Abstract
A physico-chemical characterization of seawater taken from the fishing harbour of Sfax, Tunisia, revealed a contamination by organic and inorganic micropollutants. An aerobic marine halotolerant Bacillus stratosphericus strain FLU5 was isolated after enrichment on fluoranthene, a persistent and toxic polycyclic aromatic hydrocarbon (PAH). GC-MS analyses showed that strain FLU5 was capable of degrading almost 45 % of fluoranthene (100 mg l−1), without yeast extract added, after 30 days of incubation at 30 g l−1 NaCl and 37 °C. In addition, the isolate FLU5 showed a remarkable capacity to grow on a wide range of aliphatic, aromatic and complex hydrocarbons. This strain could also synthesize a biosurfactant which was capable of reducing the surface tension of the cell-free medium, during the growth on fluoranthene. The biodegradative abilities of PAHs are promising and can be used to perform the bioremediation strategies of seawaters and marine sediments contaminated by hydrocarbons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal marine ecosystems were progressively affected by environmental stress and degradation because of pollution and other anthropogenic factors (Viaroli et al. 2004). In fact, industrial, agricultural and urban inputs may cause high levels of contamination within these ecosystems (D’Adamo et al. 2008). Harbours were among the most altered coastal areas. They usually represent polluted areas with high concentrations of pollutants such as organochlorines, heavy metals and polycyclic aromatic hydrocarbons (PAHs). Anthropogenic discharges into harbours could have severe effects on local pelagic and benthic communities (Danulat et al. 2002). As many Mediterranean coastal harbours, the fishing harbour of Sfax, Tunisia, which is one of the most important harbours in the country (30 % of the Tunisian halieutic fishing resources come from the Sfax harbour), is a polluted ecosystem subject to both urbanization and industrialization including outfall of untreated domestic sewage and wastewaters, fishery activities, as well as ship traffic and boat pollution (petroleum, engine oil…). This contamination has harmful consequences for natural resources (Louati et al. 2001; Zaghden et al. 2005). Among a long list of various pollutants, PAHs have an important impact on marine ecosystems because some of them are highly carcinogenic, genotoxic and cytotoxic (Cerniglia 1992).
PAHs are ubiquitous compounds found worldwide in air, soils, sediments and water, as a result of both natural and anthropogenic production (Hilyard et al. 2008). The anthropogenic PAHs, which were released into the marine environment, may originate from many sources, including atmospheric deposition, industrial and domestic outfalls, direct spillage of petroleum or petroleum products and river runoff (Witt 1995; Zaghden et al. 2007). Due to their high toxicity, many PAHs including naphthalene, anthracene, phenanthrene, fluoranthene and pyrene are classified as priority pollutants by the US Environmental Protection Agency (Cerniglia 1992). Fluoranthene, one of the principal polycyclic aromatic hydrocarbons (PAHs), is often chosen as a model compound for research on the biodegradation of PAH because it is widespread in the environment, genotoxic, mutagenic and in many cases, carcinogenic (Xu et al 2011). Moreover, only a few studies have focused on biodegradation of fluoranthene in marine environmental and many aspects of fluoranthene biodegradation, especially for marine-sourced degraders, remain unclear (Cao et al. 2015). The use of some typical remediation approaches, such as dredging and incineration, can be costly and harmful to the environment by dispersing PAHs and making them more bioavailable (Hilyard et al. 2008), while using microbial technology to clean up PAH-contaminated sites has been considered as an economical, eco-friendly and efficient choice (Kumar et al. 2011). For that, the use of microorganisms for bioremediation of PAH-contaminated environments seemed to be an attractive technology for restoration of polluted sites.
In the present study, we investigated the contamination of seawater of the fishing harbour of Sfax, Tunisia. Furthermore, we isolated an efficient hydrocarbonoclastic bacterium capable of degrading fluoranthene, a model of PAH composed of three aromatic rings linked to a cyclopentane. The isolate FLU5 could be helpful in improving the efficiency of biodegradation of fluoranthene and other PAHs and in remediating hydrocarbon-contaminated environments.
Materials and methods
Sampling
Seawater samples were collected in January 2013 from four distinct locations in the fishing harbour of Sfax (site 1: 34°71′58.63″ N. 10°76′12.61″ E; site 2: 34°71′58.39″ N. 10°76′51.64″ E; site 3: 34°71′31.91″ N. 10°76′52.02″ E and site 4: 34°71′30.96″ N. 10°76′15.00″ E). It is an oil polluted harbour located on the southern coastal of Sfax, Tunisia, Mediterranean sea. The samples were directly collected in sterile bottles and stored in the dark at 4 °C. A representative sample was composed equally of the four previous samples and was subsequently used for the physico-chemical characterization and for bacterial isolation.
Culture media
A modified Luria-Bertani medium (LB) contained (g l−1): 10 peptone, 5 yeast extract and 30 NaCl. The composition of the basal medium (BM) was (g l−1) as follows: 0.3 KH2PO4, 0.4 NH4Cl, 0.33 MgCl2.6H2O, 0.05 CaCl2.2H2O, 30 NaCl and 1 ml l−1 trace-element solution (Widdel and Pfennig 1981). The pH was adjusted to 7–7.2. The medium was sterilized by autoclaving at 121 °C for 20 min.
Chemicals
Simple hydrocarbons used in this study included aliphatic hydrocarbons, octane and decane; monoaromatic compounds, benzoic acid, toluene, ethylbenzene, o-, m- and p-xylenes, protocatechuic acid, gentisic acid, vanillic acid, syringic acid, gallic acid, caffeic acid, ferulic acid, phenol and tyrosol; and polycyclic aromatic hydrocarbons, naphthalene, phenanthrene, fluoranthene and pyrene. All hydrocarbons were obtained from Sigma-Aldrich Company (98–99 % purity). Motor oil and diesel fuel (complex hydrocarbons) were obtained from Shell company (Sfax, Tunisia) while crude oil was collected from “Thyna Petroleum Services” (Sfax, Tunisia).
