Abstract
Chlorpyrifos (CP) is one of the organophosphate insecticides most used worldwide today. Although the main target organ for CP is the nervous system triggering predominantly neurotoxic effects, it has suggested other mechanisms of action as cytotoxicity and endocrine disruption. The risk posed by the pesticide metabolites on non-target organisms is increasingly recognized by regulatory agencies and natural resource managers. In the present study, cytotoxicity and estrogenic activity of CP, and its principal metabolite 3,5,6-trichloro-2-pyridinol (TCP) have been evaluated by in vitro assays, using two mammalian cell lines (HEK293 and N2a), and a recombinant yeast. Results indicate that TCP is more toxic than CP for the two cell lines assayed, being N2a cells more sensitive to both compounds. Both compounds show a similar estrogenic activity being between 2500 and 3000 times less estrogenic than 17β-estradiol. In order to find new toxicity measurement models, yeasts isolated from marine sediments containing CP residues have been tested against CP and TCP by cell viability assay. Of the 12 yeast strains tested, 6 of them showed certain sensitivity, and a concentration-dependent response to the tested compounds, so they could be considered as future models for toxicity tests, although further investigations and proves are necessary.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorpyrifos (CP) is one of the organophosphate insecticides most used worldwide today. Its greatest use is in agriculture on fruits, grains, vegetables, cotton, sugar cane, and coffee (Solomon et al. 2014), although it is also used in livestock, ornamental plants, and the grass of golf courses. As a consequence of its widespread use as well as its physical-chemical properties (solubility), it is easily washed into ground and surface waters, even marine environment, leading to accumulation in sediments given its hydrophobicity (Giesy et al. 1999; Gebremariam et al. 2012). The main degradation product of CP is 3,5,6-trichloro-2-pyridinol (TCP) (Xu et al. 2008) produced by hydrolytic and photolytic mechanisms (Baskaran et al. 2003). TCP is more water-soluble than the parent compound which provokes a high mobility causing contamination in soils and aquatic environments.
Insecticide action of CP is based on inhibiting acetylcholine esterase activity. It produces an excess of acetylcholine in the synapse resulting in hyperactivity, muscle spasms which can lead to paralysis, respiratory failure, and even death (Barron and Woodburn 1995; Slotkin 2004, 2005). Although the main target organ for CP is the nervous system, triggering predominantly neurotoxic effects, other mechanisms of action as cytotoxicity, effects on synthesis of macromolecules (Qiao et al. 2001; Howard and Pope 2002; Slotkin et al. 2008; Gupta et al. 2010) and endocrine disruption (Viswanath et al. 2010; Ventura et al. 2012, 2016) have been suggested. There is evidence of genotoxicity and mutagenicity of CP in numerous studies carried out in rats, fish, toad, and human cells (Ojha and Gupta 2015; Sandal and Yilmaz 2011; Muller et al. 2014; Ezzi et al. 2016). The carcinogenic properties of CP have been evidenced through a variety of epidemiological studies, particularly lung and rectal cancer (Alavanja et al. 2004; Lee et al. 2007). Likewise, CP has also been related to breast cancer in rats and human cells (Nishi and Hundal 2013; Ventura et al. 2016, 2019), provokes abnormalities in the immunologic system of workers and laboratory animals (Gotoh et al. 2001; Navarro et al. 2001) and numerous cases of reproductive toxicity have been reported (Nandi et al. 2009; Farag et al. 2010; Bernabò et al. 2011) such as teratogenic effects in rats (Farag et al. 2003; Tian et al. 2005) and abnormalities in human sperm cells (EPA 2008).
Recently, several organizations as European Food Safety Agency (EFSA), Plant, Animal, Food and Feed (PAFF) Committee, Pesticide Action Network (PAN) or US EPA have reviewed the use of CP, which has led many countries to restrict or ban it. So, the European Union (EU) has adopted the non-approval of the active substance CP (EC 2020); Canada proposed a ban of CP on May 31, 2019; in USA, State governments have taken steps to regulate it (Backstrom and Garson 2020). However, during many years it has been one of the most common used organophosphate pesticide (John and Shaike 2015), detecting its residues in many agricultural commodities as vegetables and fruits (Guerrero 2003; García et al. 2017; Rey et al. 2018). Its extensive use in Colombia has led to the contamination of aquatic systems, fresh and saltwater, sediments, and accumulation in organisms (Tobón-Marulanda et al. 2012).
An important aspect of pesticides is derived from possible endocrine disruptor activity provoking effects in the organism development. Since 2007, the year the United States Environmental Protection Agency (US EPA) included the insecticide CP in a draft initial list of chemicals for Endocrine Disruptor screening (EPA 2007), limited studies indicate that CP may affect the endocrine system (De Angelis et al. 2007; Viswanath et al. 2010; Ventura et al. 2012, 2016) but it still needs more evidence (Yu et al. 2015). Most of these studies, related to toxicity in humans, depend to a great extent on the use of animals, but in recent years there has been a clear tendency to replace these with other studies that represent faster and cost-effective alternatives (Braconi et al. 2011; Heinonen and Tähti 2013). One of the most common alternatives is the use of artificially grown cells which respond quickly to different adverse environmental conditions. These in vitro tests are inexpensive, easy to carry out and, can be used as preliminary tests that can lead the scientist to decide whether further testing is necessary (Meneau 2014; Mushtaq et al. 2018). Furthermore, they are useful for testing a large number of samples (Aslantürk 2017). They can use cells varying from microorganisms to mammalian and human cells. The evaluation of cytotoxicity focus on simple tests, where cell viability and/or proliferation of cells are measured. Chemical agents can affect cell health and metabolism via different mechanisms such as membrane destruction, inhibition of protein synthesis, irreversible binding to receptors, inhibition of nucleic acid elongation, and enzymatic reactions (Ishiyama et al. 1996; Aslantürk 2017). There are different classifications for cytotoxicity and cell viability assays, the most used are according to measurement types of endpoints (colorimetric fluorescence, luminescent, etc.) (O’Brien et al. 2000; Hamid et al. 2004; Rampersad 2012; Gilbert and Friedrich 2017), using a wide variety of cells. In the cytotoxicity evaluation, cell lines such as human kidney embryonic cells (HEK) and mouse neuroblasts (N2a) have been used for neurotoxic and neurodegenerative effects, being biological models of great importance (LePage et al. 2005; Provost 2010; Wang et al. 2015; Qiu et al. 2016; Sindi et al. 2016; Acevedo et al. 2018). These cell lines has been chosen to assess organophosphate pesticide toxicity, like parathion (Wang et al. 2019), paraquat (Cai et al. 2019) and CP (Van Emon et al. 2018).
Microbiological indicators (prokaryotic and eukaryotic organisms) are also used widely in the study of environmental contamination in order to determine viability of cell cultures (Grela et al. 2018). Among them, yeast model Saccharomyces cerevisiae is one of the most used. The yeasts are potentially good models for assessing toxicity of environmental pollutants (Ribeiro et al. 2000; Cabral et al. 2003; Papaefthimiou et al. 2004), they have similarities to mammalian cells, especially regarding the functionality of homologous proteins (Braconi et al. 2011). They are easy to maintain and culture, reducing the variability found with more complex organisms (Esteve et al. 2009). In addition, yeasts can provide information of direct relevance to other eukaryotes and can be isolated from a wide range of environments such as marine, anaerobic sediments and contaminated sites (Baronian 2004). Given the high number of yeasts that are likely to exist it is probable that wild-type yeast will prove to be a considerable source of toxicity indicators (Braconi et al. 2011). Short-term toxicity assays based on the measurement of changes in yeast cultures to estimate the impact of toxic compounds are used increasingly. These assays are relatively simple, rapid, cost-effective and require small sample volumes so they can be of interest as alternative tools for preliminary screening and for inclusion in a test battery. Fai and Grant (2009a, 2009b) examined the effects of several fungicides and other contaminants to a wide range of yeast species and proposed the inclusion of the resorufin fluorescence inhibition bioassay in a battery with other biomarkers in the rapid screening of environmental samples.
