Abstract
Sediments are the ultimate sink for many toxic organic contaminants released into aquatic environment. The present study evaluated the toxicity effect of 13 surface sediment samples from Huangpu River and Suzhou River, East China using two-hybrid yeast bioassays for estrogenic and thyroidal effects and H4IIE rat hepatoma cell bioassay for ethoxyresorufin O-deethylase (EROD) activity. Toxicity was expressed as 17β-estradiol equivalent (E2-EQ), 3,3′,5-triiodothyronine equivalent (T3-EQ), and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) equivalent (TEQ). At the same time, the causality between the observed EROD activity and concentrations of polycyclic aromatic hydrocarbons (PAHs) was examined. The results showed that the total estrogenic effects in sediments ranged from 0.06 to 1.21 μg E2-EQ kg−1 dry weight (dw), the thyroidal effects ranged from 4.68 to 69.9 μg T3-EQ kg−1 dw, and significantly positive correlations were found between lgT3-EQs and lgE2-EQs. The AhR agonist effects varied from 26.5 to 148.3 ng TEQ kg−1 dw. Chemical analysis-derived TEQs contributed by PAHs ranged from 13.8 to 66.0 ng kg−1 dw accounting for 27.2–109.9 % with mean of 48.9 % of TEQbio, indicating that PAHs made important contributions to the EROD effects of sediment extracts from the two rivers. The present study would provide meaningful information for further analysis and risk evaluation for organic pollutants in Huangpu River and Suzhou River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For a large number of dangerous organic pollutants, the aquatic environment serves as the major route of distribution, and the sediments represent the ultimate sink (Peck et al. 2004; Luo et al. 2009). Various classes of contaminants in sediment are biologically available to aquatic organisms and can have adverse effects (Kwok et al. 2010; Li et al. 2016). However, most evaluations on pollutants in water and sediment are carried out by chemical analysis, which can hardly gain information on the risk stressor affection of the system. To assess the hazardous effect and identify causative chemicals in the contaminated sediments, a link between effect data and hazardous compounds based on a combination of biotesting and chemical analysis is developed (Floehr et al. 2015; Hong et al. 2016). In this combination system, the main aim of bioassays is to identify the total biological activity of the compounds with the same mode of action (MOA) in the extracts of environmental samples (Luo et al. 2011; Floehr et al. 2015; Li et al. 2016; Hong et al. 2016). Therefore, bioassays are very important research tools in estimating complex toxicity effects of samples and identifying causative chemicals in environmental samples. In particular, in vitro bioassays based on the action mechanism of chemicals have the advantages of being rapid, sensitive, and relatively inexpensive and are widely used to screen ecological risk stressors of concern (Liu et al. 2014a; Floehr et al. 2015). In the past decades, numerous studies have reported the use of in vitro assays to assess the toxicity of riverine sediment samples (Luo et al. 2011; Lei et al. 2015; Floehr et al. 2015).
Contaminated sediment represents an important environmental issue as they can contain complex mixtures of both known and unknown organic contaminants (Floehr et al. 2013). Among the contaminants, environmental endocrine disrupting chemicals (EDCs) draw attention due to their potential adverse effects on human and various animal species at low dose. Mounting evidence suggests that EDCs can interfere with the organism’s endocrine system and adversely affect their reproduction and development, neural network, and cardiovascular, metabolic, and immune systems. Environmental endocrine ligands of estrogen receptor (ER), thyroidal hormone receptor (TR), and aryl hydrocarbon receptor (AhR) have been known to be essential for normal growth and development in both humans and animals, and increasing evidence suggests that a number of traditional EDCs and many emerging organic pollutants may disrupt these endocrine ligands signaling (Lei et al. 2013; Liu et al. 2014a; Li et al. 2014). A number of in vitro bioassays have been developed to characterize the corresponding toxic effects (Liu et al. 2014a, 2015; Lei et al. 2015; Floehr et al. 2015; Zhang et al. 2016a). The present study mainly focused on estrogenic, thyroidal, and Ah receptor-mediated effects evaluated by a two-hybrid yeast bioassay and H4IIE rat hepatoma cell bioassay. The two-hybrid yeast bioassay systems rely on recombinant yeast cells expressing an estrogen or thyroidal receptor, which, upon binding with hormones, acts as a transcriptional enhancer for an estrogen- or thyroid-responsive DNA element-controlled reporter gene, and β-galactosidase activity serves as a measure for the estrogenic or thyroidal potency caused by chemicals (Li et al. 2014). The H4IIE cell bioassay used in the present study is based on Ah receptor-dependent ethoxyresorufin O-deethylase (EROD) induction. This enzymatic assay can serve as a sensitive biomarker for characterizing and assessing the exposure to dioxin-like contaminants such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs) in environmental samples (Luo et al. 2009; Floehr et al. 2015).
Shanghai is located in the Yangtze River Delta and is a large industrial and commercial city with a total population of over 19 × 106 in 2010. Huangpu River, the largest river flowing through Shanghai, is about 114 km long and originates in the lake district (Dianshan Lake) of Shanghai municipality and flows northeast past Shanghai into the Yangtze River at Wusong port. Suzhou River is the largest tributary of Huangpu River and is known as the second largest river in Shanghai after Huangpu River. Huangpu River and Suzhou River are important for water supply, cultivation irrigation, navigation, and tourism. However, in recent years, because the two sides of the rivers gather a large number of industrial enterprises, many domestic sewages and industrial wastewaters flow into the rivers, seriously reducing the water quality. Therefore, their pollution status should cause attention. Especially, Huangpu River is the last tributary of the Yangtze River before empting into the East Sea and the confluence is near the Yangtze Estuary which exhibited stronger pollution than other sections of the Yangtze River (Floehr et al. 2013). Therefore, the pollution status of Huangpu River to study the pollution mass transfer of the Yangtze River for the environmental quality assessment of its estuary and the East China Sea is very important.
