity, pH, turbidity, and temperature) were measured in situ using a Horiba U-10 multisensor probe.
Sample treatments and analysis
Organotin compounds
In a first step, all the composite samples from sediment obtained in each sampling were lyophilized during 48 h; then, they were powdered and sieved through stainless steel meshes (250 mesh). The lower particle size fraction (< 63 μm) was used for OTCs analysis. According to Buggy and Tobin (2006), the effects of variable grain size are negligible working with a grain size less than 63 μm since sample variance is markedly reduced.
The extraction and determination of OTCs in sediments were carried out following the procedure proposed by Quintas et al. (2016). Water samples were immediately filtered through a polycarbonate membrane (Millipore HTTP 04700) with a pore size of 0.4 μm and 47 mm in diameter. Filtered fractions were acidified to pH 2 with concentrated analytical quality HCl, placed in amber conditioned glass bottles by triplicate, bagged and stored at 4 °C in darkness until the corresponding analyses were carried out. Briefly, the extraction of OTCs in water samples consisted of placing 500 mL of sample in a separating funnel and then adding tripropyltin chloride—as recovery control standard, methanolic NaOH solution, hexane, and finally, NaBH4. Afterwards, funnels were stirred for 5 min at 0 °C in an orbital shaker and then the aqueous residues were removed by adding anhydrous sodium sulfate. The hexane layer was transferred to an amber glass vial and the volume was reduced by applying a gentle stream of pure nitrogen. Lastly, tetrabutyltin standard solution (internal standard) was added and immediately injected into the gas chromatograph. OTCs in both samples, sediment and water, were quantified by capillary gas chromatography (Agilent 7890 B) coupled with a mass spectrometer (Agilent 5977A), using an HP-5MS fused silica column (30 m; 0.25 mm i.d.; 0.25-μm film thickness). GC-MS instrumental condition was the same as detailed in previous works carried out in our laboratory (Quintas et al. 2017).
Nutrients and particulate organic matter
Seawater samples (~ 250 mL) for dissolved inorganic nutrients and particulate organic matter (POM) determination were filtered through muffled (450–500 °C, 1 h) glass fiber membranes (47 mm diameter and 0.7 μm) and frozen at − 20 °C in plastic bottles until analyzed (Grasshoff 1976; APHA 1998). Porewater samples for nutrient determination were treated in the same way as seawater samples. Both types of samples were immediately filtrated in the laboratory to avoid the oxidation–reduction processes.
Samples for ammonium were preserved with a specific chemical reagent (APHA 1998), kept in the dark, and frozen.
Nitrate (NO3−) (Treguer and Le Corre 1975a), nitrite (NO2−) (Grasshoff et al. 1983), ammonium (NH4+) (Koroleff 1969; Treguer and Le Corre 1975b), dissolved inorganic phosphorous as PO43− (Eberlein and Kattner 1987), and dissolved silicates (DSi) (Technicon 1973) concentrations were determined using an automatized and five channels upgraded Technicon Autoanalyzer II. The limits of quantification (LOQ) were 0.10 μmol L−1, 0.02 μmol L−1, 0.01 μmol L−1, 0.01 μmol L−1, and 1.00 μmol L−1 for NO3−, NO2−, NH4+, PO43−, and DSi respectively. POM content was analyzed following the Strickland and Parsons method (1968) (range 10–4000 mgC m−3) using a Jenway 6715 UV–vis spectrophotometer.
Chlorophyll a
Surface sediments were separated and seawater samples were filtered through glass fiber membranes (47 mm diameter and 0.7 μm). Sediment samples and filters were kept at − 20 °C in foil envelopes until analysis. Chl-a was extracted with acetone 90%, immersed in an ultrasonic bath at a controlled temperature, and then refrigerated in the dark at − 4 °C for 24 h (APHA 1998; Gómez et al. 2009). Chl-a concentrations were determined spectrophotometrically (Jenway 6715 UV–vis) by means of the equations of Lorenzen (1967). The results were expressed in micrograms per gram and micrograms per liter for sediment and seawater samples, respectively.
Organic matter and moisture content
The organic matter content (%OM) in sediments was calculated from weight loss of ignition (LOI), in samples previously dry at 105 ± 5 °C for 12–24 h or up to constant weight (Santisteban et al. 2004), calcining samples at 450 ± 50 °C for 1 h in a muffle furnace (Dean 1974; Buhl-Mortensen 1996).
Quality control
For sediment samples, the method traceability for OTCs determination was evaluated using a certified reference material (PACS-2). Precision was evaluated under repeatability conditions and was estimated as percent relative standard deviation, RSD (%), n = 3 and was found to vary between 1.1 and 1.9%. Trueness was assessed in terms of recovery of extraction/derivatization reaction. The recoveries varied between 82.5 and 97.0% (Table 1). For water samples, in order to estimate the accuracy of the applied method, selected samples were spiked with known concentrations of TBT, DBT, and MBT and passed through the entire analytical procedure. Recoveries ranged between 82.1 and 105.7%, whereas precision—evaluated under repeatability conditions and estimated as RSD (%)—ranged between 2.2 and 19.3%. Overall results were in agreement with international analytical validation recommendations (EURACHEM 1998; IUPAC 2002).
Detection limits (LODs) were calculated as three times the baseline noise of chromatograms (signal to noise ratio, S/N) (Cassi et al. 2008; Choi et al. 2014; Dominguez et al. 2014; Vidal et al. 2003) and the limit of quantifications (LOQs) as ten times the abovementioned ratio. In sediment samples, LODs were 0.78, 1.08, and 3.5 ng Sn g−1 dry weight for TBT, DBT, and MBT, respectively. In addition, LOQs were 2.7, 3.7, and 11.7 ng Sn g−1 dry weight for TBT, DBT, and MBT, respectively. In water samples, LODs were 2.1 ng Sn L−1 for TBT, 5.4 ng Sn L−1 for DBT, and 8.4 ng Sn L−1 for MBT. While LOQs were 7.0 ng Sn L−1 for TBT, 17.7 ng Sn L−1 for DBT, and 28.0 ng Sn L−1 for MBT.