More precise assays have been developed in order to detect specific activities of chemical compounds such as endocrine disrupting activity as a RYA (Recombinant Yeast Assay) using a genetically modified S. cerevisiae strain (Routledge and Sumpter 1996; García-Reyero et al. 2001) that contains the human estrogen receptor (hER) gene in the main chromosome linked to a reporter gene lacZ encoding for the β-galactosidase enzyme which is produced and secreted to the medium, and whose reaction specific reaction is easy to follow using a convenient (chromogenic or fluorogenic) enzyme substrate.
The main purpose of the present study is to contribute to a greater knowledge of the toxicity of the CP and its major metabolite TCP, through rapid in vitro tests using two mammalian cell lines and yeasts isolated in our laboratory from marine sediments exposed to CP with the aim of providing new microbiological indicators for use in toxicity tests. Likewise, with the intention of providing new data on the potential estrogenic activity of the compounds, these have been subjected to RYA tests.
Material and methods
Test compounds
Chlorpyrifos (CAS: 2921-88-2) (O, O-diethil O-3,5,6-trichloropyridin-2-il phosphorothionate; CP) 99.7% analytical standard and its metabolite 3,5,6-trichloro-2-pyridinol (TCP) (CAS: 6515-38-4) 99.3%, analytical standard were obtained from Sigma-Aldrich. Stock solutions of compounds and dilution series were prepared in the appropriate solvent or culture medium according to toxicity assays performed. Water employed in the experiments was Milli-Q grade. All reagents used were of analytical grade.
Cytotoxicity test with HEK293 and N2a cell lines
Cytotoxicity of compounds has been evaluated using two cell lines, N2a (Mouse Neuroblastoma) and HEK293 (Human Embryonic Kidney) kindly provided by Biomedicine Institute (Valencia, Spain). Assays are based in the reduction of resazurin, by metabolic activity of cells, transformed in resorufin, a highly fluorescent compound which allowing spectroscopic measurement (Czekanska 2011). Culture cells were maintained in DMEM (Dubelcco’s Modified Eagle Medium) appropriately supplemented with glutamine, penicillin-streptomycin and fetal bovine serum and incubated at 37 °C, and 5% CO2 humidified atmosphere (Koppikar et al. 2010). When cells achieve a 70–90% confluency, they were harvested by trypsinization and counted. A suspension of 1 × 105 cells/mL was prepared in DMEM supplemented, with which a tissue culture plate was inoculated (100 µL/well). After 24 h, cells were exposed to 5 µL of test compounds to obtain a range of final concentrations of 6.3–1600 mg/L which were assayed in quadruplicate. Negative controls and blanks were run simultaneously. Plates were incubated again (24 h), the medium was removed and 100 µL of 15 μM resazurin solution in DMEM supplemented was added. Finally, the plate was incubated for 4 h newly and fluorescence (Ex560/Em590) was measured using a Tecan Infinite M200 spectrofluorometer. All culture cell reagents and plates were supplied by VWR® International Eurolab S.L. (Barcelona, Spain). Results were expressed as percentage of viability, used to calculate the IC50 (concentration that inhibits cellular viability by half).
Toxicity test with marine yeasts
Isolation and conservation of marine yeasts
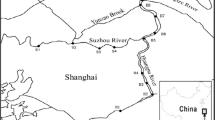
Yeasts were isolated from fresh sediment samples from nine locations in Cartagena Bay (Colombia) (Fig. 1). Sediments were collected with a dredger, Ekman type, in sterile containers, which were transported refrigerated to the laboratory where were kept at −20 °C until analysis. To isolate yeasts the following procedure was carried out: 1 g of sediment was suspended in 10 mL of 0.9% NaCl containing 0.05% Tween 20, homogenized in vortex for 2 min and standing 2 min more for decanting particles, then 100 μL of dilution were spread onto plates with MEA (Malt Extract Agar, Difco) in triplicate and incubated at 25 °C for 5–7 days. Presumptive yeasts were subcultured on MEA plates and identified by API 20 C AUX® System (BioMérieux). Yeast isolates were cultured in tubes containing MEA, kept to 4 °C for further studies and preserved −80 °C in MicrobankTM-Blue criovials (Pro-Lab Diagnostics).
Yeast viability test
One loop of yeast colonies grown onto MEA tubes was cultured in 50 mL SD medium supplemented with glucose (2% final concentration) and incubated overnight at 25 °C in an orbital shaker (150 rpm). The resulting culture was diluted with the same medium until an optical density of 0.5 (1 × 106 cells/mL) at 600 nm and used for the microplate assay. Stock solutions of CP and TCP were prepared, at a concentration of 250 mg/L, in SD medium with DMSO (1%). Yeast culture was added to white flat bottom 96-well microplates (Costar, Corning Inc., New York, USA) as described below. First, plate was filled with 100 µL/well of yeast culture; 100 µL/well of the stock solutions of CP or TCP were added to the second row and 1:2 serial dilutions were made by transferring 100 µL from the second to the third row and so on until 1:64. For each yeast, four replicates of controls (SD yeast culture) and four replicates of serial dilutions of pollutants (CP or TCP solutions) were made. The total volume per well was 100 µL. SD plus DMSO control was also run in the last row.
The covered plates were incubated on a shaking incubator for 6 h at 25 °C. After this time, plates were taken out and centrifuged at 1200 rpm. The aqueous content was eliminated with a Pasteur pipette coupled to a vacuum pump, and 100 µL of resazurin solution (12.5 µM in PBS) were added to each well. Then, plates were incubated at 25 °C but the incubation time required to measure the effect of compounds varied from one yeast species to another due to the different capacity to reduce resazurin. As indicated by Fai and Grant (2009a), the maximum resorufin fluorescence inhibition relative to the control were used in comparison of effects. Some species caused reduction of resazurin to the pink resorufin and then to colorless and non-fluorescent hydroresorufin in the first minute and another species between 20 and 60 min of incubation. Fluorescence were determined by the microplate reader Tecan Infinite M200 at 530 nm (λex) and 590 nm (λem). Cell viabilities were reported respect to control using fluorescence data. For the bioassay, sensitivity controls were performed using the salt K2Cr2O7 (Sigma-Aldrich, Madrid, Spain) in the same conditions. IC50 values of CP and TCP to yeast species were calculated.
Recombinant yeast assay (RYA)
RYA was performed to assess estrogenic activity. It uses a genetically modified strain of S. cerevisiae that contains the human estrogenic hormone receptor gene. If the tested substances have estrogenic activity, they bind to hER, activating lacZ gene, which expresses the β-galactosidase enzyme, generating a reaction that is measured spectrophotometrically by adding the appropriate enzyme substrate.
Recombinant Yeast Assay was performed as described by Noguerol et al. (2006). Briefly, an overnight culture of yeast in SD medium [6.7 g/L of YNBAA/AS - yeast nitrogen base without amino acids of Difco (Basel, Switzerland) and 5 g/L of (NH4)2SO4] plus glucose, histidine and methionine was used at a final optical density of 0.1 (600 nm). Assays were conducted in a 96 well microplate. The first column was filled with a 1:20 v/v dilution of compound to test in the yeast culture and then diluted in a 1:2 series across the plate. Assay concentrations of each compound was 8 mg/L. Columns 10, 11 and 12 are filled with toxicity, positive (17β-estradiol) and negative controls, respectively. Once filled, plate was incubated during 6 h at 30 °C at 120 rpm in orbital shake. The, in order to release active proteins from cells, a Y-PER Yeast Protein Extraction Reagent (PIERCETM, Rockford, IL, USA) was added and further incubated at same temperature during 30 min. Then, 50 µL of enzymatic substrate, previously prepared in an appropriate buffer was added, and β-galactosidase activity was read by fluorescence using a spectrofluorometer (TECAN Infinite M2000) at 360 nm excitation and 460 nm emission wavelengths. Fluorescence was recorded for 20 min (one measurement per 42 s). Enzymatic activity was calculated as slope of linear regression of fluorescence units plotted vs. time, and, then a relative activity derived of positive and negative controls was calculated. Estrogenic activity was calculated from dose-response curve (β-galactosidase relative activity vs. chemical concentration) and expressed as an apparent EC50 values for each compound. These values were converted to EEQ, equivalents of estradiol using the following equation (Esteban et al. 2014; Balsiger et al. 2010), where EC50 (17β-estradiol) is 72.73 × 10−6 mg/L (Piña et al. 2009) and C is the assay concentration of the compound:
Statistical analysis
Data were analyzed by using a one-way ANOVA followed by a post-hoc analysis using the Fisher’s least significant difference (LSD) test using Statgraphics program v.6.0. The IC50 values were calculated using Probit analysis (SPSS Statistics program v. 16.0). A p < 0.05 was taken to indicate statistical significance.