Physico-chemical analyses and analytical methods
The pH, chemical oxygen demand (COD), five-day biological oxygen demand (BOD5), total organic carbon (TOC) and total Kjeldahl nitrogen (TKN) were performed as described elsewhere (Chebbi et al. 2016). The amounts of ions Fe, Cu, Mn, Ni, Zn, Cr, Na, Ca, Mg and K were determined by using an AAnalyst 200 Atomic Absorption Spectrometer, PerkinElmer. The purity, shape and mobility of bacteria were examined using an OLYMPUS BX51 phase contrast microscope equipped with an OLYMPUS DP70 digital camera. A Shimadzu model UV-1800 spectrophotometer was used to assess bacterial growth by measuring the absorbance at 600 nm.
Gas chromatography-mass spectrometry (GC-MS) was carried out to identify hydrocarbons present in seawater samples and to evaluate the hydrocarbon biodegradation by the hydrocarbonoclastic isolated strain. Seawater sample (50 ml) was extracted three times with dichloromethane (DCM), (v/v). The organic fraction was evaporated, dissolved in equal volume of DCM and then analyzed. GC-MS analytical procedure was performed as described previously (Mnif et al. 2009). The surface tension of the cell-free culture supernatant was followed with a digital tensiometer TSD (Gibertini, Milano, Italy). Values given are the mean of three replicates ± standard deviation.
Enrichment and isolation of PAH-degrading bacteria
A 5-ml sample of the polluted seawater was inoculated into separate Erlenmeyer flasks containing 45 ml of basal medium with 200 mg lc1 of a PAH (naphthalene, phenanthrene, fluoranthene or pyrene), as carbon and energy sources. The enrichment cultures were incubated at 37 °C and 180 rpm for 7 days. Additional BM flasks were set up for each PAH, under the same conditions, to serve as abiotic controls. The enrichment culture on fluoranthene was subcultured three times under the same conditions prior to isolation, until the substrate was metabolized. The cell growth was measured based on absorbance at 600 nm. After successful enrichment, aliquots (100 μl) of 10−1 to 10−10 dilutions were plated onto fluoranthene (200 mg l−1) agar basal medium. The plates were incubated at 37 °C under aerobic conditions, for 4–5 days until colony formation. Single colonies that were surrounded by a clear zone were picked up and serially diluted in the fresh basal media containing 100 mg l−1 fluoranthene. The purity of the isolates was checked microscopically. Strain FLU5 was chosen for further investigation because it showed the maximum growth in the basal medium containing fluoranthene, without yeast extract addition.
Growth of strain FLU5 on fluoranthene
Growth studies were conducted in flask cultures containing 50 ml basal medium with 30 g l−1 NaCl and 100 mg l−1 fluoranthene, under agitation of 180 rpm at 37 °C. Experiments were carried out in duplicate with an inoculum proportion of 3 % (v/v) which has been subcultured at least once under the same conditions. The cell growth was verified by monitoring the OD at 600 nm and by determination of colony-forming unit (CFU), at different times of culture. Values given are the mean of three replicates ± standard deviation.
Biodegradation experiments
To study the biodegradation capability of fluoranthene, used as the sole carbon and energy source, by strain FLU5, this substrate was added from a stock solution (100 g 1−1 dissolved in ethyl acetate) into 50 ml basal medium at a final concentration of 100 mg 1−1. Before bacterial inoculation, the ethyl acetate in PAH-BM was evaporated by shaking the flask on a rotary shaker (180 rpm) at 37 °C for 2 h. Similar sterilized flasks, without bacterial inoculation, were used as abiotic controls. All flasks were shaken under 180 rpm at 37 °C for various culture times. Samples (50 ml) of culture FLU5 containing fluoranthene and an abiotic control were extracted three times with dichloromethane (DCM). The organic fraction was evaporated, dissolved in equal volume of DCM and then analyzed by GC-MS using the same program as described above.
Characterization of strain FLU5
The Gram reaction was determined using the BioMérieux Gram stain Kit according to the manufacturer’s instructions. Oxidase activity was determined by oxidation of 1 % p-aminodimethylaniline oxalate. Catalase activity was determined by bubble production in 3 % (v/v) hydrogen-peroxide solution. Optimal growth temperature was done by culture incubation on LB medium at temperature range between 8 and 55 °C in the presence of 30 g l−1 NaCl and at pH 7. Whereas, the growth of strain FLU5 at various pH was studied by incubation of adjusted cultures ranging from pH 3 to pH 12, using 5 M HCl or 5 M NaOH solutions, at 37 °C and 30 g l−1 NaCl. The NaCl concentration range for growth was determined by weighing in flasks different amounts of NaCl prior to dispensing 50 ml on LB medium at pH 7 to obtain the desired NaCl concentration (from 0 to 15 %, w/v), then, cultures were incubated at 37 °C. Optical density at 600 nm was followed as a measurement of growth, and data from the mid-log phase growth of the strain were selected for calculating the specific growth rates. Other phentotypic characteristics were screened by test strips of the API system (BioMérieux, France). The API 50 CHB and API 20 E kits were used following the methods of Logan and Berkeley 1984.
The ability of strain FLU5 to degrade different hydrocarbons was studied by adding substrates into flasks containing 50 ml BM without yeast extract added. Aromatic compounds including protocatechuic, gentisic, vanillic, syringic, gallic, caffeic and ferulic acids, tyrosol and phenol were used at 5 mM. The aromatic compound stock solutions were neutralized when required and were sterilized by filtration (pore size 0.2 mm; Millipore). The aliphatic hydrocarbons (octane and decane) and the monoaromatics (benzene, toluene, ethylbenzene and o-, m-, and p-xylenes (BTEX)) were added at a concentration of 0.5 % (v/v). Polyaromatic hydrocarbons including naphthalene, phenanthrene and pyrene were added at a concentration of 200 mg l−1. The following complex hydrocarbons were used at concentration of 1 % (v/v): crude oil, fuel oil and motor oil. Olive oil and glycerol were used at a concentration of 1 % (v/v). Positive growth was demonstrated by an increase in the OD600 nm in substrate-containing cultures compared with control flasks lacking substrates.