Result and discussion
Organotin compounds
OTCs were detected in all sediments and water samples analyzed, suggesting a ubiquity of TBT, DBT, and MBT in the study area, RP. The organotin compound levels obtained are the average concentrations of three replicates (n = 3; mean value ± standard deviation). In water column, TBT levels ranged from 22.6 ± 2.4 (spring—November) to 216 ± 16 ng Sn L−1 (winter—August), with 105 ± 10 ng Sn L−1 in autumn (May) (Table 2). While the levels of the first degradation product (DBT) were quantified only during the spring season (November) (288 ± 12 ng Sn L−1), i.e., in the other months of sampling, the DBT levels were under the LOQ (17.7 ng Sn L−1). MBT levels range between 289 ± 2.0 and 455 ± 5 ng Sn L−1 (Table 2). The detection of TBT in water samples during the whole sampling period would indicate a permanent entry of this compound into the RP (Table 2). A first rationale for this could be attributed to the main route of entry of TBT by direct leaching of antifouling paints since there is a shipyard in the surrounding coasts apart from the great maritime traffic and fishing pressure. Secondly, the continuous dredging of the main navigation channel of the estuary originates a great resuspension of sediments, which—as a secondary source—could potentially redistribute these compounds over the area (Santos et al. 2004; Buggy and Tobin 2006). Under the same environmental conditions, di- and tributylated compounds quickly degrade in water column, with a half-life ranging between days and weeks (Ranke and Jastorff 2000; Cruz et al. 2015). Conversely, MBT have a longer half-life than TBT and DBT and it does not show an apparent degradation even after 280 days (Cima et al. 2003; Furdek et al. 2012).
As it can also be seen in Table 2, TBT levels decreased throughout the sampled period. A priori, it was possible to attribute this behavior to various degradative processes that are known to occur faster in water than in sediment, triggered by temperature (Filipkowska et al. 2014; Furdeck et al. 2016). As previously mentioned, DBT could be quantified only during the spring season. Add to that, DBT has the shortest half-life in the water column between studied OTCs; the described behavior could be the result of a very slow degradation of TBT at the low temperatures in autumn and winter. Some authors have reported slower TBT degradation rates in water during these seasons due to weaker solar irradiation and lower microbial activity (Furdek et al. 2012; Tang et al. 2010). On the opposite, the observed decrease in TBT concentration and simultaneous increase in DBT levels in the spring season could be the result of a TBT extensive degradation triggered by biological processes taking place during the late winter-recent spring (Guinder et al. 2015). Further, as shown in Table 2, MBT levels were much higher than TBT and DBT. This could be the result of the TBT degradation directly to MBT in the column water, as some literatures suggest (Adelman et al. 1990). However, MBT concentration did not increase from autumn to spring as would have been expected. Probably, this behavior is resulting from a degradation rate of MBT to inorganic tin accelerated—in spring—due to high temperatures and Chl-a levels recorded in this season. In fact, the MBT degradation rate of almost 300 days mentioned above has been extrapolated from in vitro studies so, a possibility exists by which this rate is not achieved at the area of study. This conduct could be attributing this to specific local conditions as pH, microbial communities, etc. Furthermore, probably some secondary sources of MBT were increasing these compound levels during winter. One of them could possibly be wastewater discharges from the city, where domestic water (sewage) is drained without treatment (Hoch et al. 2003; Buggy and Tobin 2006). Another punctual MBT source could be the plastic industry sewage and drainage water pipes since MBT is used in the production process of PVC (Hoch 2001; Dong et al. 2015).
In regard to sediments (Table 3), TBT levels were quantifiable only in May—autumn (29.7 ± 1.2 ng Sn g−1). In winter and spring, TBT level were under the LOQ. DBT concentration appeared to decrease during the sampling period; its highest concentration was recorded also in autumn (67.4 ± 7.5 ng Sn g−1) while the minimum one was in November—spring (value higher than LOD but lower than LOQ). Finally, MBT was found to be above the LOQ along the entire sampling period. While the highest MBT levels were quantified in autumn and spring (463 ± 6 ng Sn g−1 and 134 ± 5 ng Sn g−1, respectively) winter levels were the lowest (47.0 ± 6.2 ng Sn g−1) (Table 3). Despite the high recorded levels of MBT, no quantifiable levels of TBT were found during winter and spring seasons. This scenario points to a more probable MBT source inputs to the area vs. the TBT degradation pathway. As mentioned above, MBT inputs could be originated from sewage discharges, landfill leachates, and diverse discharges of industrial effluents that are close to the study area (Hoch et al. 2003; Quintas et al. 2016).
In order to estimate TBT input age, we used the butyltin degradation index (BDI), originally proposed by Diez et al. (2002) for sediments and used in water samples (Choi et al. 2010):
BDI values > 1 suggest recent inputs of TBT, while BDI values < 1 suggest no recent inputs (Diez et al. 2002). In both, sediments and estuarine water samples, the ratios were less than one and, in some cases, values were closer to zero (Tables 2 and 3). In principle, these values indicate that if the presence of DBT and MBT in both compartments (sediment and water) comes only from the degradation of TBT, the contamination of OTCs is not recent or is under a strong process of degradation.
Physicochemical parameters
Temperature
The relationship between sedimentary OTCs levels and surface sediment temperature was studied for the first time at the area of study. At first sight, there was no clear pattern between the OTCs concentrations and temperature in both sediment and water samples (Tables 2 and 3); however, it was possible to observe significant differences between water column temperature (average 12.6 °C ± 4.8) and surface sediment temperature (average 25.2 °C ± 5.6) during the period of study. In addition, a second remark by which TBT levels were much lower than their respective breakdown compounds (DBT and MBT), even in August—winter and November—spring when TBT was not possible to quantify, leading to a new hypothesis: higher temperatures in surface sediments (21.2–29.1 °C) contribute to faster degradation of TBT in the sediment. In agreement with this, several authors have reported that increased temperature has been proven to accelerate degradation on account of that the metabolic activity of the sediment’s microorganisms increases with the temperature (Sakultantimetha et al. 2011a, b; Filipkowska et al. 2014; Cruz et al. 2015). Then, it is probable that the particularly high MBT levels recorded in the area could be originated because of the biodegradation process, triggered by the high temperature values in surface sediments.
Lastly, in water samples, when TBT degradation takes place at a faster rate than sediment samples, a similar trend was observed for during November—spring: when the water column temperature increased, TBT levels decreased (Table 2).
pH
OTCs adsorption/desorption is controlled, in other factors, by the pH (Hoch et al. 2002; Bangkedphol et al. 2009). For instance, some authors have reported that the maximum adsorption of TBT and DBT in sediments occurs between pH 6.0 and 6.5 (Hoch et al. 2003; Bangkedphol et al. 2009), while that the maximum desorption of these compounds takes place at pH 8 (Hoch et al. 2002, 2003).