Results and discussion
Cytotoxicity
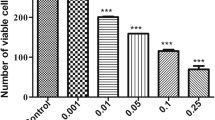
The cytotoxic potential of CP and TCP, and an equimolar mixture of CP:TCP (1:1) was also assessed by the Alamar Blue assay using cell-based systems with two mammal HEK293 and N2a cell lines. The exposure to these compounds and their mixture produced a significant cytotoxicity on the cell viability (Figs 2 and 3).
As expected, the viability of cells decreased with increasing concentrations of CP and TCP. The IC50 values of pure compounds, with their 95% confidence limits, to cell lines are presented in Table 1. CP and TCP at concentrations of 451 mg/L and 295 mg/L, respectively, gave 50% cytotoxicity with HEK293 and at concentrations of 90.0 mg/L and 61.6 mg/L, respectively, gave 50% cytotoxicity with N2a. IC50 values of the mixture CP:TCP (1:1) were 71.8 (54.5–94.4) mg/L for HEK293 and 16.7 (12.3–21.4) mg/L for N2a.
After 24 h cells exposure, the metabolite TCP has been found to be more cytotoxic than the parent compound CP and mixture was more cytotoxic than CP and TCP. After exposed to CP at concentrations from 0 to 400 mg/L, the viability of HEK293 cells decreased up to 45.8% slowly but for N2a decreased rapidly until 16.0%. In the same way, TCP at the same concentrations, produced a decrease in viability of HEK293 and N2a up to 42.3% and 11.6%, respectively. In CP:TCP (1:1) exposure (0–400 mg/L), the viability decreased rapidly, to 29.2% for HEK293 and 8.3% for N2a. At concentrations of CP and TCP from 0 to 100 mg/L, HEK293 cell viability underwent a little variation; the same effect was observed from 0 to 25 mg/L for N2a cells. Results obtained in cytotoxicity assay indicated a greater tolerance of HEK293 cells to CP, TCP and mixture in the concentration range of 0–1600 mg/L.
In this paper, cytotoxicity of CP and its metabolite TCP was probed in two cell-based systems with mammalian cells. Results indicate that TCP is more toxic than CP for the two cell lines assayed. A different sensitivity was observed between cell lines, being N2a cells more sensitive which could be due to different mechanism of action of compounds. A cytotoxicity test was performed by Álvarez-Navarro (2017) on the same cell lines for the toxicity screening of several environmental pollutants, also detecting, a higher sensitivity for N2a cells. Similarly, Lovecka et al. (2015) in a cytotoxic assay using the two cell lines HEK293 and HepG2 exposed to herbicides, bromoxynil, chloroxynil, and ioxynil, demonstrated a lower sensitivity for HEK293.
It is known that the acute toxicity of CP is mediated through inhibition of acetylcholinesterase by the production of the active metabolite CP oxon (Barron and Woodburn 1995; Mangas et al. 2016), but several studies suggests that CP may influence cell replication and differentiation directly (Das and Barone 1999; Dam et al. 2000; Qiao et al. 2001) which also extend to their major metabolites CP oxon and TCP. The current work is addressed in this line. Cell lines were selected as suitable models for use with in vitro cytotoxicity assays because are representative of two target tissues for many pesticides. The mouse N2a neuroblastoma cell line has been regularly used in neurotoxicity and pesticide research for mechanistic and screening studies (Veronesi 1992; Perreault et al. 2011; Pawlowiez et al. 2013; Pisapia et al. 2017). On the other hand, HEK293, human embryonic kidney cell line, is widely used as in vitro model for cytotoxicity assays to probe oxidative stress effects and other related properties (Waly et al. 2013; Lovecka et al. 2015).
A high activity to arthropods and relatively low toxicity to mammals in animal models has been largely probed for CP (Giddings et al. 2014; Ezzi et al. 2016). Results obtained here support this statement with IC50 values for cell lines about 90 to 400 times higher compared to D. magna (Echeverri et al. 2020) (Table 1). In addition, the degree of cytotoxicity of several insecticides on human cells (HEK293, HeLa and HepG2) reported by Yun et al. (2017) was significantly lower than that on insect cells (Tn5b1-4, Sf-9, and S2). In this paper, CP showed relatively little cytotoxicity on HEK293 cells during 24 h exposure. This might be related to the neurotoxic activity of CP, resulting in a lower cytotoxicity expression on non-neuronal cells.
Toxicity test with marine yeasts
Identification of marine yeasts
A total of 26 yeasts were isolated from marine sediment of Cartagena bay (Colombia). Eleven marine yeasts were randomly selected for toxicity bioassay. Their identification is presented in Table 2.
Effects of CP and TCP on marine yeasts
To investigate the capability of yeasts as bioindicators of toxicity, a bioassay using the yeast isolates from marine sediments were performed. Eleven marine yeasts and the species CECT 1891 S. cerevisiae were exposed to different concentrations of CP and TCP; viability of treated and untreated organisms was assessed using resazurin method (Figs 4 and 5). The maximum resorufin fluorescence inhibition relative to the control was obtained usually within 20–60 min of incubation for MY1, MY2, MY3, MY6, MY11 strains and CECT 1891 S. cerevisiae. The strains MY4, MY5, MY7, MY8, MY9 and MY10 metabolized the fluorescent resorufin to the transparent hydroresorufin quickly, in the first minute and, at this time, effect could not be observed at studied concentrations.
Experimental data with the six most sensitive yeasts to CP and TCP are presented as concentration–response curves (Figs 6 and 7). The IC50 values for both compounds are presented in Table 1. MY3, MY6 and MY11 strains were more sensitive to TCP than MY1, MY2 and CECT 1891 S. cerevisiae, being MY11 strain the most sensitive to this compound, and CECT 1891 S. cerevisiae the most resistant. For yeasts exposed to CP, MY1, MY2, MY3, MY8 strains and CECT 1891 S. cerevisiae were more resistant than MY11 strain. As observed, the latter showed greater sensitivity to CP and TCP than the other yeast strains, with IC50 values of 3.4 mg/L and 0.15 mg/L, respectively. TCP was more toxic for yeasts than CP with IC50 ranged from 0.15 to 3.4 mg/L for the former and from 3.4 to 26.3 mg/L for the latter. The yeast strains MY1, MY2 and CECT 1891 S. cerevisiae showed a similar sensitivity to TCP; these same strains plus MY3 showed it against CP with IC50 values from 1.17 mg/L to 3.4 mg/L and from 10.0 mg/L to 14.9 mg/L, respectively.
In order to assess sensitivity of resorufin inhibition bioassay, response of most sensitive yeasts studied in this work was compared with cytotoxicity assay and other standardized tests (Aliivibrio fisheri, Pseudokirchneriella subcapitata and Daphnia magna) (Echeverri et al. 2020). There seemed to be considerable differences in sensitivity between the cell lines and the other organisms (Table 1). The bacteria A. fisheri, the cladoceran D. magna and the yeast MY11 R. minuta were the most sensitive organisms to CP, and A. fisheri, MY11 R. minuta and the alga P. subcapitata were to TCP. HEK293 and N2a cell lines were the most tolerant to studied compounds with IC50 values ranged from 61.6 to 451 mg/L. TCP was more toxic than CP for all organisms and cell lines being IC50 0.15 to 295 mg/L and 3.4 to 451 mg/L, respectively.