However, to date, most of the studies mainly focused on the distribution and sources of pollutants (Wu et al. 2012, 2013; Shi et al. 2014; Zhang et al. 2014) in these two rivers are mainly based on instrument analysis. Far fewer studies investigated the toxicity effects especially endocrine disrupting effects of organic pollutants in the water or sediments in Huangpu River and Suzhou River by bioassay (Zhang et al. 2015). In this study, the ER-mediated estrogenic effect, TR-mediated thyroidal hormone effect, and AhR-mediated EROD effect of the sediments from Huangpu River and Suzhou River were investigated. The contribution of PAHs to the EROD effects of sediment extracts based on a combination of the H4IIE rat hepatoma cell bioassay and chemical analysis were identified.
Methods and materials
Chemicals
17β-estradiol (E2), 3,3’,5-triiodo-l-thyronine (T3, 95 %) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) were purchased from Sigma (St. Louis, MO, USA). For all compounds, stock solutions were prepared in dimethyl sulfoxide (DMSO, 99.5 %, AMRESCO, USA).
Sample collection
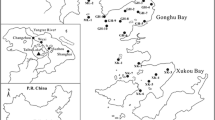
In July 2010, the 13 sampling sites were set along Huangpu River and Suzhou River according to the location of raw water supply for drinking purposes (H1), river confluence reaches (S2, H2, H5, H8, and H9), near the drainage outlets of the sewage treatment plants (S1 and S4), near the industrial district (S3 and H3), and densely populated area (H4, H5, and H6) as shown in Fig. 1. The latitude and longitude of the sampling sites were measured by global positioning systems (GPS) and are listed in Table 1. The detailed collection and the storage methods of sediments were described elsewhere (Wu et al. 2013; Lei et al. 2015).
A detailed description of the sample preparation was provided by Lei et al. (2015). Briefly, the freeze-dried and sieved sediments (20 g) were extracted with 200 mL of n-hexane/acetone mixture (1/1, v/v) using a Soxlet extractor for 48 h. The extractions were subsequently concentrated to approximately 10 μL followed by a solvent exchange with 400 μL DMSO. Five series of concentrations obtained by twofold dilution with DMSO from stock solutions were used in the bioassays. The final concentrations of DMSO used in the bioassays were 1 % for estrogenic and thyroidal hormone effect assay and 0.5 % for EROD effect assay respectively, which was shown to be non-toxic to yeast cells and H4IIE cells (Hu et al. 2002; Luo et al. 2009). At the same time, the preexperiment also proved that DMSO at 1 % was not toxic to yeast cells by detecting optical density (OD) at 595 nm and at 0.5 % was also non-toxic to H4IIE cells by MTT cytotoxicity assay.
Two-hybrid yeast bioassay
Sediment samples were analyzed for thyroid hormone receptor- or estrogen receptor-mediated endocrine disrupting potential using a previously developed two-hybrid yeast-based screen system, and a detailed description of the bioassay was provided elsewhere (Hu et al. 2002). Briefly, the estrogen or thyroid agonist activities of the sediment samples were assayed by measuring β-galactosidase activity. E2 for estrogenic activity and 3,3′–5-triiodothyronine (T3) for thyroidal activity were used as positive controls, and DMSO was used as a negative control. All experiments were carried out in triplicate. β-Galactosidase activity was calculated according to the method provided by Hu et al. (2002) and was expressed as the means and standard deviations (SDs) of the triplicate results.
The standard solutions were prepared by serial dilution of E2 or T3 stock solution that could yield dose–response curves for the quantitative determination of endocrine activity expressed in μg kg−1 as E2 equivalent (E2-EQ) or T3 equivalent (T3-EQ) by comparing the induction of activity caused by environmental sample extracts with that caused by authentic E2 or T3 standards. The minimum detectable concentrations were 6 pg E2-EQ well−1 for E2 and 30 pg T3-EQ well−1 for T3. Blanks for the extraction procedure were included, and their estrogenic and thyroidal effect levels were all below the detection limits.
EROD activity analysis
Rate hepatoma cells (H4IIE) were purchased from the Cell Culture Center, Institute of Basic Medical Chinese Academy of Medical Sciences, China. The routine maintenance of cells was conducted as described elsewhere (Luo et al. 2009; Lei et al. 2015). The standard solutions were prepared by serial dilution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) standard solutions (TCDD 0–140 pg mL−1 in DMSO culture ) that could yield a full dose–response curve of EROD induction. A detailed description of the bioassay was provided elsewhere (Qiao et al. 2006; Lei et al. 2015). EROD enzyme activity was analyzed according to the method described by Qiao et al. (2006). The data from the EROD measurements were inputted to a sigmoid curve-fitting program:
where y is the EROD response; A is the minimum EROD response; B is the relative slope of middle region; C is the EC50 which is the concentration of TCDD eliciting 50 % of the maximal inducible EROD activity; and D is the maximum EROD response. Generally, TEQbio could be calculated according to the method described by Eichbaum et al. (2014) and the EC25 or EC50 value was usually chosen as effect level. In the present study, the bioassay-derived TCDD equivalents (expressed as TEQbio) in the sediment samples were obtained by comparing with the dose–response curve of the standard solution on the same plate (Qiao et al. 2006). If necessary, the extract of the sediment sample was diluted to fit the linear part of the TCDD dose–response curve. Therefore, we did not need to select the EC25 or EC50 value as effect level in the calculation of TEQbio. The minimum detectable concentrations were 0.10 pg TEQ well−1. Blanks for the extraction procedure were included, and their AhR agonistic effect levels were all under the detectable limit.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 (SPSS, Chicago, IL, USA) and Origin 8.0 (OriginLab, Northampton, MA, USA). Prior to data analysis, the experimental data were checked for the assumptions of normality using the Kolmogorov–Smirnov one-sample test. The final data were presented as mean ± standard deviation. The spatial distribution differences in E2-EQs and T3-EQs among the two rivers were compared using nonparametric two-sample Kruskal–Wallis tests. In addition, to evaluate the relationship of the two effects, E2-EQs, T3-EQs, and TEQs were logarithmically transformed for normality (one-simple Kolmogorov–Smirnov test) (p > 0.05), and the linear correlations and Spearman correlations were analyzed. The results of all tests were accepted as significant at p < 0.05.