During the period of study, the pH values in surface sediments for a whole tidal flat varied from 6.2 to 8.3 (Table 3). The highest pH value was recorded in November—spring when only MBT was quantified. Then, the rationale could be related to the maximum desorption of TBT and DBT at those pH values in the sediment (Hoch et al. 2002, 2003). In this way, under these conditions, TBT and DBT desorb from sediments to the overlaying water, increasing these analyte’s levels in the water column. On the contrary, the highest concentrations of TBT and DBT were recorded at pH 6.2—during the autumn season (May). In conclusion, while pH seemed to influence drastically the distribution of TBT and DBT between water and sediments, it did not appear to be an influential variable on MBT partitioning (Marcic et al. 2006).
Finally, in regard to the water column pH values (Table 2, ranging between 7.9 and 8.1), they were in agreement with the historical values for the area (Spetter et al. 2015; Fernández 2017) and showed no evidence of correlation with OTCs levels.
Eh
The Eh regulates much of the biogeochemical reactions involved in marine sediments, such as availability nutrients and re-mineralization of OM. The obtained Eh values for the area ranged from 123 to 175 mV. This indicated that the sediments were reducing during the study period (i.e., represent anaerobic conditions) and the oxidation of organic matter was probably being performed by the reduction of Fe (~ 100–180 mV) (Spetter et al. 2015; Fernández et al. 2016 and references therein). This assumption is based on the reported presence of FeS (pyrite) and the accumulation of Fe in sediments due to biofilms and microbial mats at the area (Cuadrado et al. 2012; Fernández 2017). Briant et al. (2016) argue that when there is a reduction of Fe in sediments, they are released up to 40 ng Sn L−1 through porewater to the water column. In fact, according to several authors, the availability of Fe (III) in sediments contributes to the abiotic degradation of TBT because these elements act as a photocatalyst (Tessier et al. 2007; Furdeck et al. 2016). This could probably explain the low TBT levels and simultaneous high MBT levels in sediment samples throughout the sampling period (Table 3).
Salinity
In seawater, the TBT and DBT affinity to the solid phase decreases strongly with increasing salinity (Hoch et al. 2003). Such behavior is explained in terms of the ion exchange competition of seawater cations with butyltin molecules for the binding sites on the clay mineral surfaces (Hoch et al. 2003). It is possible to observe in Table 2 that in May—autumn, when the salinity value was maximum, TBT also reached its maximum concentration in water. This fact points a positive correlation between TBT in water vs salinity which is in agreement with the literature. Similarly, when the lowest value of salinity was registered (November—spring), a greater adsorption of the TBT to the solid phase is observed and simultaneous decrease of the TBT levels in the water column was expected, which was in fact observed in the field (Table 2).
Turbidity
Turbidity showed maximum values (35 mg L−1) in June—winter and minimum during May—autumn (19 mg L−1). In general, these values describe highly turbid environments and are characteristic from the study area; in concordance, with diverse authors, they are a result of the combined effect of both winds and tidal currents (Gómez et al. 2005; Perillo et al. 2007; Carbone et al. 2016). During winter, when turbidity levels were high, TBT levels in the water column were lower than those recorded in autumn, when turbidity decreased (Table 2). As discussed earlier, this singular observation could be part of the general rule by which the TBT adsorption in the particulate material gives rise to a decrement of the TBT levels in the water column (Hoch and Schwesig 2004).
Organic matter
Organic matter performs a significant role in the persistence of OTCs within marine-coastal ecosystems in two different ways (Hoch and Schwesig 2004). It has a great responsibility on the TBT adsorption and in the composition of the microbial community present in the sediments (Furdeck et al. 2016). In fact, organic matter and dissolved organic matter content have been identified as the main adsorbent component of the matrix in sediments and water, respectively (Tessier et al. 2007; Quintas et al. 2016). In this sense, several authors have related the TBT persistence in contaminated sediments with the organic matter content (Hoch and Schwesig 2004; Pinochet et al. 2009). Other studies have also demonstrated that in sediments, it is possible the formation of volatile methylated butyltin compounds leads to the transference of these compounds to the aquatic environment (Tessier et al. 2007). The natural derivatization pathway that produces the potential biomethylation is through the presence of sulfate-reducing bacteria and different natural methyl donor species in sediments, especially humid substances. On the one side, an increase in %OM for sediments can be observed during June—winter and November—spring and a simultaneous decrement in OTCs levels (Table 3). Following Tessier et al. (2007), a rationale can be found in a probable formation of volatile methylated compounds. On the other side, Table 2 shows a trend by which increasing POM in the water column leads to TBT and MBT decrement and DBT increment. This behavior could be explained through the partition coefficient octanol-water (Kow). Several precedents showed that Kow value found for TBT is much higher than for DBT and MBT (Berg et al. 2001). Thus, TBT is more lipophilic and shows a higher affinity for organic matter. Further, DBT has a lower Kow, and a consequent lower affinity for organic matter (Hongxia et al. 1996).
Chlorophyll a and nutrients
Chl-a concentration was used as an estimation of phototrophic population biomass. Chl-a contents were the highest during November—spring (7.09 μg L−1 and 32.29 μg g−1, water and sediment, respectively) but only in water column this maximum was accompanied with a decrease of all macronutrients analyzed (Fig. 2). For sediments, this maximum could be associated with an extensive benthonic-pelagic interaction that exists in these types of environments (Guinder et al. 2015; Zapperi et al. 2016; Fernández 2017). Coincidentally, a decrease in TBT and DBT levels was recorded in sediments during November—spring, probably due to an accelerated biodegradation process caused for the microphytobenthos activity (Fig. 2d). This degradation appeared to act to a lesser extent for MBT, which showed a slight increase with respect to winter records.
In water column, Chl-a concentrations were lower than those recorded for sediments; this could be probably due to the fact that in shallow environments as the BBE, the biomass of benthic microalgae normally exceeds the biomass of phytoplankton in the water column (Fig. 2a, d) (MacIntyre et al. 1996). In addition, during spring (November), the increase of Chl-a levels matched with the detection of DBT, pointing to an accelerated TBT biodegradation by the presence of phytoplankton in the estuarine environment, as DBT can be extruded from the cell and released into the medium (Tsnag et al. 1999).