Esteve et al. (2009) evaluated the metabolic activity of yeast S. cerevisiae exposed to three pesticides and compared the toxic effect with D. magna and A. fischeri standard bioassays. They concluded that the yeast bioassay was 96 times faster than the D. magna toxicity bioassay, but had lower sensitivity; however, A. fischeri was the most tolerant to pesticides. Several authors reported toxicity values to several fungicides to D. magna between <0.01 and 4.3 mg/L (Ferrando et al. 1992; Freeman and Nizani 1997; Bartlett et al. 2001; Fai and Grant 2009b). In our study, D. magna bioassay was more sensitive for the insecticide CP than yeast, bacteria and algae bioassays, which is evident considering its mode of action; however, for TCP, the microcrustacean bioassay was the less sensitive. Unlike CP, TCP does not inhibit acetylcholinesterase activity (Qiao et al. 2001). CP and TCP toxicity on A. fischeri was of the same order as the most sensitive yeast MY11 R. minuta.
Sensitivity of the type yeast CECT 1891 S. cerevisiae to CP was not significantly different from the mean of CP IC50 values of six most sensitive yeasts (MY1, MY2, MY3, MY6, MY11 and CECT 1891). Instead, this type yeast was 2.3 times more tolerant than the average for TCP. The mean yeast IC50 values for CP (13.2 mg/L) and TCP (1.5 mg/L) were 4 and 10 times higher than CP and TCP IC50 of the most sensitive yeast MY11, respectively (Fig. 8).
Although, S. cerevisiae is an experimental model proposed by several authors for assessing effects of environmental contaminants to non-target fungi because wide distribution, fast growing cells, ease of cultivation, no pathogenic and fully sequenced genome (Kitagawa et al. 2003; Papaefthimiou et al. 2004; Fay and Grant 2009a) there are another yeast species that could been used in environmental studies (Baronian 2004; Fai and Grant 2009a; Vadkertiová and Slavikova 2011). In this study, MY11 showed a high sensitivity to studied compounds comparable to sensitivity of A. fischeri and P. subcapitata (Fig. 8). Nevertheless, further assays were necessary to establish MY11 as representative yeast in a battery of toxicity tests to assess environmental contaminants.
The yeast bioassay has been sensitive giving inhibitory concentrations comparable to lower IC50 values of several fungicides reported in the literature for S. cerevisiae and other fungi (Zerva et al. 1996; Freeman and Nizani 1997; López et al. 2003). We found that CP and TCP toxicity, as mean of IC50 of the yeast species, were one hundred thirty (CP) and fifteen (TCP) fold higher than the lowest IC50 value founded by the authors above mentioned.
Estrogenic activity of compounds
The estrogenic activity of the selected compounds was determined by RYA. Dose-response curves were obtained for every compound and are presented in Fig. 9 as plots of relative β-galactosidase activity, in arbitrary units, vs. compound concentration. Results showed that both compounds were able to bind to the estrogen receptor with similar affinities. Table 3 shows the 50% effective concentration (EC50) values for each compound, calculated using standard linear regression methods as well as the lowest concentration at which estrogenic activity was detected (CL). Both compounds show a similar estrogenic activity as verified from EC50 values. In order to compare with 17β-estradiol standard, equivalents were calculated (EEQ) as described in point 2.4 of Material and methods section. These values represent the concentration of 17β-estradiol that elicit the same response as the compounds at initial assay concentration. In terms of relative potency, the studied compounds represent between 2500 and 3000 times less estrogenicity than17β-estradiol. On the other hand, CP and TCP caused estrogenic activity at concentrations as low as 0.04 mg/L and 0.07 mg/L, respectively, whose estrogenic activities correspond to 14.5 ng/L and 25.5 ng/L of EEQ, respectively, values below of EC50 of 17β-estradiol (72.73 ng/L). However, considering that CP and TCP are detected in the water bodies at levels ranging from 0.5 µg/L to 700 µg/L (Mazanti et al. 2003; Bonifacio et al. 2017), endocrine disruption activity should be considered for a complete risk assessment in environmental samples suspected of containing CP and TCP. RYA has been demonstrated to be an excellent tool for screening of natural samples for their content of substances with estrogenic activity (García-Reyero et al. 2001, 2005; Brix et al. 2010).
The endocrine disruption activity of CP has been evidenced in recent years through studies varying from in vivo and in vitro assays until epidemiological studies. Several investigations describe CP as a potent antiandrogenic compound (Usmani et al. 2003; Joshi et al. 2007; Viswanath et al. 2010), impairing reproductive capacity in men. Recent epidemiological studies have demonstrated significant associations between maternal and paternal exposures to CP and testicular damages (Uchendu et al. 2013). Estrogenic activity of CP has been also verified by means a several studies: Ventura et al. (2012) demonstrated that CP at environmental concentrations promotes breast cancer cell proliferation through estrogen receptor (ER-α). Other authors also found it to be estrogenic using Chinese hamster ovarian cells (Kojima et al. 2004). Thyroid effects have also been demonstrated, causing harmful effects on brain development of fetus (Ghisari and Bonefeld 2005). Haviland et al. (2010) detected increased thyroid hormone levels in CP exposed female mice, whose learning behavior was consequently altered. Furthermore, the presence of CP residues in food has been widespread interest, considering that low doses could alter the function of the hormonal system in human and wildlife, leading to adverse effects (Yu et al. 2015).
Studies performed on animals stand out, however, these in vivo assays are time-consuming and laborious. Taking account endocrine disruption is a form of toxicity that it is often difficult to prove, it is necessary that a number of in vitro assays be proposed to be used as a first screen for endocrine disruptor (Bishop and Willett 2014). Most of these trials fall into three categories (Kinnberg 2003): (1) estrogen receptor (ER) competitive ligand binding assays that measure the binding affinity of a chemical for the ER; (2) cell proliferation assays that measure the increase in cell number of estrogen sensitive cells (E-screen); and (3) reporter gene assays that measure ER binding-dependent transcriptional and translational activity. The features, performance, and use of these assays in screening for estrogenic activities of endocrine disruptors have been reviewed and discussed elsewhere (Zacharewski 1998; Andersen et al. 1999; Fang et al. 2000; Kinnberg 2003). Of the three types of in vitro assays, type 3, reporter gene assays, have more advantages compared to the others: ER competitive assays (1) are significantly less sensitive, and cell proliferation assays (2) are more time consuming. However, reporter gene assays are considered more specific and reliable for a first level screening of estrogenic activity. Furthermore, it can be carried out with mammalian or yeast cells, although former have the main drawback that their cells are more difficult and expensive to culture and are more susceptible to cytotoxic effects. Recombinant yeast assay (RYA), classified as type 3 assays, can be considered as a robust, rapid, and sensitive tool for assessing of putative endocrine disruption activity in environment as well as in the screening of the new chemical compounds at moderate cost (Noguerol et al. 2006; Brix et al. 2010).
CP was included in lists of chemicals for screening endocrine disruptor activity (EPA 2007; EC 2002). Although, endocrine disruptor activity of CP has been shown by some authors as above mentioned, international organizations as EPA (EPA 2015) and EFSA (EFSA 2019) have stated it still needs more evidence to prove that CP is an endocrine disruptor. Although, results obtained here indicate a weak estrogenicity of the tested compounds compared to estradiol, they must be considered in further risk assessment studies since they are ubiquitous and may accumulate in organisms at high concentrations, enough to induce similar effects to estradiol.
Conclusion
In summary, this study was conducted with three in vitro model systems. The suitability of mammal cell-based assays has been investigated. The variability of cellular responses observed in this work supports the need to use cell lines representative of different target tissues for chemicals and environmental pollutants, such as HEK293 and N2a. Yeasts are widely distributed and play important roles in the ecosystems. Moreover, they are easy to maintain and cultivate in laboratory. Koch et al. (1993) has already proposed yeasts as alternative toxicity models and more recently, several authors support it (Ribeiro et al. 2000; Cabral et al. 2003; Braconi et al. 2011, 2016). Present results also suggest yeast as a reliable model for a preliminary screening for environmental pollutants toxicity, although further investigations and proves are necessary in order to establish its accuracy and precision. Recombinant yeast assay (RYA) showed a slight estrogenic activity of CP and TCP so it can be considered as an alternative to in vivo assays. RYA has been used to demonstrate estrogenic activity of both, individual compounds, and environmental samples (García-Reyero et al. 2005; Puy-Azurmendi et al. 2014). And it is aimed to the rapid screening for potential ligands for hormone receptors and to the identification of endocrine disruption signals at lower concentrations and shorter exposition times (Piña et al. 2009).