Results and discussion
The bioassay-derived E2-EQs, T3-EQs, and TEQs of the sediment from Huangpu River and Suzhou River are listed in Table 1.
Estrogenic effects of sediment extractions
The E2-EQ values varied greatly among the 13 sediment samples collected from the 13 sites in Huangpu River and Suzhou River, with the highest and the lowest E2-EQ values in the S1 and S3 sites from the Suzhou River, respectively. The total estrogenic activity in the 13 sediment samples ranged from 0.06 ± 0.011 to 1.21 ± 0.27 μg E2-EQ kg−1 dw with a mean value of 0.37 ± 0.33 μg E2-EQ kg−1 dw (Table 1). The mean values of estrogen effects in Huangpu River and Suzhou River were 0.23 ± 0.077 and 0.69 ± 0.47 μg E2-EQ kg−1 dw, respectively. The significant difference between Huangpu River and Suzhou River in the spatial distribution of E2-EQs was observed by nonparametric two-sample Kruskal–Wallis tests. A global comparison revealed that the ER-mediated activity in the sediment from Huangpu River and Suzhou River is of medium levels, which is close to that in the Lake Karlskopf quarry pond in Germany (Schulze-Sylvester et al. 2016), the Three Gorges Reservoir and Liaohe River in China (Wang et al. 2014; Ke et al. 2015), and is higher than that in the Taihu Lake in China (Lei et al. 2015), Lake Shihwa in Korea (Koh and Khim 2005), and two United Kingdom rivers (Peck et al. 2004) (Table 2). However, the estrogenic activity in the sediments from the Huangpu River and Suzhou River were lower than that in the sediments from the Wenyu, Haihe, and Dagu drainage rivers in China (Song et al. 2006a; Luo et al. 2011) and from industrialized areas in the Dutch Wadden Sea ranges in Netherlands (Legler et al. 2002). This may be because sediments in these studies inducing estrogenicity are collected from the rivers which receive sewage discharge. The sources of various ER agonists in sediments are hypothesized to primarily originate from sewage effluents and industrial discharges (Luo et al. 2011; Liu et al. 2014b). In recent years, part reaches of Huangpu River are set as the drinking water source for Shanghai City, and the amounts of sewage effluents released into Huangpu River are strictly controlled. This can explain that the estrogenicity detected in Huangpu River sediments is lower than that observed in the aforementioned studies. Suzhou River had been seriously polluted for the last century because a large number of industrial, agricultural, and domestic wastewaters from the Shanghai area are directly or indirectly discharged into Suzhou River. However, to improve the water quality of Suzhou River, the government began to clear contaminated sediments in Suzhou River and control sewage release into Suzhou River at the beginning of the 90s of the last century. Therefore, this may be the reason for the lower estrogen effect in the sediments from Suzhou River compared with that from other studies.
Some investigators have found that the estrogen fractions of organic extracts from aquatic sediment have high potency in inducing estrogenic activity. For example, Luo et al. (2011) found that the chemicals in the sediment from Wenyu River, Beijing, China, inducing significant estrogenic effects based on two-hybrid yeast bioassay, were six estrogens (estrone (E1), E2, estriol (E3), estradiol valerate (EV), 17α-ethinylestradiol (EE2) and diethylstilbestrol (DES)) and two phenols (bisphenol A (BPA) and 4-nonylphenol (4-NP)). Ke et al. (2015) found that E1, E2, and EE2 were the predominant ER agonists in the sediments from Liaohe River in China. Through causality analysis of combination, the EEQ values of yeast assay and chemical analysis, Rao et al. (2013) also found that EE2 and E2 were the main contributors to the estrogenic effects of river samples from three rivers, Tianjin, China, accounting for 63.0–185.7 % of the whole estrogenic activities. These estrogens are also found in Huangpu River and Suzhou River. For example, Zhang et al. (2014) found that E1, E2, E3, EE2, NP, and BPA were detected in all 11 sampling sites from Huangpu River and the values of EEQ suggest a high possibility of endocrine effects on exposed organisms in Huangpu River. Wu et al. (2013) reported that Huangpu River and Suzhou River were polluted by estrogenic compounds such as 4-NP and BPA, which are found in surface sediments with 4-NP concentrations in the range of 13.2–337.7 μg kg−1 for Huangpu River, 145.0–197.6 μg kg−1 for Suzhou River and BPA concentrations in the range of 0.96–13.5 μg kg−1 for Huangpu River and 6.28–7.68 μg kg−1 for Suzhou River, respectively. In addition, Shi et al. (2014) investigated the distribution of E1, E2, EE2, and BPA in the Yangtze River Estuary and the adjacent East China Sea and found that E1 and BPA were the dominant compounds detected and the zones of the highest E1 and BPA concentrations in water were located at the mouth of the Huangpu River. Therefore, the estrogenic effects in this area may be caused by these estrogenic chemicals. Of course, further studies should be conducted to identify specific ER agonists in the sediments from Huangpu River and Suzhou River and predict the ecological risks of ER agonists in this area.