As shown in Fig. 2d, the higher the PO43− concentration in interstitial water is, the higher the sedimentary OTC levels are (Table 2). As it is well stated, antifouling paints are not only a source of OTCs but also of PO43−. In fact, the main organotin compound used as an industrial biocide is Tris (tributylstannyl) phosphate (Clark et al. 1988). Then, results point to a probable secondary source of PO43− via the interstitial water.
In November—spring, when the environmental conditions were favorable for NO3− accumulation, the presence of NO3− in porewater indicated a continuous process of nitrification (Kaspar 1983). At both samples, pore and column water, the concentration of NO3− increased from autumn (May) to spring, while TBT levels decreased (Fig. 2b, e). In this sense, several authors have shown that debutylation is promoted by the nitrate-reducing activity (Yonezawa et al. 1994; Briant et al. 2016); then, results were consistent with an active debutylation process triggered by nitrification.
High DSi concentrations recorded in porewater (151.4–156.3 μmol L−1) and water column (44.36–71.69 μmol L−1) may be due to the dissolution of silica compound present in the sediments (Fig. 2c, d), as previously reported by Spetter et al. (2015). In this way, the OTCs could spread to the water column via porewater through their hydrophobic interaction with mineral faces of sediments that include Si among other (Huang and Matzner 2004).
Conclusions
Water TBT levels throughout the sampling period indicated a continuous entry of this compound into the study area. While relatively high levels of MBT were found in sediments and water samples, they could not be uniquely attributed to a TBT degradation pathway; in fact, results outlined a possible MBT additional input in the area.
Physicochemical parameters demonstrated to be critical for the fate, mobility, and availability of OTCs within the marine-coastal ecosystem. In particular, it was possible to outline two general processes strongly ruled by them:
First is a general degradative process, headed by debutylation steps. In this sense, evidence shows that temperature triggers MBT production as a consequence of TBT and DBT biodegradation process. In addition, the presence of Fe (III) in sediments promotes the abiotic degradation of TBT while biotic debutylation is triggered by nitrification.
In the second place, the chemical partition process is critically affected by pH, salinity, turbidity, and organic matter contents. Indeed, a high salinity favors the TBT desorption from sediment particles to the water column while pH drastically influences the distribution of TBT and DBT between water and sediments. In addition, POM decreases the TBT levels in the water column while %OM in sediments favors the TBT accumulation.
Future tiered studies are necessary; however, the current work sets the first field evidence for OTCs fate and mobility within a marine-coastal environment, filling a gap in the knowledge of OTCs dynamics and increasing the awareness of the South American environments as a part of the international OCTs burden.
References
Adams, D. D. (1994) Sediment pore water sampling. In A. Mudroch & S. D. MacKnight (Eds), Handbook of techniques for aquatic sediments sampling, Second Edition CRC Press (pp. 171-202).
Adelman, D., Hinga, K. R., & Pilson, M. E. (1990). Biogeochemistry of butyltins in an enclosed marine ecosystem. Environmental Science & Technology, 24, 1027–1032. https://doi.org/10.1021/es00077a012.
APHA, AWWA, WEF. (1998). In L. S. Clesceri, A. E. Greenberg, & A. D. Eaton (Eds.), Standard methods for the examination of water and wastewater (20th ed.). Washington, 996: American Public Health Association.
Ayanda, O. S., Fatoki, O. S., Adekola, F. A., & Ximba, B. J. (2012). Fate and remediation of organotin compounds in seawaters and soils. Chemical Science Transactions, 1, 470–481. https://doi.org/10.7598/cst2012.177.
Bancon-Montigny, C., Lespes, G., & Potin-Gautier, M. (2000). Improved routine speciation of organotin compounds in environmental samples by pulsed flame photometric detection. Journal of Chromatography A, 896, 149–158. https://doi.org/10.1016/S0021-9673(00)00595-1.
Bangkedphol, S., Keenan, H. E., Davidson, C., Sakultantimetha, A., & Songsasen, A. (2009). The partition behavior of tributyltin and prediction of environmental fate, persistence and toxicity in aquatic environments. Chemosphere, 77, 1326–1332. https://doi.org/10.1016/j.chemosphere.2009.09.046.
Berg, M., Arnold, C. G., Müller, S. R., Mühlemann, J., & Schwarzenbach, R. P. (2001). Sorption and desorption behavior of organotin compounds in sediment−pore water systems. Environmental Science & Technology, 35, 3151–3157. https://doi.org/10.1021/es010010f.
Briant, N., Bancon-Montigny, C., Freydier, R., Delpoux, S., & Elbaz-Poulichet, F. (2016). Behaviour of butyltin compounds in the sediment pore waters of a contaminated marina (Port Camargue, South of France). Chemosphere, 150, 123–129. https://doi.org/10.1016/j.chemosphere.2016.02.022.
Buggy, C. J., & Tobin, J. M. (2006). Seasonal and spatial distributions of tributyltin in surface sediment of the Tolka Estuary, Dublin, Ireland. Environmental Pollution, 143, 294–303. https://doi.org/10.1016/j.envpol.2005.11.025.
Buhl-Mortensen, L. (1996). Amphipod fauna along offshore-fjord gradient. Journal of Natural History, 30, 23–49. https://doi.org/10.1080/00222939600770031.
Carbone, M. E., Spetter, C. V., & Marcovecchio, J. E. (2016). Seasonal and spatial variability of macronutrients and chlorophyll a based on GIS in the South American estuary (Bahía Blanca, Argentina). Environmental Earth Sciences, 75. https://doi.org/10.1007/s12665-016-5516-6.
Cassi, R., Tolosa, I., & de Mora, S. (2008). A survey of antifoulants in sediments from Ports and Marinas along the French Mediterranean coast. Marine Pollution Bulletin, 16, 355–359. https://doi.org/10.1002/aoc.315.
Castro, I. B., & Fillmann, G. (2012). High tributyltin and imposex levels in the commercial muricid Thais chocolata from two Peruvian harbor areas. Environmental Toxicology and Chemistry, 31, 955–960. https://doi.org/10.1002/etc.1794.
Choi, M., Moon, H. B., Yu, J., Eom, J. Y., & Choi, H. G. (2010). Temporal trend of butyltins in seawater, sediments, and mussels from Busan Harbor of Korea between 2002 and 2007: tracking the effectiveness of tributylin regulation. Archives of Environmental Contamination and Toxicology, 58, 394–402. https://doi.org/10.1007/s00244-009-9428-2.