On the other hand, results also confirm the need to study the toxicity not only parent compounds but also their metabolites as we can see with the metabolic product TCP, which was more toxic than CP. Additionally, levels that produce toxic effects of CP in all model systems assayed here were higher than environmental concentrations reported by some authors in water bodies (Mazanti et al. 2003; Bonifacio et al. 2017).
References
Acevedo R, Sabater C, Olivero J (2018) Ecotoxicological assessment of perchlorate using in vitro and in vivo assays. Environ Sci Pollut Res 25:13697–13708. https://doi.org/10.1007/s11356-018-1565-6
Alavanja MC, Dosemeci M, Samanic C, Lubin J, Lynch CF, Knott C, Barker J, Hoppin JA, Sandler DP, Coble J, Thomas K, Blair A (2004) Pesticides and lung cancer risk in the agricultural health study cohort. Am J Epidemiol 160:876–885. https://doi.org/10.1093/aje/kwh290
Álvarez C (2017) Evaluación de los riesgos ambientales de contaminantes de preocupación emergente en la Unión Europea. TFG, Universitat Politècnica de València, Valencia, Spain. https://riunet.upv.es
Andersen HR, Andersen AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R, Bonefeld E, Korsgaard B, Le Guevel R, Leffers H, McLachlan J, Møller A, Nielsen JB, Olea N, Oles-Karasko A, Pakdel F, Pedersen KL, Perez P, Skakkebœk NE, Sonnenschein C, Soto AM, Sumpter JP, Thorpe SM, Grandjean P (1999) Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect 107:89–108. https://doi.org/10.1289/ehp.99107s189
Aslantürk OS (2017) In vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages. By Özlem Sultan Aslantürk, Submitted: May 16th 2017 Reviewed: October 24th 2017 Published: December 20th 2017. https://doi.org/10.5772/intechopen.71923
Backstrom TD, Garson KN (2020) European Union to ban chlorpyrifos after January 31, 2020. The National Law Review X (4). https://www.natlawreview.com/article/european-union-to-ban-chlorpyrifos-after-january-31-2020
Balsiger HA, De la Torre R, Lee WY, Cox MB (2010) A four-hour yeast bioassay for the direct measure of estrogenic activity in wastewater without sample extraction, concentration, or sterilization. Sci Total Environ 408:1422–1429. https://doi.org/10.1016/j.scitotenv.2009.12.027
Baronian KHR (2004) The use of yeast and moulds as sensing elements in biosensors. Biosens Bioelectron 19:953–962. https://doi.org/10.1016/j.bios.2003.09.010
Bartlett DW, Clough JM, Godfrey CRA, Godwin JR, Hall AA, Heaney SP, Maund SJ (2001) Understanding the strobilurin fungicides. Pesticide Outlook 12:143–148. https://doi.org/10.1039/B106300F
Barron MG, Woodburn KB (1995) Ecotoxicology of chlorpyrifos. Rev Environ Contam Toxicol 144:1–93. https://doi.org/10.1007/978-1-4612-2550-8_1
Baskaran S, Kookana RS, Naidu R (2003) Contrasting behaviour of chlorpyrifos and its primary metabolite, TCP (3,5,6-trichloro-2-pyridinol), with depth in soil profiles. Aust J Soil Res 41:749–760. https://doi.org/10.1071/SR02062
Bernabò I, Gallo L, Sperone E, Triperi S, Brunelli E (2011) Survival, development, and gonadal differentiation in Rana dalmatina chronically exposed to chlorpyrifos. J Exp Zool A Ecol Genet Physiol 315:324–327. https://doi.org/10.1002/jez.678
Bishop PI, Willett CE (2014) The use of Other Scientifically Relevant Information (OSRI) in the U.S. Environmental Protection Agency (EPA) Endocrine Disruptor Screening Program, Birth deffects. Res B Dev Reprod Toxicol 101:3–22. https://doi.org/10.1002/bdrb.21077
Bonifacio AF, Ballesteros ML, Bonansea RI, Filippi I, Amé MV, Hued AC (2017) Environmental relevant concentrations of a chlorpyrifos commercial formulation affect two neotropical fish species, Cheirodon interruptus and Cnesterodon decemmaculatus. Chemosphere 188:486–493. https://doi.org/10.1016/j.chemosphere.2017.08.156
Braconi D, Bernardini G, Millucci L, Jacomelli G, Micheli V, Santucci A, Kortekamp A (2011) Saccharomyces cerevisiae as a tool to evaluate the effects of herbicides on Eukaryotic life. In: Kortekamp A (ed.) Herbicides and Environment, chapter 24. InteechOpen, Rijeka, 10.5772/13237
Braconi D, Bernardini G, Santucci A (2016) Saccharomyces cerevisiae as a model in ecotoxicological studies: a post-genomics perspective. J Proteom 137:19–34. https://doi.org/10.1016/j.jprot.2015.09.001
Brix R, Noguerol TN, Piña B, Balaam J, Nilsen AJ, Tollefsen KE, Levy W, Schramm KE, Barceló D (2010) Evaluation of the suitability of recombinant yeast-based estrogenicity assays as a pre-screening tool in environmental samples. Environ Int 36:361–367. https://doi.org/10.1016/j.envint.2010.02.004
Cabral MG, Viegas CA, Teixeira MC, Sá I (2003) Toxicity of chlorinated phenoxyacetic acid herbicides in the experimental eukaryotic model Saccharomyces cerevisiae: role of pH and of growth phase and size of the yeast cell population. Chemosphere 51:47–54. https://doi.org/10.1016/s0045-6535(02)00614-8
Cai Z, Zheng F, Ding Y, Zhan Y, Gong R, Li J, Aschner M, Zhang Q, Wu S, Li H (2019) Nrf2-regulated miR-380-3p Blocks the Translation of Sp3 Protein and Its Mediation of Paraquat-Induced Toxicity in Mouse Neuroblastoma N2a Cells. Toxicol Sci 171:515–529. https://doi.org/10.1093/toxsci/kfz162
Czekanska EM (2011) Assessment of cell proliferation with resazurin-based fluorescent dye. Methods Mol Biol 740:27–32. https://doi.org/10.1007/978-161779-108-6_5
Dam K, Seidler FJ, Slotkin TA (2000) Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev Brain Res 121:179–187. https://doi.org/10.1016/s0165-3806(00)00044-4
Das KP, Barone S (1999) Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetyl-cholinesterase inhibition the site of action? Toxicol Appl Pharmacol 160:217–230. https://doi.org/10.1006/taap.1999.8767
De Angelis S, Tassinari R, Maranghi F, Eusepi A, Di Virgilio A, Chiarotti F, Ricceri L, Venerosi A, Gilardi E, Moracci G, Calamandrei G, Olivieri A, Mantovani A (2007) Developmental exposure to chlorpyrifos induces alterations in thyroid and Thyroid Hormone Levels Without Other Toxicity Signs in Cd1 Mice. Toxicol Sci 108:311–319. https://doi.org/10.1093/toxsci/kfp017
European Commission (2002) Endocrine Disrupters: Study on gathering information on 435 substances in insuficient dates. Final Report DG ENV. EUDGENVIRONMENT: B4-3040/2001/325850/MARC/C2
Echeverri G, Jaramillo B, Sabater C, Castillo MÁ (2020) Acute toxicity of chlorpyrifos and its metabolite 3,5,6-trichloro-2-pyridinol alone and in combination using a battery of bioassays. Environ Sci Pollut Res 27:32770–32778. https://doi.org/10.1007/s11356-020-09392-x
EFSA (2019) Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos. EFSA J 17(8):5809. https://doi.org/10.2903/j.efsa.2019.5809
EPA (2007) Draft list of initial pesticide active ingredients and pesticide inerts to be considered for screening under the Federal Food, Drug, and Cosmetic Act, Federal Register: US. p. 72 (116). http://www.epa.gov/endo/pubs/draft_list_frn_061807.pdf
EPA (2008) Evidence on the developmental and reproductive toxicity of chlorpyrifos. Draft. California Environmental Protection Agency. http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/ChlorpyrifosHID0908.pdf.