Thyroidal hormone effects of sediment extractions
Total thyroidal activity in the 13 sediment samples ranged from 4.68 ± 1.37 to 69.9 ± 13.8 μg T3-EQ kg−1 dw, with a mean value of 19.6 ± 17.1 μg T3-EQ kg−1 dw. The lowest and highest T3-EQ values were found in Huangpu River and Suzhou River, respectively. The mean values of thyroidal effects in Huangpu River and Suzhou River were 13.5 ± 7.60 and 33.5 ± 25.3 μg T3-EQ kg−1 dw, respectively. However, there was no significant difference in the spatial distribution of T3-EQs in Huangpu River and Suzhou River according to the nonparametric two-sample Kruskal–Wallis tests. In recent years, several studies have focused on determining substances with estrogenic activity in river sediments. Far less attention has been given to identifying thyroid-disrupting activity for sediment samples. Gutleb et al. (2005) reported that some apolar sediment extracts showed TR agonist activities using T-screening, whereas other sediment extracts showed TR antagonistic activities. However, in that study, specific T3-EQs were not calculated. In our previous study, we found that total thyroidal activities (0.35 ± 0.60–24.8 ± 3.02 ng T3-EQ kg−1 with a mean value of 7.74 ± 5.93 ng T3-EQ kg−1 dw in 28 sediment samples from Taihu Lake, China) were lower than those of our study. Other studies investigate the TR agonistic or antagonistic activity of sewage samples. Murata and Yamauchi (2008) detected strong TR agonistic activity in the dichloromethane and dichloromethane/methanol fractions of effluents from a sewage treatment plant (STP) in Japan. Li et al. (2011) reported TR antagonistic activity of organic extracts of sewage in all processes from the wastewater treatment plants (WWTPs) in Beijing, China. These results indicated the potential thyroid hormone disrupting effects for extracts from matrices such as sewage. In addition, a few studies have investigated thyroid-disrupting activity in the environmental samples and successfully identify thyroidal hormone disrupting chemicals. For example, Li et al. (2014) found that a major cause of the TR antagonism, not agonism, of the water from Guanting Reservoir, Beijing, China is dibutyl phthalate (DBP). Li et al. (2010) also suggested that DBP is the main contributor to the antagonistic TR activity in drinking water. Environmental chemicals such as poly brominated diphenyl ethers (PBDEs), PCBs, pesticides, phenolic compounds, and phthalates (Boas et al. 2012) have been reported to have adverse disruption effect on the thyroid system of various vertebrates. Some studies have reported the disruption of thyroid hormone levels in fish just because of the presence of xenobiotics in polluted water bodies (Boas et al. 2012). Environment matrices can also exert agonistic and or antagonistic effects on the growth and development of organisms by directly interfering with TR-mediated pathways. Gutleb et al. (2007) found that PCBs and apolar sediment extracts significantly decrease the percentage of frogs that successfully completed metamorphosis and significantly increased the number of tadpoles that remained at early and late metamorphic stages through thyroid hormone-dependent pathways. However, for many chemicals, mixtures, and environment matrices taken in the field, the potential effects on thyroid hormone functions are still unclear. Therefore, further study is required to investigate the thyroidal potential of sediments from different study areas and to identify the causative chemicals in sediments by a combination between bioassay and chemical analysis.
EROD effects of sediment extractions and PAH contribution
The bioassay-derived TCDD equivalents in the 13 sediment samples ranged from 26.5 ± 2.15 to 148.3 ± 7.31 ng TEQ kg−1 dw, with a mean value of 66.0 ± 37.1 ng TEQ kg−1 dw. The lowest and highest TEQ values were all found in Suzhou River. The mean values of EROD activity in Huangpu River and Suzhou River were 67.8 ± 28.2 and 61.8 ± 58.0 ng TEQ kg−1 dw, respectively. Similar to T3-EQs, no significant difference in the spatial distribution of TEQs in Huangpu River and Suzhou River was observed. The EROD assay has been applied using different cell lines, such as permanent fish cell line rainbow trout liver-waterloo 1 (RTL-W1) or rat hepatoma cell line H4IIE, which led to the title “golden standard of in vitro bioassays” (Eichbaum et al. 2014). There were some reports on the TEQbio values of crude extracts from other locations (Table 2). Generally, in national and international comparison, AhR-induced activity appeared to be low to medium level. However, the obtained TEQbio values exceeded those of crude extracts from the Taihu Lake (Lei et al. 2015) and the Yellow Sea (Hong et al. 2012). These comparisons indicated that EROD effect was accumulated in the sediment of Huangpu River and Suzhou River to a relatively moderate degree.