16S rRNA sequence determination and phylogenetic analysis
The 16S rRNA gene of the strain FLU5 was amplified by PCR using a stratagene PCR system (Robocycler gradient 96) with GoTaq DNA polymerase (Promega, WI, USA), as described previously (Chamkha et al. 2002). The universal bacterial primers Fd1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGTTACCTTGTTACGACTT -3′) (Proligo Primers and Probes, Paris, France) were used to obtain a PCR product of approximately 1.5 Kb corresponding to base position 8-1542, based on Escherichia coli numbering of the 16S rRNA gene (Winker and Woese 1991). Sequence data were imported into the sequence editor BioEdit version 5.0.9 (Hall 1999). The consensus sequences were manually adjusted in accordance with the 16S rRNA secondary structure model (Winker and Woese 1991). Sequences used in the phylogenetic analysis were obtained from GenBank database (Benson et al. 1999). Positions of sequence and alignment ambiguity were omitted and pairwise evolutionary distances based on 1478 unambiguous nucleotides were calculated using the method of Jukes and Cantor 1969. A dendrogram was constructed using the neighbour-joining method (Saitou and Nei 1987) with the Molecular Evolutionary Genetic Analysis (MEGA) program version 6 (Tamura et al. 2013). Confidence in the tree topology was determined using 100-bootstrapped trees (Felsenstein 1985).
Results
Physico-chemical characterization of the seawater
Physico-chemical parameters including pH, electrical conductivity (EC), COD, BOD5, TOC, TKN, ions, heavy metals and total hydrocarbons (TH) were determined to estimate the pollution of the studied seawater (Table 1). The contaminated seawater was subjected to extractions with dichloromethane, concentrated and then analyzed by GC-MS (Fig. 1). GC-MS analysis showed the presence of various hydrocarbons, including essentially n-alkanes from C12 to C25, as well as the individual isoprenoid hydrocarbons, pristane and phytane (Fig. 1).
Isolation of fluoranthene-degrading bacterium
An aerobic enrichment culture method was used according to the protocol described in above to isolate marine PAH-degrading bacteria at 37 °C and 30 g l−1 NaCl. After several dilutions and subculturing in the basal medium containing 200 mg l−1 of fluoranthene, a positive aerobic enrichment culture showed a stable bacterial population with a cellular morphology dominated by a motile and rod-shaped bacterium. The latter culture was then used for the isolation of pure strains on solid media containing 200 mg l−1 fluoranthene. Seven colonies were picked and a pure culture designed FLU5 was selected on the basis of its high capacity to grow on fluoranthene, as the sole carbon and energy source, without yeast extract added, in solid and liquid media. The selected strain FLU5 was used for further characterization.
Characterization of strain FLU5
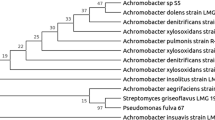
The bacterial strain FLU5 was aerobic, Gram-positive, rod-shaped, motile, sporulated bacterium, oxidase positive and catalase positive. Colonies of this isolate were white, irregular, raised and 3–5 mm in diameter, after overnight culture on LB agar plate at 37 °C. Strain FLU5 grew over a temperature range between 8 and 40 °C, with an optimum at 30 °C. It was also able to grow over a pH range between 6 and 10.6, with an optimum growth at 7.1. The NaCl concentration range for growth was between 0 and 120 g l−1, with an optimum at 30 g l−1 NaCl. The 16S r RNA gene sequence of strain FLU5, consisting of 1478 nucleotides, was determined and deposited in the GenBank nucleotide database under accession number KR676443. The phylogenetic analysis indicated that strain FLU5 was closely related to members of the genus Bacillus with Bacillus stratosphericus, strain 41KF2aT (Shivaji et al. 2006) being the most closely related species (99.66 % 16S rRNA gene sequence similarity). The phylogenetic tree based on related Bacillus species is shown in Fig. 2. Differential phenotypic characteristics of strain FLU5 and its closest phylogenetic neighbours in the genus Bacillus are given in Table 2. Therefore, according to its phylogenetic and phenotypic characteristics, the isolated strain FLU5 was identified as a member of the genus Bacillus in the Bacillaceae family and in particular as a strain of the species of B. stratosphericus.
Phylogenetic tree based on 1478 unambiguous nucleotides of the 16S rRNA gene sequence indicating the position of strain FLU5 among related members of the genus Bacillus. Reference type strain organisms are included and GenBank sequence accession numbers are given in parentheses. Micrococcus luteus is used as an out group. Bootstrap values, expressed as percentage of 1000 replications, are given at the nodes. Bar, two substitutions per 100 nucleotides
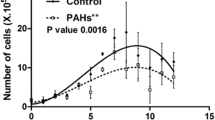
Biodegradation of fluoranthene by strain FLU5
Strain FLU5 was able to degrade fluoranthene (100 mg l−1), used as the sole source of carbon and energy, in basal liquid medium, in the presence of 30 g l−1 NaCl and at 37 °C. The growth of this strain on fluoranthene was followed by measuring the OD600 nm at different culture’s time (Fig. 3). These results were also confirmed by enumeration of viable cells, indicating positive growth in the presence of this substrate (Fig. 3). GC-MS analyses were also carried out to monitor the biodegradation of fluoranthene after 0, 3, 15, 21 and 30 days of incubation, compared with abiotic controls incubated at the same conditions. GC-MS analyses showed that strain FLU5 was capable of degrading almost 30 % of fluoranthene (retention time = 11.482 min, m/z = 202.1) after 15 days and 44 % after 30 days. This result was confirmed by diminution of the peak areas of fluoranthene, as shown in Fig. 4. In order to study the effect of addition of a synthetic surfactant on fluoranthene biodegradation by strain FLU5, Tween 80 (0.05 %, v/v) was added to the culture medium. Adding Tween 80 has no effect on either growth or biodegradative activity (data not shown). In addition, during the growth of strain FLU5 on fluoranthene, the surface tension decreased from 55 to 42 mN m−1, after 3 days of incubation at 37 °C and remained stable during the stationary phase (Fig. 5). This result suggested that strain FLU5 has the capacity to secrete a biosurfactant that enhances fluoranthene degradation.