Choi, J. Y., Hong, G. H., Ra, K., Kim, K. T., & Kim, K. (2014). Magnetic characteristics of sediment grains concurrently contaminated with TBT and metals near a shipyard in Busan, Korea. Marine Pollution Bulletin, 85, 679–685. https://doi.org/10.1016/j.marpolbul.2014.03.029.
Cima, F., Craig, P. J., & Harrington, C. (2003). Organotin compounds in the environment. In P. J. Craig (Ed.), Organometallic compounds in the environment (pp. 101–149). John Wiley & Sons: Chichester.
Clark, E. A., Sterritt, R. M., & Lester, J. N. (1988). The fate of tributyltin in the aquatic environment. Environmental Science & Technology, 22, 600–604.
Cole, R. F., Mills, G. A., Hale, M. S., Parker, R., Bolam, T., Teasdale, P. R., Bennett, W. W., & Fones, G. R. (2018). Development and evaluation of a new diffusive gradients in thin-films technique for measuring organotin compounds in coastal sediment pore water. Talanta, 178, 670–678. https://doi.org/10.1016/j.talanta.2017.09.081.
Commendatore, M. G., Franco, M. A., Gomes Costa, P., Castro, I. B., Fillmann, G., Bigatti, G., Esteves, J. L., & Nievas, M. L. (2015). BTs, PAHs, OCPs and PCBs in sediments and bivalve mollusks in a mid-latitude environment from the Patagonian coastal zone. Environmental Toxicology and Chemistry, 34, 2750–2763. https://doi.org/10.1002/etc.3134.
Cruz, A., Anselmo, A. M., Suzuki, S., & Mendo, S. (2015). Tributyltin (TBT): a review on microbial resistance and degradation. Critical Reviews in Environmental Science and Technology, 45, 970–1006. https://doi.org/10.1080/10643389.2014.924181.
Cuadrado, D. G., Carmona, N. B., & Bournod, C. (2011). Biostabilization of sediments by microbial mats in a temperate siliciclastic tidal flat, Bahia Blanca estuary (Argentina). Sedimentary Geology, 237, 95–101. https://doi.org/10.1016/j.sedgeo.2011.02.008.
Cuadrado, D. G., Carmona, N. B., & Bournod, C. N. (2012). Mineral precipitation on modern siliciclastic tidal flats colonized by microbial mats. Sedimentary Geology, 271-272, 58–66. https://doi.org/10.1016/j.sedgeo.2012.06.005.
de Castro, Í. B., Perina, F. C., & Fillmann, G. (2012). Organotin contamination in South American coastal areas. Environmental Monitoring and Assessment, 184, 1781–1799. https://doi.org/10.1007/s10661-011-2078-7.
Dean, W. E. J. R. (1974). Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. Journal of Sedimentary Petrology, 44, 242–248.
Del Brio, F., Commendatore, M., Castro, I. B., Gomes Costa, P., Fillmann, G., & Bigatti, G. (2016). Distribution and bioaccumulation of butyltins in the edible gastropod Odontocymbiola magellanica. Marine Biology Research, 12, 608–620. https://doi.org/10.1080/17451000.2016.1169296.
Delucchi, F., Narvarte, M. A., Amin, O., Tombesi, N. B., Freije, H., & Marcovecchio, J. (2011). Organotin compounds in sediments of three coastal environments from the Patagonian shore, Argentina. International Journal of Environment and Waste Management, 8, 3. https://doi.org/10.1504/IJEWM.2011.040962.
Di Bonito, M., Breward, N., Crout, N., Smith, B., & Young, S. (2008). Overview of selected soil pore water extraction methods for the determination of potentially toxic elements in contaminated soils: operational and technical aspects. In B. De Vivo, H. E. Belkin, & A. Lima (Eds.), Environmental geochemistry: site characterization, data analysis and case histories (pp. 213–249). Elsevier: Amsterdam.
Diez, S., Abalos, M., & Bayona, J. M. (2002). Organotin contamination in sediments from the Western Mediterranean enclosures following 10 years of TBT regulation. Water Research, 36, 905–918. https://doi.org/10.1016/S0043-1354(01)00305-0.
Dominguez, L. A., Caldas, S. S., Primel, E. G., & Fillmann, G. (2014). The influence of salinity and matrix effect in the determination of antifouling biocides in estuarine waters of Patos Lagoon (southern Brazil). Journal of the Brazilian Chemical Society. https://doi.org/10.5935/0103-5053.20140110.
Dong, C. D., Chen, C. F., & Chen, C. W. (2015). Composition and source of butyltins in sediments of Kaohsiung Harbor, Taiwan. Estuarine, Coastal and Shelf Science, 156, 134–143. https://doi.org/10.1016/j.ecss.2014.08.002.
Eberlein, K., & Kattner, G. (1987). Automatic method for determination of orthophosphate and total dissolved phosphorus in the marine environment. Fresenius’ Journal of Analytical Chemistry, 326, 354–357. https://doi.org/10.1007/BF00469784.
Eurachem (1998) The fitness for purpose of analytical methods. A laboratory guide to method validation and related topics. LGC (Teddington) Ltd, London, p 75.
Fang, L., Xu, C., Li, J., Borggaard, O. K., & Wang, D. (2017). The importance of environmental factors and matrices in the adsorption, desorption, and toxicity of butyltins: a review. Environmental Science and Pollution Research, 24, 9159–9173. https://doi.org/10.1007/s11356-017-8449-z.
Fernández, E. M. (2017) Dinámica de nutrientes, material orgánica y clorofila a en planicies de marea cubiertas por matas microbianas. http://repositoriodigital.uns.edu.ar/handle/123456789/3601.
Fernández, E. M., Spetter, C. V., Martinez, A. M., Cuadrado, D. G., Avena, M. J., & Marcovecchio, J. E. (2016). Carbohydrate production by microbial mats communities in tidal flat from Bahía Blanca Estuary (Argentina). Environmental Earth Sciences, 75(8), 641. https://doi.org/10.1007/s12665-016-5405-z.
Ferrer, L., Contardi, E., Andrade, S., Asteasuain, R., Pucci, A., & Marcovecchio, J. (2000). Environmental cadmium and lead concentrations in the Bahía Blanca estuary (Argentina). Potential toxic effects of Cd and Pb on crab larvae. Oceanologia, 43, 493–504.
Filipkowska, A., Kowalewska, G., & Pavoni, B. (2014). Organotin compounds in surface sediments of the southern Baltic coastal zone: a study on the main factors for their accumulation and degradation. Environmental Science and Pollution Research, 21, 2077–2087. https://doi.org/10.1007/s11356-013-2115-x.