At present, many studies successfully identify AhR agonists in sediments or soils from different countries by combination bioassay and chemical analysis. Generally, polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), PCBs, and PAHs are found to be responsible for AhR-agonist effects, respectively, in the environment samples (Qiao et al. 2006; Luo et al. 2009; Floehr et al. 2015). Among them, PAHs are especially concerned due to their wide distributions and higher concentrations in water bodies (Floehr et al. 2013). The mean concentrations of 16 priority PAHs in the sediments collected from Huangpu River and Suzhou River at the same sampling sites and the same sampling time were 3480 and 3040 ng/gdw, respectively (Wu et al. 2012). Floehr et al. (2015) found that PAHs with the concentrations of 165–1653 ng/g in the sediments from the Yangtze River had a pivotal role in the observed ecotoxicological impacts such as EROD activity, embryotoxic/teratogenic effects. A quality goal of 1000–4000 ng/g for EPA–PAHs in sediments were proposed for the assessment of sediment quality and values that exceed this goal require further analysis or immediate action (Floehr et al. 2013). These results indicated that the pollution of PAHs is very serious in Huangpu River and Suzhou River and is likely to cause a serious damage to the ecosystem. PAHs which have AhR agonistic effect, i.e., benz[a]anthracene (B[a]A), benzo[b]fluorrranthene (B[b]F), chrysene (Chry), benzo[a]pyrene (B[a]P), benzo[k]fluoranthene (B[k]F), indeno[1,2,3-cd]pyrene (I[cd]P) and dibenz[a,h]anthracene (D[ah]A)] are commonly metabolized through cytochrome P4501A1 and have been shown to elicit dioxin-like responses or to induce cytochrome P4501A1 activity in vitro (Luo et al. 2009). In the present study, the contribution of these seven PAHs to EROD effect in the sediment from Huangpu River and Suzhou River was also evaluated according to their concentrations reported by Wu et al. (2012). The TEQs for individual PAHs were calculated by multiplying the measured concentration by the corresponding toxicity equivalent factors (TEFs) (Qiao et al. 2006). The TEQcal was calculated as the sum of TEQ for an individual compound by assuming additive responses to chemicals in the mixture. It was observed that TEQs contributed by PAHs ranged from 13.81 to 66.0 ng kg−1 dw. The contribution rate of the seven PAHs to EROD effects of the sediments was calculated as follows: CR = TEQcal × 100 % / TEQbio. The data showed that for the 13 sampling sites, the CRs of PAHs ranged from 27.2 to 109.9 % with mean of 48.9 %, where congener B[k]F accounted for 21.8–90.9 % of TEQbio (Fig. 2). In addition, TEQbio was significantly correlated with TEQcal (R = 0.67, p = 0.035). Given the potential uncertainty in the TEQ estimates, our results indicated that PAHs especially B[k]F in Huangpu River and Suzhou River could provide important contribution to the AhR-agonistic effects of sediment organic extraction fraction observed in the H4IIE cell bioassay.
EROD effects of about 40 % sediment samples were caused by PAHs whose CRs were more than 50 %, while in other sampling sites of Huangpu River and Suzhou River, the CRs of PAH to TEQbio were basically below 30 % (Fig. 2). These findings showed that the EROD effects of sediment extracts from Huangpu River and Suzhou River were also caused by other compounds, such as dioxins, polychlorinated biphenyls, etc., together. The past work indicated that the TEQ is mainly contributed by dioxin-like PAHs in the sediment from Meiliang Bay, Taihu Lake, China (Qiao et al. 2006). While in other lake regions of Xukou Bay and Gonghu Bay, the CRs of PAH to TEQbio was only 40 %, indicating that other chemicals also contributed to EROD effect in the sediments (Lei et al. 2014). Some investigators found that the polyaromatic fractions of organic extracts from aquatic sediment had high potency in inducing EROD activity. However, these studies attempts to attribute the potency to identified priority PAHs had failed (Luo et al. 2009; Kaisarevic et al. 2009). For example, Liu et al. (2014a) found that the priority polycyclic aromatic hydrocarbons only accounted for a low portion of TEQbios ranging from 1 to 10 % of crude extracts in the Yangtze River Estuary. Wang et al. (2014) also found that the known AhR agonists PAH, PCB, and PCDD/F only explained up to 8 % of the more persistent AhR agonist activity in the samples, which suggested that unidentified AhR-active compounds represented a great proportion of the TEQ in sediments from the Three Gorges Reservoir (TGR). Similarly, in the present work, PAHs in some sampling sites could also not account for most EROD induction potency. The recent studies showed that hydroxylated and methoxylated polybrominated diphenyl ethers (HO-/MeO-PBDEs) and polychlorinated diphenyl sulfides (PCDPSs) have also AhR-mediated activities (Zhang et al. 2016a, b).
Relationships between bioassay responses
Significantly positive correlations were only found between lgT3-EQs and lgE2-EQs (Fig. 3). Their positive correlation coefficients, R = 0.59 (p = 0.03) for linear correction and R = 0.69 (p = 0.009) for Spearman correlation, indicate fair agreement of the spatial distribution patterns of E2-EQs and T3-EQs. In our previous study, we also found significantly linear positive correlations between ERα and TRα agonistic activity of sediments from Taihu Lake, China by a two-hybrid yeast bioassay (Lei et al. 2015). In addition, Valdehita et al. (2014) found that extracts from poultry manure exhibited simultaneously thyroidal activity and estrogenic activity using estrogen receptor and thyroid receptor transactivation assays. These results are similar to those in the present study.
ER and TR are ligand-dependent nuclear transcription factors. Endocrine disrupters are widely known for their estrogenic and thyroidal hormone effects by binding to ER or TR (Li et al. 2014). Their importance, especially TR in cell differentiation and growth in various tissues as well as crucial role in the development of gonads and the metamorphosis of amphibians, cannot be replaced (Gutleb et al. 2007). However, the action mechanisms of endocrine disrupters are actually complicated and are still unknown. There is growing evidence that estrogen and thyroid hormones can interact to regulate target gene expression and consequently physiological and behavioral functions (Rajoria et al. 2012). Some chemicals such as nonlyphenol have both estrogenic and thyroidal effects. Jugan et al. (2009) reported that most of the thyroidal effects of the influents from wastewater treatment plants were associated with compounds with low polarity and could be partly attributed to 4-NP. 4-NP is also a traditionally estrogenic compound (Hu et al. 2002) and is widespread in Chinese rivers, lakes, estuaries, and oceans, which is also present in the sediments from the study areas with the highest concentration of 337.7 ng g−1 dw (Wu et al. 2013). In addition, many other chemicals such as bisphenol analogs (BPs) and tectoridin are also considered to have both estrogenic and thyroidal effects (Boas et al. 2012; Shim et al. 2014). If these compounds are also present in the sediment of Huangpu River and Suzhou River, they can also contribute to the measured effects. Therefore, a significantly positive relationship between the estrogenic and the thyroidal effects is possible when the ER- or TR-inducing contaminants are from the same chemicals. However, which compounds induce simultaneously measured estrogenic and thyroidal effects needs to be further identified.