Biodegradation potential of some hydrocarbons by strain FLU5
The ability of strain FLU5 to grow on a variety of hydrocarbons, as sole carbon and energy sources, in the presence of 30 g l−1 NaCl and at 37 °C, was studied (Table 3). Strain FLU5 was able to grow on naphthalene, phenanthrene and pyrene, PAHs with 2, 3 and 4 aromatic rings, respectively. Strain FLU5 was also capable of growing on mono-aromatics, benzene, toluene, ethylbenzene and o-, m- and p-xylenes. Protocatechuic, gentisic and gallic acids, derivatives of benzene, were metabolized by strain FLU5. In contrast, syringic and vanillic acids were not used. Caffeic acid, a cinnamic acid derivative, was used by strain FLU5. However, ferulic acid was not degraded. Moreover, strain FLU5 was capable of growing on octane, an n-alkane with 8 carbon atoms, but it was not able to grow on decane, an n-alkane with 10 carbon atoms. Strain FLU5 showed also a better growth on tyrosol, a natural phenolic antioxidant, than phenol, a toxic aromatic compound. In addition, crude oil, diesel fuel and motor oil, which are complex hydrocarbons containing aromatic, aliphatic and polyaromatic compounds, could be degraded by this strain. Furthermore, strain FLU5 showed an important growth on olive oil and on glycerol, a simple polyol compound (Table 3).
Discussion
Determination of physico-chemical parameters is very important to get an idea about the quality of the seawaters and the degree of pollution in the studied harbour. The pH is one of the most important factors in the coastal ecosystems, in fact, many biological activities can occur only within a narrow range. The wide variety of aquatic animals prefers pH ranging from 6.5 to 8 (Faragallah et al. 2009), such as the pH found in the studied seawater (7.4) (Table 1). The water temperature influences also the physico-chemical characters of coastal water. From the results found, it could be understood that the surface water temperature is governed by the atmospheric temperature (17.4 °C, Table 1). Besides, results showed that the studied seawater is rich in minerals Na, Ca, Mg and K (Table 1). This may explain the significant EC and salinity (36.2 g l−1, Table 1). In fact, the salinity is quantified as the total concentration of soluble salts and is expressed in terms of electrical conductivity. In addition, results found indicate the abundance of various heavy metals in the studied seawater (Table 1). This contamination may be due to industrial effluents, fishery activities, ship traffic, inputs associated with municipal and industrial sewage water, etc. (Zaghden et al. 2007). In connection with the COD parameter, the TOC provides information on the type and origin of organic contamination of the water. Analysis showed that organic carbon value is greater than inorganic carbon value (Table 1). The BOD is an indicator of organic load of water. If BOD/COD ratio is between 0.3 and 0.6, there is a chemical pollution and it is required to treat it biologically (Srinivas 2008). In the present study, BOD/COD ratio is equal to 0.3 (Table 1); hence, the studied seawater was characterized by difficult biodegradable organic pollutants.
A noticeable contamination of seawater sample was shown. It may be due to industrial effluents, fishing and commerce boats that generate various pollutants, especially hydrocarbons (0.6 mg l−1) (Table 1). GC-MS data indicate the presence of a large variety of hydrocarbons, including essentially n-alkanes from C12 to C25 as well as the individual isoprenoid hydrocarbons, pristane and phytane, suggesting a petroleum contamination of seawater of the fishing harbour of Sfax (Fig. 1). The hydrocarbons pristane (2. 6. 10. 14-tetramethylpentadecane, Pr: R t = 9.49 min, m/z = 269.2) and phytane (2. 6. 10. 14-tetramethylhexadecane, Ph: R t = 10.07 min, m/z = 282.3) are common isoprenoid detected in coastal marine sediments (Readman et al. 2002). They are commonly present in most petroleum, usually as the major constituents among a much wider distribution of isoprenoid alkanes. Therefore, they are often considered as good indicators of petroleum contamination (Volkman et al. 1992; Zaghden et al. 2007). Some studies dealing with sources of contamination of coastal sediments from the Sfax area have been published. Indeed, Louati et al. 2001 reported that the hydrocarbon concentrations are relatively higher in the coastal area of the Sfax city compared to other coastal Mediterranean sites. Furthermore, the impact of this hydrocarbon contamination becomes real in areas of harbour activities, where the concentration of total petroleum hydrocarbons reaches 1729 μg g−1 and total of PAHs approaches 11 μg g−1 (Zaghden et al. 2007). The region receives hydrocarbons from several origins such as fishery activities, ship traffic, inputs associated with municipal and industrial sewage waters, especially from storage of crude oil and phosphogypsum at the coast.
The physico-chemical characterization of the seawater showed a heavy contamination by organic and inorganic micropollutants. These are excellent tracers of urban and industrial pollution, and they are among the most toxic compounds due to their low biodegradation. Their presence requires regular monitoring, preventive measures and a total ban on discards along the south coast of Sfax. The increasing of human population and industrial activity generates an organic contamination which is the most serious problem for seawater quality.
Strain FLU5 isolated from seawater of the fishing harbour after enrichment on fluoranthene was an aerobic, mesophile and halotolerant bacterium. The phenotypic and phylogenetic characteristics of the marine isolate FLU5 indicate that it was closely related to the species of B. stratosphericus (Shivaji et al. 2006). Organisms belonging to B. stratosphericus are widespread worldwide and have been isolated frequently and from almost every environment, such as marine areas (Siddikee et al. 2010; Wang et al. 2010; Lima et al. 2013; Pandey et al. 2013), soil (Yadav et al. 2011; Hosseini-Abari et al. 2014), estuarine sediments (Zhang et al. 2012) and human cerumen (Gerchman et al. 2012). B. stratosphericus was originally isolated from cryogenic tubes used for collecting air samples from high altitudes (Shivaji et al. 2006).
Many studies have been reported on the involvement of Bacillus strains in biodegradation of components of petroleum products and crude oil including PAHs (da Cunha et al. 2006), but there are no reports that specifically mention the use of B. stratosphericus strains for PAHs or fluoranthene degradation. Among Bacillus species capable of degrading PAHs, B. fusiformis degraded naphthalene (Lin et al. 2010), B. cereus (Fuchedzhieva et al. 2008) and B. thuringiensis (Maiti et al. 2012) degraded fluoranthene, and B. subtilis was able to degrade pyrene (Hunter et al. 2005; Das and Mukherjee 2007a) and benzo(a)pyrene (Hunter et al. 2005; Su et al. 2006). A crude oil-degrading B. stratosphericus strain has been isolated from seawater around Xiamen Island, China (Wang et al. 2010). Lima et al. 2013 reported the presence of cytochrome P450 and glutathione transferase suggesting the capability of B. stratosphericus strain LAMA 585 to metabolize recalcitrant compounds, such as benzopyrene and naphthalene.