Furdek, M., Vahčič, M., Ščančar, J., Milačič, R., Kniewald, G., & Mikac, N. (2012). Organotin compounds in seawater and Mytilus galloprovincialis mussels along the Croatian Adriatic Coast. Marine Pollution Bulletin, 64, 189–199. https://doi.org/10.1016/j.marpolbul.2011.12.009.
Furdeck, M., Mikac, N., Bueno, M., Tessier, E., Cavalheiro, J., & Monperrus, M. (2016). Organotin persistence in contaminated marine sediments and porewaters: In situ degradation study using species-specific stable isotopic tracers. Journal of Hazardous Materials, 307, 263–273. https://doi.org/10.1016/j.jhazmat.2015.12.037.
Gelós, E. M., Marcos, O. A., Spagnuolo, J. O., & Schillizzi, R. A. (2004). Textura y Mineralogía de los sedimentos. In M. C. Piccolo & M. Hoffmeyer (Eds.), Ecosistema del Estuario de Bahía Blanca (3rd ed., pp. 43–50). Bahía Blanca: Ediuns.
Gómez, E. A., Cuadrado, D. G., & Federici, G. A. (2005). Environmental impact assessment in a harbour area, Argentina. Thalass, An international Journal of Marine Science, 21, 31–38.
Gómez, N., Donato, J. C., Giorgi, A., Guasch, H., Mateo, P., & Sabater, S. (2009). La biota de los ríos: los microorganismos autótrofos. In A. Elosegi & S. Sabater (Eds.), Conceptos y Técnicas en Ecología Fluvial (pp. 234–236). España: Valant.
Grasshoff, K. (1976). Filtration and storage. In Methods of seawater analysis (pp. 21–24). Weinheim: Verlag Chemie.
Grasshoff, K., Erhardt, M., & Kremling, K. (1983). Methods of seawater analysis, 2nd Edition (pp. 365–366). Weinheim: Verlag-Chemie.
Guinder, V. A., López-Abbate, M. C., Berasategui, A. A., Negrin, V. L., Zapperi, G., Pratolongo, P. D., Férnandez Severini, M. D., & Popovich, C. A. (2015). Influence of the winter phytoplankton bloom on the settled material in a temperate shallow estuary. Oceanologia, 57, 50–60. https://doi.org/10.1016/j.oceano.2014.10.002.
Hoch, M. (2001). Organotin compounds in the environment—an overview. Applied Geochemistry, 16, 719–743. https://doi.org/10.1016/S0883-2927(00)00067-6.
Hoch, M., & Schwesig, D. (2004). Parameters controlling the partitioning of tributyltin (TBT) in aquatic systems. Applied Geochemistry, 19, 323–334. https://doi.org/10.1016/S0883-2927(03)00131-8.
Hoch, M., Alonso Azcarate, J., & Lischick, M. (2002). Adsorption behavior of toxic tributyltin to clayrich sediments under various environmental conditions. Environmental Toxicology and Chemistry, 21, 1390–1397. https://doi.org/10.1002/etc.5620210709.
Hoch, M., Alonso-Azcarate, J., & Lischick, M. (2003). Assessment of adsorption behavior of dibutyltin (DBT) to clay-rich sediments in comparison to the highly toxic tributyltin (TBT). Environmental Pollution, 123, 217–227. https://doi.org/10.1016/S0269-7491(02)00402-5.
Hongxia, L., Guolan, H., & Shugui, D. (1996). Transport of butyltins at the water-air interface and the adsorptive behavior of tributyltin in the surface microlayer. Toxicological & Environmental Chemistry, 55, 257–265. https://doi.org/10.1080/02772249609358340.
Huang, J. H., & Matzner, E. (2004). Adsorption and desorption of organotin compounds in organic and mineral soils. European Journal of Soil Science, 55, 693–698. https://doi.org/10.1111/j.1365-2389.2004.00634.x.
IMO, International Maritime Organization. (2005). Antifouling systems. International convention on the control of harmful antifouling systems on ships. London: International Maritime Organization.
Iribarne, O., Martinetto, P., Schwindt, E., Botto, F., Bortolus, A., & Borboroglu, P. G. (2003). Evidences of habitat displacement between two common soft-bottom SW Atlantic intertidal crabs. Journal of Experimental Marine Biology and Ecology, 296, 167–182. https://doi.org/10.1016/S0022-0981(03)00318-6.
IUPAC- International Union of Pure and Applied Chemistry. (2002). Analytical, applied, clinical, inorganic, and physical chemistry divisions interdivisional working party for harmonization of quality assurance schemes for analytical laboratories. Pure and Applied Chemistry, 74, 835–855.
Jin, J., Yang, L., Chan, S. M., Luan, T., Li, Y., & Tam, N. F. (2011). Effect of nutrients on the biodegradation of tributyltin (TBT) by alginate immobilized microalga, Chlorella vulgaris, in natural river water. Journal of Hazardous Materials, 185, 1582–1586. https://doi.org/10.1016/j.jhazmat.2010.09.075.
Kaspar, H. F. (1983). Denitrification, nitrate reduction to ammonium, and inoganic nitrogen pools in intetidal sediments. Marine Biology, 74(2), 133-139. https://doi.org/10.1007/BF00413916
Koroleff, F. (1969) Direct determination of ammonia in natural waters as indophenol blue. ICES C.M. 1969/C: 9. Hydrol Commun p. 4.
La Colla, N. S., Botté, S. E., Oliva, A. L., & Marcovecchio, J. E. (2017). Tracing Cr, Pb, Fe and Mn occurrence in the Bahía Blanca estuary through commercial fish species. Chemosphere, 175, 286–293. https://doi.org/10.1016/j.chemosphere.2017.02.002.
Laitano, M. V., Castro, Í. B., Costa, P. G., Fillmann, G., & Cledón, M. (2015). Butyltin and PAH contamination of Mar del Plata port (Argentina) sediments and their influence on adjacent coastal regions. Bulletin of Environmental Contamination and Toxicology, 95, 513–520. https://doi.org/10.1007/s00128-015-1637-y.
López Abbate, M. C., Molinero, J. C., Guinder, V. A., Perillo, G. M., Freije, R. H., Sommer, U., Spetter, C. V., & Marcovecchio, J. E. (2017). Time-varying environmental control of phytoplankton in a changing estuarine system. Science of the Total Environment, 609, 1390–1400. https://doi.org/10.1016/j.scitotenv.2017.08.002.