No correlations were observed between lgTEQs and lgE2-EQs and between lgTEQs and lgT3-EQ. Spink et al. (1992) reported that EROD activity was induced by exposure to PAHs and other dioxin-like compounds, and the induction of antiestrogenic activity by most of these chemicals was mediated by the Ah-receptor. However, Kummer et al. (2008) found that PAHs such as B[a]P, B[a]A, and fluoranthene (Fla) behaved as estrogen-like compounds which can significantly increase uterine weight and hypertrophy of luminal epithelium in immature Wistar rats when applied for three consecutive days at 10 mg/kg/day, and these estrogen effects are likely to be mediated by ERα. However, they also indicated that the exposure to B[k]F did not significantly affect the uterine weight and a weak suppression of ERα immunostaining was observed in luminal and glandular epithelium, possibly related to its AhR-mediated activity (Kummer et al. 2008). Two mechanisms (Arcaro et al. 1999) have been proposed for the antiestrogenicity induced by PAHs. One mechanism is enhanced E2 depletion through induction of PAHs and another possible mechanism is the competition for ER by PAHs. In addition, some studies suggest that estrogen-receptor hijacking may be the major interaction between AhR and ER (Giesy et al. 2002; Van Lipzig et al. 2005). PAHs had been identified to be important EROD-inducing contaminants in Huangpu River and Suzhou River in the present study. Therefore, it is possible that no correlations were found between TEQs and E2-EQs or T3-EQs.
Conclusions
In this study, the toxicity of surface sediment from Huangpu River and Suzhou River, China was investigated using a battery of in vitro bioassays. The results demonstrated the presence of contaminants that would cause estrogenic, thyroidal, and EROD effects. The distribution patterns between thyroidal effects and estrogenic effects in surface sediments were positively correlated with each other, while the distribution patterns of AhR agonist effects in surface sediment were different from those of both estrogenic and thyroidal hormone effects. The combination of bioassays and chemical analysis through comparing instrumentally derived TEQcals to bioassay-derived TEQbios suggested that PAHs were the main contributors to TEQbio only in some sediment samples from Huangpu River and Suzhou River. The proposed approach using a battery of in vitro bioassays encompassing different toxic mechanisms in this study can provide useful information regarding ecological risk assessment and enable the identification of ecological risk stressors on a regional scale.
References
Arcaro KF, O’Keefe PW, Yang Y (1999) Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology 133:115–127
Boas M, Feldt-Rasmussen U, Main KM (2012) Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355:240–248
Eichbaum K, Brinkmann M, Buchinger S, Reifferscheid G, Hecker M, Giesy JP, Engwall M, van Bavel B, Hollert H (2014) In vitro bioassays for detecting dioxin-like activity-application potentials and limits of detection, a review. Sci Total Environ 487:37–48
Floehr T, Xiao HX, Scholz-Starke B, Wu LL, Hou JL, Yin DQ, Zhang XW, Ji R, Yuan XZ, Ottermanns R, Roß-Nickoll M, Schäffer A, Hollert H (2013) Solution by dilution?-a review on the pollution status of the Yangtze River. Environ Sci Pollut Res 20:6943–6971
Floehr T, Scholz-Starke B, Xiao HX, Hercht H, Wu LL, Hou JL, Schmidt-Posthaus H, Segner H, Kammann U, Yuan XZ, Roß-Nickoll M, Schäffer A, Hollert H (2015) Linking Ah receptor mediated effects of sediments and impacts on fish to key pollutants in the Yangtze Three Gorges Reservoir, China-a comprehensive perspective. Sci Total Environ 538:191–211
Giesy JP, Hilscherova K, Jones PD, Kannan K, Machala M (2002) Cell bioassays for detection of aryl hydrocarbon (AhR) and estrogen receptor (ER) mediated activity in environmental samples. Mar Pollut Bull 14:3–16
Gutleb AC, Meerts IATM, Bergsma JH, Shriks M, Murk AJ (2005) T-Screen as a tool to identify thyroid hormone receptor active compounds. Environ Toxicol Pharmacol 19:231–238
Gutleb AC, Schriks M, Mossink L, Van den Berg JHJ, Murk AJ (2007) A synchronized amphibian metamorphosis assay as an improved tool to detect thyroid hormone disturbance by endocrine disruptors and apolar sediment extracts. Chemosphere 70:93–100
Hilscherova K, Kannan K, Kang Y-S, Holoubek I, Machala M, Masunaga S, Nakanishi J, Giesy JP (2001) Characterization of dioxin-like activity of sediments from a Czech River basin. Environ Toxicol Chem 20:2768–2777
Hong S, Khim JS, Naile JE, Park J, Kwon BO, Wang T, Lu Y, Shim WJ, Jones PD, Giesy JP (2012) AhR-mediated potency of sediments and soils in estuarine and coastal areas of the Yellow Sea region: a comparison between Korea and China. Environ Pollut 171:216–225
Hong S, Yim HU, Ha SY, Shim WJ, Jeon S, Lee S, Kim C, Choi K, Jung J, Giesy JP, Khim JS (2016) Bioaccessibility of AhR-active PAHs in sediments contaminated by the Hebei Spirit oil spill: application of Tenax extraction in effect-directed analysis. Chemosphere 144:706–712