Strain FLU5 was capable of degrading aerobically almost 30 and 45 % of fluoranthene (100 mg l−1) in the presence of 30 g l−1 NaCl and without yeast extract added, after 15 and 30 days of incubation at 37 °C, respectively. Comparing these results with other studies in the same context, we can conclude that the strain FLU5 has significant biodegradative potential on fluoranthene (Table 4). In fact, Pasteurella ssp. strain B-2 isolated from an activated sludge was able to degrade 40 % of fluoranthene after 30 days of incubation, but only in the presence of a concentration of 20 mg l−1 (Šepič et al. 1997). A Herbaspirillum chlorophenolicum strain FA1, isolated from an activated sludge, was also capable of degrading 13.7 % of fluoranthene with only an initial concentration of 20 mg l−1, after 30 days of incubation (Xu et al. 2011). Recently, a Bacillus thuringiensis strain NA2, isolated from a petroleum oil contaminated site, can degrade almost 30 % of fluoranthene, with an initial concentration of 50 mg l−1, after 12 days of incubation (Maiti et al. 2012). Metabolic pathways of fluoranthene have been proposed for several bacterial fluoranthene degraders, such as bacteria belonging to the genera Mycobacterium (Kelley et al. 1993; Šepič et al 1998; Rehmann et al. 2001; López et al. 2005; Kweon et al. 2007; Zhong et al. 2011), Pasteurella (Šepič et al. 1998), Sphingomonas (van Herwijnen et al. 2003), Alcagligenes (Weissenfels et al. 1991), Pseudomonas (Gordon and Dobson 2001) and Celeribacter (Cao et al. 2015). However, little is known regarding the metabolic pathways of fluoranthene by bacteria belonging to the Bacillus genus. The main pathway for bacterial degradation of fluoranthene is usually initiated by dioxygenation at the C-1,2, C-2,3 C-7,8 and C-8,9 positions using dioxygenase enzymes, which incorporate two atoms of molecular oxygen leading to the production of dihydrodiols. Dihydrodiols are then converted through either an ortho or a meta cleavage type of pathway into a key intermediates as catechol, salicylate, and protocatechuate, which are then transformed to tricarboxylic acid cycle intermediates (Weissenfels et al. 1991; Šepič et al. 1998; Rehmann et al. 2001; López et al. 2005; Kweon et al. 2007; Cao et al. 2015). In other case, some bacteria are also capable of oxidizing fluoranthene via cytochrome P450 monooxygenase enzyme to form monohydroxyfluoranthene (Cao et al. 2015; van Herwijnen et al. 2003). In fact, van Herwijnen et al. 2003 reported the initial monooxygenation of fluoranthene by Sphingomonas sp. LB126 which was capable to cooxidize fluoranthene, anthracene and phenanthrene without the accumulation of intermediates. Lima et al. 2013 reported the presence of cytochrome P450 suggesting the capability of B. stratosphericus strain LAMA 585 to metabolize recalcitrant compounds, such as HAPs. Accordingly, it might be possible that strain FLU5 could degrade fluoranthene using cytochrome P450. Although important advances have been made, many aspects of metabolism of fluoranthene, especially for marine bacteria still demands more explorations due to limited information on biodegradation of PAHs containing a cyclopentane moiety (Story et al. 2001; Cao et al. 2015).
Addition of the synthetic surfactant, Tween 80, has no effect on the biodegradation of fluoranthene by the strain FLU5. Some studies showed that the surfactants can improve the bioavailability of hydrocarbons including PAHs, for microorganisms and then have been reported to facilitate and enhance biodegradation (Hickey et al. 2007; Mnif et al. 2009; Chamkha et al. 2011; Xu et al. 2011; Maiti et al. 2012), while others reported that addition of surfactants might inhibit the biodegradation of PAHs (Tiehm 1994). In other cases, the addition of surfactants can have negligible effect on hydrocarbon biodegradation as it is the case for our study (Fernando Bautista et al. 2009). In the case of culture FLU5 on fluoranthene, the Tween 80 does not respond to its characteristics which basically enable it to increase the solubility and the bioaccessibility of the hydrocarbons to the microbial attack.
The reduction of surface tension during growth of the culture FLU5 on fluoranthene supported the secretion of biosurfactant by this strain (Fig. 5), as shown for strains of Streptomyces spp., isolated from a plain soil sample, capable of utilizing naphthalene and crude oil as the sole source of carbon (Ferradji et al. 2014) and strains of Bacillus subtilis and Pseudomonas aeruginosa, isolated from a petroleum-contaminated soil, capable of degrading pyrene (Das and Mukherjee 2007a). These strains were able to secrete a large amount of biosurfactants while growing on hydrocarbon-rich medium. The criterion used for selecting biosurfactant produces is the ability to reduce the surface tension (Kebbouche-Gana et al. 2009). In previous study, a clear association between hydrocarbon biodegradation and biosurfactant production was found for several bacterial strains isolated from Tunisian oilfields (Mnif et al. 2011). The production of biosurfactants by hydrocarbonclastic bacteria is considered as a biological strategy to facilitate the biodegradation of hydrocarbons. Biosurfactants could, significantly, enhance the apparent solubility of hydrocarbons, and then, improve the bioavailability of these compounds into microorganisms. In general, Bacillus species are mostly known to produce biosurfactant belonging to the family of lipopeptides such as fengycin, iturin and surfactin. These groups of microbial biosurfactants are interesting due to their several important properties including oil degradation, bioremediation, as well as their anti-bacterial, fungal and viral properties (Sang-cheol et al. 2010).