EPA (2015) EDSP Weight of Evidence Conclusions on the Tier 1 Screening Assays for the List 1 Chemicals. Chemical: Chlorpyrifos. https://www.epa.gov/endocrine-disruption/status-endocrine-disruptor-screening-program-tier-1-screening-results-and-data
Esteban S, Gorga M, Petrovic M, González-Alonso S, Barceló D, Valcárcel Y (2014) Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci. Total Environ. 466–467:939–951
Esteve K, Poupot C, Dabert P, Mietton M, Milistic V (2009) A Saccharomyces cerevisiae -based bioassay for assessing pesticide toxicity. J Ind Microbiol Biotechnol 36:1529–1534. https://doi.org/10.1007/s10295-009-0649-1
European Commission (2020) Commission implementing Regulation (EU) 2020/18. Off J Eur U 13.1.2020: L7/14-L7/16
Ezzi L, Belhadj I, Haouas Z, Sakly A, Grissa I, Chakroun S, Kerkeni E, Hassine M, Mehdi M, Ben Cheikh H (2016) Histopathological and genotoxic effects of chlorpyrifos in rats. Environ Sci Pollut Res Int 23:4859–4867. https://doi.org/10.1007/s11356-015-5722-x
Fai PB, Grant A (2009a) A comparative study of Saccharomyces cerevisiae sensitivity against eight yeast species sensitivities to a range of toxicants. Chemosphere 75:289–296. https://doi.org/10.1016/j.chemosphere.2008.12.059
Fai B, Grant A (2009b) A rapid resazurin bioassay for assessing the toxicity of fungicides. Chemosphere 74:1165–1170. https://doi.org/10.1016/j.chemosphere.2008.11.078
Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM (2000) Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect 108:723–729. https://doi.org/10.1289/ehp.00108723
Farag AT, El Okazy AM, El-Aswed AF (2003) Developmental toxicity study of chlorpyrifos in rats. Reprod Toxicol 17:203–208. https://doi.org/10.1016/s0890-6238(02)00121-1
Farag AT, Radwan AH, Sorour F, El Okazy A, El-Agamy ES, El-Sebae AEK (2010) Chlorpyrifos induced reproductive toxicity in male mice. Reprod Toxicol 29:80–85. https://doi.org/10.1016/j.reprotox.2009.10.003
Ferrando MD, Andreu E, Fernández A (1992) Relative sensitivity of Daphnia magna and Brachionus calyciflorus to 5 pesticides. J Environ Sci Health B. 27:511–522. https://doi.org/10.1080/03601239209372798
Freeman S, Nizani Y (1997) Control of Colletotrichum acutatum in strawberry under laboratory, greenhouse and field conditions. Plant Dis 81:749–752. https://doi.org/10.1094/PDIS.1997.81.7.749
Gilbert DF, Friedrich O (2017) Cell Viability Assays. Methods and Protocols. In: D. F. Gilbert and O. Friedrich (Ed). Human Press. https://doi.org/10.1007/978-1-4939-6960-9
García A, Rodríguez C, Restrepo E, Sánchez A (2017) Residuos de plaguicidas en tomate (Solanum lycopersicum) comercializado en Armenia, Colombia. Vitae (Revista de la Facultad de Ciencias Farmacéuticas y Alimentarias) 24:68–79. https://doi.org/10.17533/udea.vitae.v24n2(2)a08
García-Reyero N, Grau E, Castillo M, López de Alda MJ, Barceló D, Piña B (2001) Monitoring of endocrine disruptors in surface waters by the yeast recombinant assay. Environ Toxicol Chem 20:1152–1158. https://doi.org/10.1897/1551-5028(2001)020<1152:moedis>2.0.co;2
García-Reyero N, Piña B, Grimalt J, Fernández P, Fonts R, Polvillo O, Martrat B (2005) Estrogenic activity in sediments from European mountain l akes. Environ. Sci. Technol. 39:1427–1435. https://doi.org/10.1021/es0400685
Gebremariam SY, Beutel MW, Yonge DR, Flury M, Harsh JB (2012) Adsorption and desorption of chlorpyrifos to soils and sediments. In: Whitacre DM (ed) Reviews of Environmental Contamination and Toxicology. Springer, New York, NY, pp 123–175. https://doi.org/10.1007/978-1-4614-1463-6_3
Ghisari M, Bonefeld EC (2005) Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol Cell Endocrin 244:31–41. https://doi.org/10.1016/j.mce.2005.01.013
Giddings JM, Williams WM, Solomon KR, Giesy JP (2014) Risks to aquatic organisms from use of chlorpyrifos in the Unites States. In: Giesy JP, Solomon KR (eds) Reviews of Environmental Contamination and Toxicology. Ecological Risk Assessment of chlorpyrifos, vol 231. Springer, New York, NY, pp 119–162
Giesy JP, Solomon KR, Coates JR, Dixon KR, Giddings JM, Kenaga EE (1999) Chlorpyrifos: ecological risk assessment in North American aquatic environments. Rev Environ Contam Toxicol 160:1–129. https://doi.org/10.1007/978-1-4612-1498-4_1
Gotoh M, Saito I, Huang J, Fukaya Y, Matsumoto T, Hosanaga N, Shibata E, Ichihara G, Kamujima M, Takeuchi Y (2001) Changes in cholinesterase activity, nerve conduction velocity, and clinical signs and symptoms in termite control operators exposed to chlorpyrifos. J Occup Health 43:157–164. https://doi.org/10.1539/joh.43.157
Grela E, Koz J, Grabowiecka A (2018) Current methodology of MTT assay in bacteria. A review. Acta Histochem 120:303–311. https://doi.org/10.1016/j.acthis.2018.03.007
Guerrero AJ (2003) Estudio de residuos de plaguicidas en frutas y hortalizas en áreas específicas de Colombia. Agronomía Colombiana 21:198–209. http://www.redalyc.org/articulo.oa?id=180317974009
Gupta SC, Mishra M, Sharma A, Deepak TGR, Kumar BR, Mishra RK, Chowdhuri DK (2010) Chlorpyrifos induces apoptosis and DNA damage in Drosophila through generation of reactive oxygen species. Ecotoxicol Environ Saf 73:1415–1423. https://doi.org/10.1016/j.ecoenv.2010.05.013
Hamid R, Rotshteyn Y, Rabadi L et al. (2004) Comparison of Alamar Blue and MTT assays for high through-put screening. Toxicol. In Vitro 18:703–710. https://doi.org/10.1016/j.tiv.2004.03.012
Haviland JA, Butz DE, Porter WP (2010) Long-term sex selective hormonal and behaviour alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol 29:74–79. https://doi.org/10.1016/j.reprotox.2009.10.008
Heinonen T, Tähti H (2013) Eläinkokeeton toksikologia kemikaalien turvallisuustestauksessa [Non-animal toxicology in the safety testing of chemicals]. Duodecim 129:1686–1694
Howard MD, Pope CN (2002) In vitro effects of chlorpyrifos, parathion, methyl parathion and their oxons on cardiac muscarinic receptor binding in neonatal and adult rats. Toxicology 170:1–10. https://doi.org/10.1016/s0300-483x(01)00498-x
Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Okhura Y, Ueno KA (1996) Combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharmaceutical Bull 19:1518–1520. https://doi.org/10.1248/bpb.19.1518
John EM, Shaike JM (2015) Chlorpyrifos: pollution and remediation. Environ Chem Lett 13:269–291. https://doi.org/10.1007/s10311-015-0513-7
Joshi SC, Mathur R, Gulati N (2007) Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol Ind Health 23:439–444. https://doi.org/10.1177/0748233707080908
Kinnberg K (2003) Evaluation of in vitro assays for determination of estrogenic activity in the environment. Working Report 43. University of Southern Denmark. Danish Environmental Protection Agency