Hu JY, Xie GH, Aizawa T (2002) Products of aqueous chlorination of 4-nonylphenol and their estrogenic activity. Environ Toxicol Chem 21:2034–2039
Jugan ML, Oziol L, Bimbot M, Huteau V, Tamisier-karolak S, Blondeau JP, Lévi Y (2009) In vitro assessment of thyroid and estrogenic endocrine disruptors in wastewater treatment plants, rivers and drinking water supplies in the greater Paris area (France). Sci Total Environ 407:3579–3587
Kaisarevic S, Varel UL, Orcic D, Streck G, Schulze T, Pogrmic K, Teodorovic I, Brack W, Kovacevic R (2009) Effect-directed analysis of contaminated sediment from the wastewater canal in Pancevo industrial area, Serbia. Chemosphere 77:907–913
Ke X, Wang CY, Zhang HJ, Zhang Y, Gui SF (2015) Characterization of estrogenic receptor agonists and evaluation of estrogenic activity in the sediments of Liaohe River protected areas. Mar Pollut Bull 100:176–181
Koh CH, Khim JS (2005) Instrumental and bioanalytical measures of dioxin-like and estrogenic compounds and activities associated with sediment from the Korean coast. Ecotoxicol Environ Saf 61:366–379
Kummer V, Masková J, Zraly Z, Neca J, Simecková P, Vondrácek J, Machala M (2008) Estrognic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature Wistar rats. Toxicol Lett 180:212–221
Kwok CK, Yang SM, Mak NK, Wong CKC, Liang Y, Leung SY, Young L, Wong MH (2010) Ecotoxicological study on sediments of Mai Po marshes, Hong Kong using organisms and biomarkers. Ecotoxicol Environ Saf 73:541–549
Legler J, Dennekamp M, Vethaak AD, Brouwer A, Koeman JH, van der Burg B, Murk AJ (2002) Detection of estrogenic activity in sediment-associated compounds using in vitro reporter gene assays. Sci Total Environ 293:69–83
Lei BL, Kang J, Yu YX, Zha JM, Li W, Wang ZJ (2013) β-estradiol17-valerate affects embryonic development and sexual differentiation in Japanese medaka (Oryzias latipes). Aquat Toxicol 134–135:128–134
Lei BL, Kang J, Wang XT, Yu YX, Zhang XL, Wen Y, Wang YP (2014) The levels of PAHs and aryl hydrocarbon receptor effects in sediments of Taihu Lake, China. Environ Sci Pollut Res 21:6547–6557
Lei BL, Kang J, Wang XT, Liu Q, Yu ZQ, Zeng XY, Fu JM (2015) The toxicity of sediments from Taihu Lake evaluated by several in vitro bioasays. Environ Sci Pollut Res 22:3419–3430
Li N, Wang DH, Zhou YQ, Ma M, Li J, Wang ZJ (2010) Dibutylphthalate contributes to the thyroid receptor antagonistic activity in drinking water processes. Environ Sci Technol 44:6863–6868
Li N, Ma M, Rao KF, Wang ZJ (2011) In vitro thyroid disrupting effects of organic extracts from WWTPs in Beijing. J Environ Sci 23:671–675
Li J, Ren SJ, Han SL, Lei BL, Li N (2014) Identification of thyroid-receptor antagonists in water from the Guanting Reservoir, Beijing, China. Arch Environ Contam Toxicol 67:68–77
Li JY, Su L, Wei FH, Yang JY, Jin L, Zhang XW (2016) Bioavailability-based assessment of aryl hydrocarbon receptor-mediated activity in Lake Tai Basin form Eastern China. Sci Total Environ 544:987–994
Liu L, Chen L, Shao Y, Zhang LL, Floehr T, Xiao HX, Yan Y, Eichbaum K, Hollert H, Wu LL (2014a) Evaluation of the ecotoxicity of sediments from Yangtze River Estuary and contribution of priority PAHs to Ah receptor-mdiated activities. PLoS ONE 9:e104748
Liu XW, Shi JH, Zhang H, Zhan XM, Shen GX, Hu SQ (2014b) Estimating estrogen release and load from humans and livestock in Shanghai, China. J Environ Qual 43:568–577
Liu L, Chen L, Floehr T, Xiao HX, Bluhm K, Hollert H, Wu LL (2015) Assessment fo the mutagenicity of sediments from Yangtze River estuary using Samonella Typhimurium/Microsome assay. PLoS ONE 10:e0143522
Luo JP, Ma M, Zha JM, Wang ZJ (2009) Characterization of aryl hydrocarbon receptor agonists in sediments of Wenyu River, Beijing, China. Water Res 43:2441–2448
Luo JP, Lei BL, Ma M, Zha JM, Wang ZJ (2011) Identification of estrogen receptor agonists in sediments from Wenyu River, Beijing, China. Water Res 45:3908–3914
Murata T, Yamauchi K (2008) 3,3′,5-Triodo-L-thyronine-like activity in effluents from domestic sewage treatment plants detected by in vitro and in vivo bioassays. Toxicol Appl Pharmacol 226:309–317
Otte JC, Keiter S, Faßbender C, Higley EB, Rocha SP, Brinkmann M, Wahrendorf DS, Manz W, Wetzel MA, Braunbeck T, Giesy JP, Hecker M, Hollert H (2013) Contribution of priority PAHs and POPs to Ah receptor-mediated activities in sediment samples from the River Elbe Estuary, Germany. PLoS ONE 8:e75596
Peck M, Gibson RW, Kortenkamp A, Hill EM (2004) Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environ Toxicol 23:945–952
Qiao M, Chen YY, Zhang QH, Huang SB, Ma M, Wang CX, Wang ZJ (2006) Identification of Ah receptor agonists in sediment of Meiliang Bay, Taihu Lake, China. Environ Sci Technol 40:1415–1419
Rajoria S, Suriano R, George AL, Shanmugam A, Jussim C, Shin EJ, Moscatello A, Geliebter J, Carpi A, Tiwari RK (2012) Estrogen activity as a preventive and therapeutic target in thyroid cancer. Biomed Pharmacother 66:151–158