Strain FLU5 has also the ability to use a wide range of hydrocarbons as sole carbon and energy sources: all compounds of the family BTEX; protocatechuic, gentisic, gallic and caffeic acids, tyrosol and phenol; PAHs including naphthalene, phenanthrene and pyrene; complex hydrocarbons including crude oil, diesel fuel and motor oil; octane and olive oil. Strain FLU5 showed also an important growth on glycerol. In this respect, de França et al. 2015 reported that glycerol can be used as low cost substrate for biosurfactant production. In fact, B. subtilis strain ICA56 and P. aeruginosa strain UCP0992 showed a capacity to produce an efficient biosurfactant by using glycerol as the sole carbon source (Silva et al. 2010; de França et al. 2015). The no-use of some hydrocarbons such as syringic, vanillic and ferulic acids and decane by the strain FLU5 could be explained by the recalcitrance of certain chemical structures and by the potential and the specific enzymes present in this strain. Previous research mentioned the effectiveness of Bacillus strains with regard to hydrocarbon biodegradation, among which we can cite: B. subtilis (Das and Mukherjee 2007b; Wang et al. 2008; Mukherjee and Bordoloi 2012), B. pumilus (Liu et al. 2010; Đokić et al. 2011), B.cereus (Wang et al. 2008; Đokić et al. 2011; Maliji et al. 2013); B. mojavensis, B. fusiformis (Wang et al. 2008), B. megaterium, B. simplex (Đokić et al. 2011), B. licheniformis (Mnif et al. 2011) and Aerobacillus pallidus (Mnif et al. 2014). Furthermore, Bacillus strains isolated from marine environment have been confirmed as potential degraders of hydrocarbons. In fact, B. subtilis, B. mojavenis, B. fusiformis and B. cereus, isolated from nearshore surface water and Pacific Ocean sediment, have been evaluated for their BTX biodegradation potential (Wang et al. 2008). In addition, strains of B. cereus isolated from an oil polluted seawater in south Lebanon showed their capacity to degrade aliphatic and aromatic hydrocarbons contained in the diesel oil (Maliji et al. 2013). Finally, a strain of B. stratosphericus was isolated from seawater around Xiamen Island, China, and was capable of degrading crude oil (Wang et al. 2010).
Conclusion
In recent years, the remediation of hydrocarbons contained in seawater has become an issue of global concern. The physico-chemical characterization of seawater sample taken from the fishing harbour of Sfax, Tunisia, showed a heavy contamination by inorganic and organic micropollutants, especially hydrocarbons. Fluoranthene is a PAH consisting of a naphthalene and a benzene unit connected by a five-membered ring, and it has often been taken as a model compound for research on the bioremediation of high-molecular-weight PAHs. A marine halotolerant bacterial strain FLU5, affiliated to B. stratosphericus, was isolated after enrichment culture on fluoranthene used as the sole carbon and energy source, without yeast extract added. Strain FLU5 showed an interesting degradative capacity of fluoranthene, and it was also able to grow on a wide range of aliphatic, aromatic and complex hydrocarbons. Further studies are required to explore the metabolic pathways of fluoranthene by strain FLU5. Furthermore, reduction of the surface tension was also observed during growth of strain FLU5 on fuoranthene. This result obtained point out the necessity of going further in this research work, in order to produce and characterize an efficient biosurfactant synthesized by strain FLU5. As well, a comparative study with the type strain of B. stratosphericus could provide more information regarding the ability of strain FLU5 to degrade hydrocarbons and produce biosurfactant. Overall, the significant ability of strain FLU5 to degrade hydrocarbons and its adaptability to saline conditions, make it a very good candidate for the development of biological methods in order to stimulate biodegradation for oil bioremediation in polluted seawater.
References
Benson DA, Boguski MS, Lipman DJ, Ostell J, Ouellette BF, Rapp BA, Wheeler DL (1999) GenBank. Nucleic Acids Res 27:12–17
Cao J, Lai Q, Yuan J, Shao Z (2015) Genomic and metabolic analysis of fluoranthene degradation pathway in Celeribacter indicus P73T. Sci Rep 5:7741
Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351–368
Chamkha M, Record E, Garcia J-L, Asther M, Labat M (2002) Isolation from a shea cake digester of a tannin-tolerant Escherichia coli strain decarboxylating p-hydroxybenzoic and vanillic acids. Curr Microbiol 44:341–349
Chamkha M, Trabelsi Y, Mnif S, Sayadi S (2011) Isolation and characterization of Klebsiella oxytoca strain degrading crude oil from a Tunisian off-shore oil field. J Basic Microb 51:580–589
Chebbi A, Jaoua H, Loukil S, Mhiri N, Ammar N, Sayadi S, Chamkha M (2016) Biodegradation of malodorous mercaptans by a novel Staphylococcus capitis strain isolated from gas-washing wastewaters of the Tunisian Chemical Group. Int J Environ Sci Technol 13:571–580
D’Adamo R, Di Stasio M, Fabbrocini A, Petitto F, Roselli L, Volpe MG (2008) Migratory crustaceans as biomonitors of metal pollution in their nursery areas. The Lesina lagoon (SE Italy) as a case study. Environ Monit Assess 143:15–24
Da Cunha CD, Rosado AS, Sebastián GV, Seldin L, von der Weid I (2006) Oil biodegradation by Bacillus strains isolated from the rock of an oil reservoir located in a deep-water production basin in Brazil. Appl Microbiol Biot 73:949–959
Danulat E, Muniz P, García-Alonso J, Yannicelli B (2002) First assessment of the highly contaminated harbour of Montevideo, Uruguay. Mar Pollut Bull 44:554–565
Das K, Mukherjee AK (2007a) Differential utilization of pyrene as the sole source of carbon by Bacillus subtilis and Pseudomonas aeruginosa strains: role of biosurfactants in enhancing bioavailability. J Appl Microbiol 102:195–203
Das K, Mukherjee AK (2007b) Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresource Technol 98:1339–1345