Lorenzen, C. J. (1967). Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnology and Oceanography, 12, 343–346. https://doi.org/10.4319/lo.1967.12.2.0343.
MacIntyre, H. L., Geider, R. J., & Miller, D. C. (1996). Microphytobenthos: the ecological role of “the secret garden” of unvegetated, shallow-water marine habitats. I: distribution, abundance and primary production. Estuaries and Coasts. https://doi.org/10.2307/1352224.
Marcic, C., Le Hecho, I., Denaix, L., & Lespes, G. (2006). TBT and TPhT persistence in a sludged soil. Chemosphere, 65, 2322–2332. https://doi.org/10.1016/j.chemosphere.2006.05.007.
Martin, J. B., Hartl, K. M., Corbett, D. R., Swarzenski, P. W., & Cable, J. E. (2003). A multilevel pore-water sampler for permeable sediments. Journal of Sedimentary Research, 73, 128–132. https://doi.org/10.1306/070802730128.
Martínez, M. L., Piol, M. N., Nudelman, N. S., & Guerrero, N. R. V. (2017). Tributyltin bioaccumulation and toxic effects in freshwater gastropods Pomacea canaliculata after a chronic exposure: field and laboratory studies. Ecotoxicology, 26, 691–701. https://doi.org/10.1007/s10646-017-1801-8.
Michel, P., & Averty, B. (1999). Distribution and fate of tributyltin in surface and deep waters of the northwestern Mediterranean. Environmental Science & Technology, 33, 2524–2528. https://doi.org/10.1021/es981254b.
Ogbomida, E. T., & Ezemonye, L. I. (2016). Tributyltin and its derivative in water samples of National Inland Water Way Authority Harbour of Warri, Delta State, Nigeria. Organic Chemistry Current Reserch. https://doi.org/10.4172/2161-0401.1000158.
Perillo, G. M. E., Piccolo, M. C., Parodi, E., & Freije, R. H. (2001). The Bahía Blanca Estuary, Argentina. In U. Seeliger & B. Kjerfve (Eds.), Coastal marine ecosystems of Latin America, ecological studies (pp. 205–217). Berlin: Springer.
Perillo, G. M. E., Piccolo, M. C., Palma, E. D., Pérez, D. E. & Pierini, J. O. (2007) Oceanografía física. In: M.C. Piccolo & M.S. Hoffmeyer (Eds.) Ecosistemas del estuario de Bahía Blanca (pp. 61-67). Bahía Blanca: ediUNS (ISBN 987-9281-96-9).
Piccolo, M. C. & Diez, P. G. (2004). Meteorología del Puerto Coronel Rosales. In: M.C. Piccolo & M.S. Hoffmeyer (Eds). Ecosistema del Estuario de Bahía Blanca (pp. 87-91). Bahía Blanca: ediUNS (ISBN 987-9281-96-9).
Piccolo, M. C., Perillo, G. M. E., & Melo, W. D. (2008). The Bahía Blanca Estuary: an integrated overview of its geomorphology and dynamics. In R. J. Neves, J. Baretta, & M. D. Mateus (Eds.), Perspectives on integrated coastal zone management in South America (pp. 221–232). Portugal: IST Press.
Pinochet, H., Tessini, C., Bravo, M., Quiroz, W., & De Gregori, I. (2009). Butyltin compounds and their relation with organic matter in marine sediments from San Vicente Bay—Chile. Environmental Monitoring and Assessment, 155, 341–353. https://doi.org/10.1007/s10661-008-0439-7.
Point, D., Monperrus, M., Tessier, E., Amouroux, D., Chauvaud, L., Thouzeau, G., & Clavier, J. (2007). Biological control of trace metal and organometal benthic fluxes in a eutrophic lagoon (Thau Lagoon, Mediterranean Sea, France). Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2006.11.013.
Pougnet, F., Schäfer, J., Dutruch, L., Garnier, C., Tessier, E., Dang, D. H., Lanceleur, L., Mullot, J. U., Lenoble, V., & Blanc, G. (2014). Sources and historical record of tin and butyl-tin species in a Mediterranean bay (Toulon Bay, France). Environmental Science and Pollution Research, 21, 6640–6651. https://doi.org/10.1007/s11356-014-2576-6.
Quintas, P. Y., Oliva, A. L., Arias, A., Domini, C. E., Alvarez, M. B., Garrido, M., & Marcovecchio, J. E. (2016). Seasonal changes in organotin compounds in sediments from the Bahía Blanca Estuary. Environmental Earth Sciences. https://doi.org/10.1007/s12665-016-5471-2.
Quintas, P. Y., Arias, A. H., Oliva, A. L., Domini, C. E., Alvarez, M. B., Garrido, M., & Marcovecchio, J. E. (2017). Organotin compounds in Brachidontes rodriguezii mussels from the Bahía Blanca Estuary, Argentina. Ecotoxicology and environmental safety, 145, 518–527. https://doi.org/10.1016/j.ecoenv.2017.07.052.
Ranke, J., & Jastorff, B. (2000). Multidimensional risk analysis of antifouling biocides. Environmental Science and Pollution Research, 7, 105–114. https://doi.org/10.1065/espr199910.003.
Sabah, A., Bancon-Montigny, C., Rodier, C., Marchand, P., Delpoux, S., Ijjaali, M., & Tournou, M. G. (2016). Occurrence and removal of butyltin compounds in a waste stabilisation pond of a domestic waste water treatment plant of a rural French town. Chemosphere, 144, 2497–2506. https://doi.org/10.1016/j.chemosphere.2015.11.006.
Sakultantimetha, A., Keenan, H. E., Beattie, T. K., Bangkedphol, S., & Cavoura, O. (2011a). Bioremediation of tributyltin contaminated sediment: degradation enhancement and improvement of bioavailability to promote treatment processes. Chemosphere, 83, 680–686. https://doi.org/10.1016/j.chemosphere.2011.02.024.
Sakultantimetha, A., Keenan, H. E., Beattie, T. K., Bangkedphol, S., & Cavoura, O. (2011b). Effects of organic nutrients and growth factorson biostimulation of tributyltin removal by sediment microorganisms and Enterobacter cloacae. Applied Microbiology and Biotechnology, 90, 353–360. https://doi.org/10.1007/s00253-010-3023-3.