Rao KF, Lei BL, Li N, Ma M, Wang ZJ (2013) Determination of estrogens and estrogenic activities in water from three rivers in Tianjin, China. J Environ Sci 25:1164–1171
Rawson CA, Tremblay LA, Warne MSJ, Ying GG, Kookana R, Laginestra E, Chapman JC, Lim RP (2009) Bioactivity of POPs and their effects in mosquitofish in Sydney Olympic Park, Australia. Sci Total Environ 407:3721–3730
Rocha SP, Azab E, Schmidt B, Storch V, Hollert H, Braunbeck T (2010) Changes in toxicity and dioxin-like activity of sediments from the Tietê River (Sāo Paulo, Brazil). Ecotoxicol Environ Saf 73:550–558
Schulze-Sylvester M, Heimann W, Maletz S, Seiler T-B, Brinkmann M, Zielke H, Schulz R, Hollert H (2016) Are sediments a risk? An ecotoxicological assessment of sediments from a quarry pond of the upper Rhine River. J Soils Sediments 16:1069–1080
Shi JH, Liu XW, Chen QC, Zhang H (2014) Spatial and seasonal distribution of estrogens and bisphenol A in the Yangtze River Estuary and the adjacent East China Sea. Chemosphere 111:336–343
Shim M, Bae JY, Lee YJ, Ahn MJ (2014) Tectoridin from Maackia amurensis modulates both estrogen and thyroid receptors. Phytomedicine 21:602–606
Song M, Jiang Q, Xu Y, Liu H, Lam PKS, O’Toole D, Zhang Q, Giesy J, Jiang G (2006a) AhR-active compounds in sediments of the Haihe and Dagu rivers, China. Chemosphere 63:1222–1230
Song M, Xu Y, Jiang Q, Lam PKS, O’Toole DK, Giesy JP, Jiang GB (2006b) Measurement of estrogenic activity in sediments from Haihe and Dagu River, China. Environ Int 32:676–681
Spink DC, Eugster HP, Lincoln DW (1992) 17β-Estradiol hydroxylation catalyzed by human cytochrome P4501A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenze-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys 293:248–342
Valdehita A, Quesada-García A, Delgado MM, Martin JV, García-González MC, Fernández-Cruz ML, Navas JM (2014) In vitro assessment of thyroidal and estrogenic activities in poultry and broiler manure. Sci Total Environ 472:630–641
Van Lipzig MMH, Vermeulen NPE, Gusinu R, Legler J, Frank H, Seidel A, Meerman JHN (2005) Formation of estrogenic metabolites of benzo[a]pyrene and chrysene by cytochrome P450 activity and their combined and supra-maximal estrogenic activity. Environ Toxicol Pharmacol 19:41–45
Wang JX, Bovee TFH, Bi YH, Bernhöft S, Schramm K-W (2014) Aryl hydrocarbon receptor (AhR) inducers and estrogen receptor (ER) activities in surface sediments of Three Gorges Reservoir, China evaluated with in vitro cell bioassays. Environ Sci Pollut Res 21:3145–3155
Wu MH, Li G, Xu G, Ma J (2012) Chlorophenols, bromophenols and polycyclic aromatic hydrocarbons in surface sediments of rivers in Shanghai, China. Environ Chem (In Chinese) 31:1750–1758
Wu MH, Wang L, Xu G, Liu N, Tang L, Zheng JS, Bu TT, Lei BL (2013) Seasonal and spatial distribution of 4-tert-octylphenol, 4-nonylphenol and bisphenol A in the Huangpu River and its tributaries, Shanghai, China. Environ Monit Assess 185:3149–3161
Yoo H, Khim J, Giesy J (2006) Receptor-mediated in vitro bioassay for characterization of Ah-R-active compounds and activities in sediment from Korea. Chemosphere 62:1261–1271
Zhang A, Li YM, Chen L (2014) Distribution and seasonal variation of estrogenic endocrine disrupting compounds, N-nitrosodimethylamine, and N-nitorsodimethylamine formation potential in the Huangpu River, China. J Environ Sci 26:1023–1033
Zhang LL, Qian L, Chen L, Zhang A, He JN, Wen ZH, Wu LL (2015) Toxicity of surface water from Huangpu River to luminous bacteria (Vibrio qinghaiensis SP. Q67) and zebrafish (Danio rerio) embryos. Ecotoxicol Environ Saf 112:137–143
Zhang JJ, Zhang XW, Xia P, Zhang R, Wu Y, Xia J, Su GY, Zhang JM, Giesy JP, Wang ZY, Villeneuve DL, Yu HX (2016a) Activation of AhR-mediated toxicity pathway by emerging pollutants polychlorinated diphenyl sulfides. Chemosphere 144:1754–1762
Zhang R, Zhang JJ, Zhang XW, Zhang JM, Su GY, Farmahin R, Giesy JP, Yu HX (2016b) In vitro dioxin-like potencies of HO- and MeO-PBDEs and inter-species sensitivity variation in birds. Ecotoxicol Environ Saf 126:202–210
Zhao JL, Ying GG, Yang B, Liu S, Zhou LJ, Chen ZF, Lai HJ (2011) Screening of multiple hormonal activities in surface water and sediment from the Pearl River system, South China, using effect-directed in vitro bioassays. Environ Toxicol Chem 30:2208–2215
Acknowledgments
We gratefully acknowledge the supports of the National Natural Science Foundation of China (No. 21507078) and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13078). At the same time, the authors are grateful for the contributions of Hu JY and Zhang H from Peking University for providing technical support to these experiments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Lou, S., Lei, B., Feng, C. et al. In vitro toxicity assessment of sediment samples from Huangpu River and Suzhou River, Shanghai, China. Environ Sci Pollut Res 23, 15183–15192 (2016). https://doi.org/10.1007/s11356-016-6683-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6683-4