De França ÍWL, Lima AP, Lemos JAM, Lemos CGF, Melo VMM, de Sant’ana HB, Gonçalves LRB (2015) Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal Today 255:10–15
Đokić L, Narančić T, Nikodinovic-Runic J, Bajkić S, Vasiljević B (2011) Four Bacillus sp. soil isolates capable of degrading phenol, toluene, biphenyl, naphthalene and other aromatic compounds exhibit different aromatic catabolic potentials. Arch Biol Sci 63:1057–1067
Faragallah H, Askar A, Okbah M, Moustafa H (2009) Physico-chemical characteristics of the open Mediterranean sea water far about 60 Km from Damietta harbor, Egypt. J Ecol Nat Environ 1:106–119
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fernando Bautista L, Sanz R, Carmen Molina M, González N, Sánchez D (2009) Effect of different non-ionic surfactants on the biodegradation of PAHs by diverse aerobic bacteria. Int Biodeter Biodegr 63:913–922
Ferradji FZ, Mnif S, Badis A, Rebbani S, Fodil D, Eddouaouda K, Sayadi S (2014) Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria). Int Biodeterior Biodegradation 86:300–308
Fuchedzhieva N, Karakashev D, Angelidaki I (2008) Anaerobic biodegradation of fluoranthene under methanogenic conditions in presence of surface-active compounds. J Hazard Mater 153:123–127
Gerchman Y, Patichov R, Zeltzer T (2012) Lipolytic, proteolytic, and cholesterol-degrading bacteria from the human cerumen. Curr Microbiol 64:588–591
Gordon L, Dobson ADW (2001) Fluoranthene degradation in Pseudomonas alcaligenes PA-10. Biodegradation 12:393–400
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid S 41:95–98
Hickey AM, Gordon L, Dobson ADW, Kelly CT, Doyle EM (2007) Effect of surfactants on fluoranthene degradation by Pseudomonas alcaligenes PA-10. Appl Microbiol Biot 74:851–856
Hilyard EJ, Jones-Meehan JM, Spargo BJ, Hill RT (2008) Enrichment, isolation, and phylogenetic identification of polycyclic aromatic hydrocarbon-degrading bacteria from Elizabeth River sediments. Appl Environ Microb 74:1176–1182
Hosseini-Abari A, Emtiazi G, Lee S-H, Kim B-G, Kim J-H (2014) Biosynthesis of silver nanoparticles by Bacillus stratosphericus spores and the role of dipicolinic acid in this process. Appl Biochem Biotech 174:270–282
Hunter RD, Ekunwe SIN, Dodor DE, Hwang H-M, Ekunwe L (2005) Bacillus subtilis is a potential degrader of pyrene and benzo[a]pyrene. Int J Environ Res Public Health 2:267–271
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Kebbouche-Gana S, Gana ML, Khemili S, Fazouane-Naimi F, Bouanane NA, Penninckx M, Hacene H (2009) Isolation and characterization of halophilic Archaea able to produce biosurfactants. J Ind Microbiol Biot 36:727–738
Kelley I, Freeman JP, Evans FE, Cerniglia CE (1993) Identification of metabolites from the degradation of fluoranthene by Mycobacterium sp. strain PYR-1. Appl Environ Microb 59:800–806
Kumar S, Upadhayay SK, Kumari B, Tiwari S, Singh SN, Singh PK (2011) In vitro degradation of fluoranthene by bacteria isolated from petroleum sludge. Bioresource Technol 102:3709–3715
Kweon O, Kim SJ, Jones RC, Freeman JP, Adjei MD, Edmondson RD, Cerniglia CE (2007) A polyomic approach to elucidate the fluoranthene-degradative pathway in Mycobacterium vanbaalenii PYR-1. J Bacteriol 189:4635–4647
Lima A de S, Cabral A, Andreote FD, Cavalett A, Pessatti ML, Dini-Andreote F, da Silva M (2013) Draft genome sequence of Bacillus stratosphericus LAMA 585, isolated from the Atlantic deep sea. Genome Announcements 1(3):e00204–e00213
Lin C, Gan L, Chen Z (2010) Biodegradation of naphthalene by strain Bacillus fusiformis (BFN). J Hazard Mater 182:771–777
Liu J-H, Maity JP, Jean J-S, Chen C-Y, Chen C-C, Ho S-Y (2010) Biodegradation of benzene by pure and mixed cultures of Bacillus spp. World J Microb Biot 26:1557–1567
Logan NA, Berkeley RCW (1984) Identification of Bacillus Strains Using the API System. Microbiology 130:1871–1882
López Z, Vila J, Grifoll M (2005) Metabolism of fluoranthene by mycobacterial strains isolated by their ability to grow in fluoranthene or pyrene. J Ind Microbiol Biotechnol 32:455–464
Louati A, Elleuch B, Kallel M, Saliot A, Dagaut J, Oudot J (2001) Hydrocarbon contamination of coastal sediments from the Sfax area (Tunisia), Mediterranean Sea. Mar Pollut Bull 42:445–452
Maiti A, Das S, Bhattacharyya N (2012) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons by Bacillus thuringiensis strain NA2. Journal of Science 1:72–75
Maliji D, Olama Z, Holail H (2013) Environmental studies on the microbial degradation of oil hydrocarbons and its application in Lebanese oil polluted coastal and marine ecosystem. Int J Curr Microbiol Appl Sci 2:1–18
Mnif S, Chamkha M, Sayadi S (2009) Isolation and characterization of Halomonas sp. strain C2SS100, a hydrocarbon-degrading bacterium under hypersaline conditions. J Appl Microbiol 107:785–794
Mnif S, Chamkha M, Labat M, Sayadi S (2011) Simultaneous hydrocarbon biodegradation and biosurfactant production by oilfield-selected bacteria. J Appl Microbiol 111:525–536
Mnif S, Sayadi S, Chamkha M (2014) Biodegradative potential and characterization of a novel aromatic-degrading bacterium isolated from a geothermal oil field under saline and thermophilic conditions. Int Biodeter Biodegr 86:258–264
Mukherjee A, Bordoloi N (2012) Biodegradation of benzene, toluene, and xylene (BTX) in liquid culture and in soil by Bacillus subtilis and Pseudomonas aeruginosa strains and a formulated bacterial. Environ Sci Pollut R 19:3380–3388
Palmisano M, Nakamura LK, Duncan KE, Istock CA, Cohan FM (2001) Bacillus sonorensis sp. nov., a close relative of Bacillus licheniformis, isolated from soil in the Sonoran Desert, Arizona. Int J Syst Evol Micr 51:1671–1679
Pandey S, Sree A, Dash SS, Sethi DP, Chowdhury L (2013) Diversity of marine bacteria producing beta-glucosidase inhibitors. Microb Cell Fact 12:35
Readman J, Fillmann G, Tolosa I, Bartocci J, Villeneuve J-P, Catinni C, Mee LD (2002) Petroleum and PAH contamination of the Black Sea. Mar Pollut Bull 44:48–62