Kitagawa E, Momose Y, Iwahashi H (2003) Correlation of the structures of agricultural fungicides to gene expression in Saccharomyces cerevisiae upon exposure to toxic doses. Environ Sci Technol 37:2788–2793. https://doi.org/10.1021/es026156b
Koch HP, Hofeneder M, Bohne B (1993) The yeast tests: An alternative method for the testing of acute toxicity of drug substances and environmental chemicals. Methods Find Clin. Pharmacol 15:141–152
Kojima H, Katsura E, Takeuchi S, Niyama K, Kobayashi K (2004) Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112:524–531. https://doi.org/10.1289/ehp.6649
Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul R (2010) Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer 10:210. https://doi.org/10.1186/1471-2407-10-210
Lee WJ, Sandler DP, Blair A, Samanic C, Cross AJ, Alavanja MCR (2007) Pesticide use and colorectal cancer risk in the agricultural health study. Int J Cancer 121:339–346. https://doi.org/10.1002/ijc.22635
LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF (2005) On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol 17:27–50. https://doi.org/10.1615/CritRevNeurobiol.v17.i1.20
López B, Veyrat A, Perez E, Gonzalez L, Marcos JF (2003) Comparison of the activity of antifungal hexapeptides and the fungicides thiabendazole and imazalil against postharvest fungal pathogens. Int J Food Microbiol 89:163–170. https://doi.org/10.1016/s0168-1605(03)00118-1
Lovecka P, Thimova M, Grznarova P, Lipov J, Knejzlik Z, Stiborova H, Nindhia TGT, Demnerova K, Ruml T (2015) Study of Cytotoxic Effects of Benzonitrile Pesticides. BioMed Res Int 381264:9. https://doi.org/10.1155/2015/381264
Mangas I, Vilanova E, Estévez J, França TCC (2016) Neurotoxic effects associated with current uses of organophosphorus compounds. J Braz Chem Soc 27:809–825. https://doi.org/10.5935/0103-5053.20160084
Mazanti L, Rice C, Bialek K, Sparling D, Stevenson C, Johnson WE, Kangas P, Rheinstein J (2003) Aqueous-phase disappearance of atrazine, metolachlor, and chlorpyrifos in laboratory aquaria and outdoor macrocosms. Arch Environ Contam Toxicol 44:67–76. https://doi.org/10.1007/s00244-002-1259-3
Meneau R (2014) Métodos Alternativos en Toxicología. Revista CENIC: Ciencias Biológicas 45:11–21
Muller M, Hess L, Tardivo A, Lajmanovich R, Attademo A, Poletta G, Simoniello MF, Yodice A, Lavarello S, Chialvo D, Scremin O (2014) Neurologic dysfunction and genotoxicity induced by low levels of chlorpyrifos. Neurotoxicology. 45:22–30. https://doi.org/10.1016/j.neuro.2014.08.012
Mushtaq S, Kursad Y, Aksoy A (2018) Alternative methods to animal experiments. Turkiye Klinikleri J Med Sci 38:161–170. https://doi.org/10.5336/medsci.2018-59993
Nandi S, Gupta PSP, Roy SC, Selvaraju S, Ravindra JP (2009) Chlorpyrifos and endosulfan affect buffalo oocyte maturation, fertilization, and embryo development in vitro directly and through cumulus cells. Environ Toxicol 26:57–67. https://doi.org/10.1002/tox.20529
Navarro HA, Basta PV, Seidler FJ, Slotkin TA (2001) Neonatal chlorpyrifos administration elicits deficits in immune function in adulthood: a neural effect. Brain Res Dev Brain Res 130:249–252. https://doi.org/10.1016/s0165-3806(01)00254-1
Nishi K, Hundal SS (2013) Chlorpyrifos induced toxicity in reproductive organs of female Wistar rats. Food Chem Toxicol 62:732–738. https://doi.org/10.1016/j.fct.2013.10.006
Noguerol T, Boronat S, Jarque S (2006) Detection of hormone receptor ligands in yeast by fluorogenic methods. Talanta 69:351–358. https://doi.org/10.1016/j.talanta.2005.09.044
O’Brien J, Wilson I, Orton T, Pognan Ë (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. https://doi.org/10.1046/j.1432-1327.2000.01606.x
Ojha A, Gupta YK (2015) Evaluation of genotoxic potential of commonly used organophosphate pesticides in peripheral blood lymphocytes of rats. Hum Exp Toxicol 34:390–400. https://doi.org/10.1177/0960327114537534
Papaefthimiou C, Cabral M, Mixailidou C, Viegas CA, Sá-Correia I, Theophilidis G (2004) Comparison of two screening bioassays, based on the frog sciatic nerve and yeast cells, for the assessment of herbicide toxicity. Environ Toxicol Chem 23:1211–1218. https://doi.org/10.1897/03-48
Pawlowiez R, Darius HT, Cruchet P, Rossi F, Caillaud A, Laurent D, Chinain M (2013) Evaluation of seafood toxicity in the Australes archipelago (French Polynesia) using the neuroblastoma cell-based assay. Food Add Contam A 30:567–586. https://doi.org/10.1080/19440049.2012.755644
Perreault F, Matias MS, Melegari SP, Silva de Carvalho CR, Creppy EE, Popovic R, Matias WG (2011) Investigation of animal and algal bioassays for reliable saxitoxin ecotoxicity and cytotoxicity risk evaluation. Ecotoxicol Environ Saf 74:1021–1026. https://doi.org/10.1016/j.ecoenv.2011.01.016
Piña B, Boronat S, Casado M, Olivares A (2009) Recombinant yeast assays and gene expression assays for the analysis of endocrine disruption. In: Barceló D, Hansen PD (Eds) Biosensors for Environmental Monitoring of Aquatic Systems. The Handbook of Environmental Chemistry, vol 5J. Springer, Berlin, Heidelberg, pp 69–113. https://doi.org/10.1007/978-3-540-36253-1_4
Pisapia F, Holland WC, Hardison DR, Litaker RW, Fraga S, Nishimura T, Adachi M, Nguyen-Ngoce L, Sécheta V, Amzila Z, Herrenknecht C, Hess P (2017) Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 63:173–183. https://doi.org/10.1016/j.hal.2017.02.005
Provost P (2010) Interpretation and applicability of microRNA data to the context of Alzheimer’s and age-related diseases. Aging (Albany NY) 2:166–169. https://doi.org/10.18632/aging.100131
Puy-Azurmendi E, Olivares A, Vallejo A, Ortiz-Zarragoitia M, Piña B, Zuloaga O, Cajaraville MP (2014) Estrogenic effects of nonylphenol and octylphenol isomers in vitro by recombinant yeast assay (RYA) and in vivo with early life stages of zebrafish. Sci Total Environ 466-467:1–10. https://doi.org/10.1016/j.scitotenv.2013.06.060
Qiao D, Seidler FJ, Slotkin TA (2001) Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect 109:909–913. https://doi.org/10.1289/ehp.01109909
Qiu XY, Li K, Li XQ, Li XT (2016) The inhibitory effects of nifedipine on outward voltage-gated potassium currents in mouse neuroblastoma N2A cells. Pharmacol Rep 68:631–637. https://doi.org/10.1016/j.pharep.2015.12.006
Rampersad SN (2012) Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 12:12347–12360. https://doi.org/10.3390/s120912347
Rey J, Otalvaro A, Chaparro M, Prieto L, López A (2018) Residuos de plaguicidas organofosforados en la cadena productiva del brócoli (Brassica oleracea L. var. italica) y coliflor (Brassica oleracea L. var. botrytis) en Colombia: aproximación a un perfil de riesgo. Rev Colomb Cienc Hortíc 12:156–165. https://doi.org/10.17584/rcch.2018v12i1.7352