Santisteban, J. I., Mediavilla, R., López-Pamo, E., Dabrio, C. J., Ruiz Zapata, M. B., Gil García, M. J., Castaño, S., & Martínez-Alfaro, P. E. (2004). Loss on ignition: a qualitative or quantitative method for organic matter and carbonate mineral content in sediments? Journal of Paleolimnology, 32, 287–299. https://doi.org/10.1023/B:JOPL.0000042999.30131.5b.
Santos, M. M., Vieira, N., Reis-Henriques, M. A., Santos, A. M., Gomez-Ariza, J. L., Giraldez, I., & Ten Hallers-Tjabbes, C. C. (2004). Imposex and butyltin contamination off the Oporto Coast (NW Portugal): a possible effect of the discharge of dredged material. Environment International, 30, 793–798. https://doi.org/10.1016/j.envint.2004.01.005.
Severini, M. D. F., Botté, S. E., Hoffmeyer, M. S., & Marcovecchio, J. E. (2009). Spatial and temporal distribution of cadmium and copper in water and zooplankton in the Bahia Blanca estuary, Argentina. Estuarine, Coastal and Shelf Science, 85, 57–66. https://doi.org/10.1016/j.ecss.2009.03.019.
Shue, M. F., Chen, T. C., Bellotindos, L. M., & Lu, M. C. (2014). Tributyltin distribution and producing androgenic activity in water, sediment, and fish muscle. Journal of Environmental Science and Health, 49, 432–438. https://doi.org/10.1080/03601234.2014.894780.
Simpson, S. L., Batley, G. E., Chariton, A. A., Stauber, J. L., King, C. K., Chapman, J. C., et al. (2005). Handbook for sediments quality assessment. Bangor: CSIRO.
Spetter, C. V., Buzzi, N. S., Fernández, E. M., Cuadrado, D. G., & Marcovecchio, J. E. (2015). Assessment of the physicochemical conditions sediments in a polluted tidal flat colonized by microbial mats in Bahía Blanca Estuary (Argentina). Marine Polluted Bulletin, 91, 491–505. https://doi.org/10.1016/j.marpolbul.2014.10.008.
Strickland, J.D.H. & Parson, T.R. (1968) A practical handbook of seawater analysis. Fisheries Research Board of Canada: Bulletin 167.
Tang, C. H., Hsu, C. H., & Wang, W. H. (2010). Butyltin accumulation in marine bivalves under field conditions in Taiwan. Marine Environmental Research, 70, 125–132. https://doi.org/10.1016/j.marenvres.2010.03.011.
Technicon Autoanalyzer II®. (1973) Industrial Methods N° 186–72 W/B.
Tessier, E., Amouroux, D., Morin, A., Christian, L., Thybaud, E., Vindimian, E., & Donard, O. F. (2007). (Tri) Butyltin biotic degradation rates and pathways in different compartments of a freshwater model ecosystem. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2007.08.047.
Treguer, P. & Le Corre, P. (1975a) Analyse des sels nutritifs sur autoanalyzer II. Manuel D‟Analyse des Sels Nutritifs dans L‟Eau de Mer (pp. 11-22), France: Univ. Bretagne Occidentale.
Treguer, P. & Le Corre, P. (1975b) Analyse des sels nutritifs sur autoanalyzer II. Manuel D‟Analyse des Sels Nutritifs dans L‟Eau de Mer, (pp. 34-49), France: Univ. Bretagne Occidentale.
Tsnag, C. K., Lau, P. S., Tam, N. F. Y., & Wong, Y. S. (1999). Biodegradation capacity of tributyltin by two Chlorella species. Environmental Pollution, 105, 289–297. https://doi.org/10.1016/S0269-7491(99)00047-0.
Vidal, J. M., Vega, A. B., Arrebola, F. J., González-Rodríguez, M. J., Sánchez, M. M., & Frenich, A. G. (2003). Trace determination of organotin compounds in water, sediment and mussel samples by low-pressure gas chromatography coupled to tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 17, 2099–2106. https://doi.org/10.1002/rcm.1152.
Yonezawa, Y., Fukui, M., Yoshida, T., Ochi, A., Tanaka, T., Noguti, Y., Kowata, T., Sato, Y., Masunaga, S., & Urushigawa, Y. (1994). Degradation of tri-n-butyltin in Ise Bay sediment. Chemosphere, 29, 1349–1356. https://doi.org/10.1016/0045-6535(94)90265-8.
Zapperi, G., Pratolongo, P., Piovan, M. J., & Marcovecchio, J. E. (2016). Benthic-Pelagic coupling in an intertidal mudflat in the Bahía Blanca Estuary (SW Atlantic). Journal of Coastal Research, 32(3), 629-637. https://doi.org/10.2112/JCOASTRES-D-14-00064.1
Zhang, C. N., Zhang, J. L., Huang, Y., Ren, H. T., Guan, S. H., & Zeng, Q. H. (2017). Dibutyltin depressed immune functions via NF-κB, and JAK/STAT signaling pathways in zebrafish (Danio rerio). Environmental Toxicology, 33, 104–111. https://doi.org/10.1002/tox.22502.
ZS-ZF. (2007) Zona Franca Buenos Aires Sur S.A., Concesionaria de la Zona Franca Bahía Blanca - Coronel Rosales. http://www.zfzonasur.com.ar. Accessed April 2018.
Acknowledgements
The authors would like to thank the IADO executive directors and Chemical Oceanography Area’s staff.
Funding
This research was supported by a doctoral grant funded by the National Council of Scientific and Technological Research (CONICET-Argentina) and was part of the PhD thesis of Pamela Y. Quintas and Eleonora M. Fernández. Funding was provided through research grants by CONICET (PIP D-738 2011), National Agency for Promotion of Science and Technology-ANPCyT (PICT 2015-0709, PICT 2012-2794), and National South University-UNS (PGI 24/Q086, PGI 24/ZQ12, PGI 24/ZQ15).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
● OTCs were analyzed in sediment and estuarine water from Rosales Port (Argentina) where a continuous input of TBT was assessed.
● MBT inputs were demonstrated to be originated apart from the rest of the OTCs.
● Several physicochemical parameters appeared to be critical in OTCs behavior.
● Butyltin Degradation Index analyses suggested aged TBT inputs possibly under a general degradation process.
Rights and permissions
About this article
Cite this article
Quintas, P.Y., Fernández, E.M., Spetter, C.V. et al. Preliminary studies about the role of physicochemical parameters on the organotin compound dynamic in a South American estuary (Bahía Blanca, Argentina). Environ Monit Assess 191, 127 (2019). https://doi.org/10.1007/s10661-019-7260-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7260-3