Rehmann K, Hertkorn N, Kettrup AA (2001) Fluoranthene metabolism in Mycobacterium sp. strain KR20: identity of pathway intermediates during degradation and growth. Microbiology 147:2783–2794
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sang-Cheol L, Kim SH, Park IH, Chung SY, Chandra MS, Yong-Lark C (2010) Isolation, purification, and characterization of novel fengycin S from Bacillus amyloliquefaciens LSC04 degrading-crude oil. Biotechnol Bioprocess Eng 15:246–253
Šepič E, Bricelj M, Leskovšek H (1997) Biodegradation studies of polyaromatic hydrocarbons in aqueous media. J Appl Microbiol 83:561–568
Šepič E, Bricelj M, Leskovšek H (1998) Degradation of fluoranthene by Pasteurella sp. IFA and Mycobacterium sp. PYR‐1: isolation and identification of metabolites. J Appl Microbiol 85:746–754
Shivaji S, Chaturvedi P, Suresh K, Reddy GSN, Dutt CBS, Wainwright M, Narlikar JV, Bhargava PM (2006) Bacillus aerius sp. nov., Bacillus aerophilus sp. nov., Bacillus stratosphericus sp. nov. and Bacillus altitudinis sp. nov., isolated from cryogenic tubes used for collecting. Int J Syst Evol Micr 56:1465–1473
Siddikee MA, Chauhan PS, Anandham R, Han G-H, Sa T (2010) Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechn 20:1577–1584
Silva SNRL, Farias CBB, Rufino RD, Luna JM, Sarubbo LA (2010) Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surfaces B Biointerfaces 79:174–183
Srinivas T (2008) Environmental Biotechnology. New Age International Publishers, New Delhi, p 10
Story SP, Parker SH, Hayasaka SS, Riley MB, Kline EL (2001) Convergent and divergent points in catabolic pathways involved in utilization of fluoranthene, naphthalene, anthracene, and phenanthrene by Sphingomonas paucimobilis var. EPA505. J Ind Microbiol Biot 26:369–382
Su D, Li P, Frank S, Xiong X (2006) Biodegradation of benzo[a]pyrene in soil by Mucor sp. SF06 and Bacillus sp. SB02 co-immobilized on vermiculite. J Environ Sci 18:1204–1209
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Tiehm A (1994) Degradation of polycyclic aromatic hydrocarbons in the presence of synthetic surfactants. Appl Environ Microb 60:258–263
van Herwijnen R, Wattiau P, Bastiaens L, Daal L, Jonker L, Springael D, Govers HAJ, Parsons JR (2003) Elucidation of the metabolic pathway of fluorene and cometabolic pathways of phenanthrene, fluoranthene, anthracene and dibenzothiophene by Sphingomonas sp. LB126. Res Microbiol 154:199–206
Viaroli P, Bartoli M, Giordani G, Magni P, Welsh DT (2004) Biogeochemical indicators as tools for assessing sediment quality/vulnerability in transitional aquatic ecosystems. Aquatic Conservation: Marine and Freshwater Ecosystems 14:19–29
Volkman J, Holdsworth D, Neil G, Bavor HJ Jr (1992) Identification of natural, anthropogenic and petroleum hydrocarbons in aquatic sediments. Sci Total Environ 112:203–219
Wang L, Qiao N, Sun F, Shao Z (2008) Isolation, gene detection and solvent tolerance of benzene, toluene and xylene degrading bacteria from nearshore surface water and Pacific Ocean sediment. Extremophiles 12:335–342
Wang W, Wang L, Shao Z (2010) Diversity and abundance of oil-degrading bacteria and alkane hydroxylase (alkB) genes in the subtropical seawater of Xiamen Island. Microb Ecol 60:429–439
Weissenfels WD, Beyer M, Klein J, Rehm HJ (1991) Microbial metabolism of fluoranthene: isolation and identification of ring fission products. Appl Microbiol Biot 34:528–535
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Arch Microbiol 129:395–400
Winker S, Woese CR (1991) A definition of the domains Archaea, Bacteria and Eucarya in terms of small subunit ribosomal RNA characteristics. Syst Appl Microbiol 14:305–310
Witt G (1995) Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Mar Pollut Bull 31:237–248
Xu HX, Wu HY, Qiu YP, Shi XQ, He GH, Zhang JF, Wu JC (2011) Degradation of fluoranthene by a newly isolated strain of Herbaspirillum chlorophenolicum from activated sludge. Biodegradation 22:335–345
Yadav S, Kaushik R, Saxena A, Arora D (2011) Genetic and functional diversity of Bacillus strains in the soils long-term irrigated with paper and pulp mill effluent. J Gen App Microbiol 57:183–195
Zaghden H, Kallel M, Louati A, Elleuch B, Oudot J, Saliot A (2005) Hydrocarbons in surface sediments from the Sfax coastal zone, (Tunisia) Mediterranean Sea. Mar Pollut Bull 50:1287–1294
Zaghden H, Kallel M, Elleuch B, Oudot J, Saliot A (2007) Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Sfax, Tunisia, Mediterranean Sea. Mar Chem 105:70–89
Zhang J, Zhang E, Scott K, Grant Burgess J (2012) Enhanced electricity production by use of reconstituted artificial consortia of estuarine bacteria grown as biofilms. Environ Sci Technol 46:2984–2992
Zhong Y, Luan T, Lin L, Liu H, Tam NFY (2011) Production of metabolites in the biodegradation of phenanthrene, fluoranthene and pyrene by the mixed culture of Mycobacterium sp. and Sphingomonas sp. Bioresource Technol 102:2965–2972
Acknowledgments
This work was supported by a grant provided by the Tunisian Ministry of Higher Education and Scientific Research and the Hubert Curien Program (CMCU 15G0808) supported by the French Ministry of Foreign Affairs and the Tunisian Ministry of Higher Education and Scientific Research. We thank Mrs. Manel Chalbi and Mrs. Najla Mhiri for their help with molecular techniques, Miss. Lobna Jlail and Mrs. Fatma Rezgui for their technical assistance in GC-MS analyses and Mr. Nidhal Baccar for his technical assistance in atomic absorption analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Hentati, D., Chebbi, A., Loukil, S. et al. Biodegradation of fluoranthene by a newly isolated strain of Bacillus stratosphericus from Mediterranean seawater of the Sfax fishing harbour, Tunisia. Environ Sci Pollut Res 23, 15088–15100 (2016). https://doi.org/10.1007/s11356-016-6648-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6648-7