Ribeiro IC, Verissimo I, Moniz L, Cardoso H, Sousa MJ, Soares AMVM, Leao C (2000) Yeasts as a model for assessing the toxicity of the fungicides Penconazol, Cymoxanil and Dichlofluanid. Chemosphere 41:1637–1642. https://doi.org/10.1016/S0045-6535(00)00039-4
Routledge EJ, Sumpter JP (1996) Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem 15:241–248. https://doi.org/10.1002/etc.5620150303
Sandal S, Yilmaz B (2011) Genotoxic effects of chlorpyrifos, cypermethrin, endosulfan and 2,4‐D on human peripheral lymphocytes cultured from smokers and nonsmokers. Environ. Toxicol. 26:433–442. https://doi.org/10.1002/tox.20569
Sindi RA, Harris W, Arnott G, Flaskos J, Lloyd C, Hargreaves AJ (2016) Chlorpyrifos- and chlorpyrifos oxon-induced neurite retraction in pre-differentiated N2a cells is associated with transient hyperphosphorylation of neurofilament heavy chain and ERK 1/2. Toxicol Appl Pharmacol 308:20–31. https://doi.org/10.1016/j.taap.2016.08.008
Slotkin TA (2004) Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 198:132–151. https://doi.org/10.1016/j.taap.2003.06.001
Slotkin TA (2005) Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC (Ed.) Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press, San Diego, pp 293–314
Slotkin TA, Seidler FJ, Ryde IT, Yanai J (2008) Developmental neurotoxic effects of chlorpyrifos on acetylcholine and serotonin pathways in an avian model. Neurotoxicol Teratol 30:433–439. https://doi.org/10.1016/j.ntt.2008.02.005
Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM, Giesy JP (2014) Properties and uses of chlorpyrifos in the United States. Rev. Environ Contam Toxicol 231:13–34. https://doi.org/10.1007/978-3-319-03865-0_2
Tian Y, Ishikawa H, Yamaguchi T, Yokoyama K (2005) Teratogenicity and developmental toxicity of chlorpyrifos maternal exposure during organogenesis in mice. Reprod Toxicol 20:267–271. https://doi.org/10.1016/j.reprotox.2005.01.012
Tobón-Marulanda FA, López-Giraldo LA, Paniagua-Suárez RE (2012) Contaminación del agua por plaguicidas en un área de Antioquia. Rev Salud Pública 12:300–307. https://doi.org/10.1590/S0124-00642010000200013
Uchendu C, Ambali SF, Ayo JO, Lasisi IO, Umosen AJ (2013) Subacute chlorpyrifos induced alterations in serum lipids and some oxidative stress biomarkers in male Wister rats: beneficial effect of acetyl‐L‐carnitine. Toxicol Environ Chem 95:483–494. https://doi.org/10.1080/02772248.2013.782029
Usmani KA, Rose RL, Hodgson E (2003) Inhibition and activation of the human liver and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metabol Disp 31:384–391. https://doi.org/10.1124/dmd.31.4.384
Vadkertiová R, Slavikova E (2011) Influence of pesticides on yeasts colonizing leaves. Z. Naturforsch 66:588–594. https://doi.org/10.5560/ZNC.2011.66c0588
Van Emon JM, Pan P, van Breukelen F (2018) Effects of chlorpyrifos and trichloropyridinol on HEK 293 human embryonic kidney cells. Chemosphere 191:537–547. https://doi.org/10.1016/j.chemosphere.2017.10.039
Ventura C, Núñez M, Miret N, Martinel D, Randi A, Venturino A, Rivera E, Cocca C (2012) Differential mechanisms of action are involved in chlorpyrifos effects in estrogen-dependent or -independent breast cancer cells exposed to low or high concentrations of the pesticide. Toxicol Lett 213:184–193. https://doi.org/10.1016/j.toxlet.2012.06.017
Ventura C, Ramos MR, Bourguignon N, Lux-Lantos V, Rodriguez H, Cao G, Randi A, Cocca C, Núñez M (2016) Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. J Steroid Biochem Mol Biol 156:1–9. https://doi.org/10.1016/j.jsbmb.2015.10.010
Ventura C, Zappia CD, Lasagna M, Pavicic W, Richard S, Bolzan AD, Monczor F, Núñez M, Cocca C (2019) Effects of the pesticide chlorpyrifos on breast cancer disease. Implication of epigenetic mechanisms. J Steroid Biochem Mol Biol 186:96–104. https://doi.org/10.1016/j.jsbmb.2018.09.021
Veronesi B (1992) In vitro screening batteries for neurotoxicants. Neurotoxicology 13:185–196
Viswanath G, Chatterjee S, Dabral S, Nanguneri SR, Divya G, Roy P (2010) Anti-androgenic endocrine disrupting activities of chlorpyrifos and piperophos. J Steroid Biochem Mol Biol 120:22–29. https://doi.org/10.1016/j.jsbmb.2010.02.032
Waly MI, Ali BH, Nemmar A (2013) Acute effects of diesel exhaust particles and cisplatin on oxidative stressin cultured human kidney (HEK 293) cells, and the influence of curcumint hereon. Toxicol in Vitro 27:2299–2304. https://doi.org/10.1016/j.tiv.2013.09.023
Wang X, Jiang L, Ge L, Chen M, Yang G, Ji F, Zhong L, Guan Y, Liu X (2015) Oxidative DNA damage induced by di-(2-ethylhexyl) phthalate in HEK-293 cell line. Environ Toxicol Pharmacol 39:1099–1106. https://doi.org/10.1016/j.etap.2015.03.016
Wang Y, Kim B, Walker A, Williams S, Meeks A, Lee YJ, Seo SS (2019) Cytotoxic effects of parathion, paraoxon, and their methylated derivatives on a mouse neuroblastoma cell line NB41A3. Fundam Toxicol Sci 6:45–56. https://doi.org/10.2131/fts.6.45
Xu G, Zheng W, Li Y, Wang S, Zhang J, Yan Y (2008) Biodegradation of chlorpyrifos and 3,5,6-trichloro-2-pyridinol by a newly isolated Paracoccus sp. strain TRP. Int Biodeterior Biodegr 62:51–56. https://doi.org/10.1016/j.ibiod.2007.12.001
Yu K, Li G, Feng W, Liu L, Zhang J, Wu W, Xu L, Yan Y (2015) Chlorpyrifos is estrogenic and alters embryonic hatching, cell proliferation and apoptosis in zebrafish. Chem-Biol Interact 239:26–33. https://doi.org/10.1016/j.cbi.2015.06.010
Yun X, Huang Q, Rao W, Xiao C, Zhang T, Mao Z, Wan Z (2017) A comparative assessment of cytotoxicity of commonly used agricultural insecticides to human and insect cells. Ecotoxicol Environ Saf 137:179–185. https://doi.org/10.1016/j.ecoenv.2016.12.002
Zacharewski T (1998) Identification and assessment of endocrine disruptors: limitations of in vivo and in vitro assays. Environ Health Perspect 106:577–582. https://doi.org/10.1289/ehp.98106577
Zerva L, Hollis RJ, Pfaller MA (1996) In vitro susceptibility testing and DNA typing of Saccharomyces cerevisiae clinical isolates. J Clin Microbiol 34:3031–3034. https://doi.org/10.1128/JCM.34.12.3031-3034.1996
Acknowledgements
The authors thank the Ecotoxicology Laboratory at the Department of Biotechnology of the School of Agricultural Engineering and Natural Environment (ETSIAMN) of the Polytechnic University of Valencia (Spain), and Universidad de San Buenaventura Cartagena and Universidad de Cartagena (Colombia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Echeverri-Jaramillo, G., Jaramillo-Colorado, B., Sabater-Marco, C. et al. Cytotoxic and estrogenic activity of chlorpyrifos and its metabolite 3,5,6-trichloro-2-pyridinol. Study of marine yeasts as potential toxicity indicators. Ecotoxicology 30, 104–117 (2021). https://doi.org/10.1007/s10646-020-02315-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02315-z