Abstract
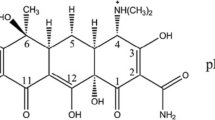
Cyanotoxins, microcystins and cylindrospermopsin, are potent toxins produced by cyanobacteria in potable water supplies. This study investigated the removal of cyanotoxins from aqueous media by magnetophoretic nanoparticle of polypyrrole adsorbent. The adsorption process was pH dependent with maximum adsorption occurring at pH 7 for microcystin-LA, LR, and YR and at pH 9 for microcystin-RR and cylindrospermopsin (CYN). Kinetic studies and adsorption isotherms reflected better fit for pseudo-second-order rate and Langmuir isotherm model, respectively. Thermodynamic calculations showed that the cyanotoxin adsorption process is endothermic and spontaneous in nature. The regenerated adsorbent can be successfully reused without appreciable loss of its original capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numbers of freshwater cyanobacteria produce potent hepatotoxins which lead to serious health risk with acute symptoms of vomiting, diarrhea, and even death (Falconer 2005). The cyanotoxin removals are important to water industries as these are hepatotoxins and promote liver tumor (Falconer 1989). Among all the cyanotoxins, microcystins (MCs) and cylindrospermopsin (CYN) are the most common hepatotoxins recorded in drinking waters produced by Microcystis aeruginosa and Cylindrospermopsin raciborskii mainly in tropical and sub-tropical regions (Ho et al. 2011). Cyanotoxins are problematic since these are highly soluble and chemically stable in water (Jones and Orr 1994; Wang et al. 2007). Moreover, the dissolved cyanotoxins have been reported to be more recalcitrant towards the conventional methods of water treatment such as, sedimentation, filtration, and coagulation-flocculation (Dixon et al. 2010; Dixon et al. 2011). However, adsorption granular and powdered activated carbons have shown to be successful in removal of cyanotoxins from drinking water (Ho et al. 2011; Hena et al. 2014).
The climate change experts have predicted increase in both the occupied water bodies and intensity of cyanobacterial blooms (Pearl and Huisman 2008); thus, it is an important topic of concern to find out better options to remove cyanotoxins together. To date, many studies have been done for the removal of microcystins (El-Sheikh et al. 2014; Wang et al. 2014; Zhang et al. 2014) and cylindrospermopsin (Fotiou et al. 2015; He et al. 2014) with different methods. Among all the reported methods, adsorption is technically easy and one of the most economically favorable method, although there are very few studies that have been conducted on removal of microcystins and cylindrospermopsin together (Ho et al. 2011). However, no studies have been yet performed involving conductive electroactive polymer as an adsorbent for removing cyanotoxins from drinking waters.
The main aim of this study was to investigate the adsorption of cyanotoxins on Fe3O4 coated with electroactive conducting polymer (ECP) polypyrrole (PPy/Fe3O4) without compromising the quality of drinking water. Polypyrrole (PPy) has been a subject of intense investigation of many research groups because of their interesting qualities such as high electrical conductivity, environmental stability, and non-toxicity, which advocate it to categorize under reusable and environmental friendly material. Its easy and relatively low cost of preparations competes over activated carbon (Bhaumik et al. 2011a; Ansari et al. 2009). PPy matrix carries positive charges due to positive nitrogen atoms which happen to be produce during polymerization reaction. The matrix maintains their neutrality via incorporating the counter ions commonly known as dopant during the course of polymerization reaction (Zhang and Bai 2003). The presence of positively charged nitrogen atoms are being used to form electrostatic bond between any other negatively charged carrying compounds. Previously, PPy have been used for removal of fluoride (Bhaumik et al. 2011b), chromium (IV) (Bhaumik et al. 2011a; Ansari and Fahim 2007), and arsenic (Eisazadeh 2008) from aqueous medium and have proven advantageous adsorbent.
The main objectives of this study were to evaluate an efficient separation method for removing the cyanotoxins from water by PPy/Fe3O4 nanoparticles. The operational parameters in this adsorption process such as temperature effect, initial concentration of adsorbates, time of contact of adsorbate and adsorbent, and solution pH were characterized, and the adsorption mechanism of PPy/Fe3O4 nanoparticles was investigated.
Methods
Materials
Fe3O4, pyrrole (Py), and FeCl3 were used from Sigma–Aldrich, Germany. The MC-LR, RR, YA, LA, and CYN were supplied by Fluka, Switzerland, and were used without further purification. Hydrochloric acid and sodium hydroxide were used to alter the pH of the solutions as per requirement. All aqueous solutions were prepared using organic molecule free Milli-Q water (18-MΩ resistance).
Synthesis of the magnetic PPy/Fe3O4 nanoparticle
The PPy/Fe3O4 nanoparticles were synthesized by chemical oxidative polymerization of freshly distilled pyrrole monomer in the presence of FeCl3 used as an oxidant, as shown in Scheme 1, where y = n × m.
For polymerization of Py, 0.1 g of Fe3O4 was added into 30-mL deionized water in a beaker and ultrasonicated for 10 min to dispersed nanoparticles of Fe3O4 homogenously into double-distilled deionized water to achieved constant size of PPy/Fe3O4. A 3 g of oxidant, FeCl3, was supplemented into the deionized water having dispersed Fe3O4 and was agitated for 10 min on shaker and followed by addition of 0.5 mL of freshly distilled Py. The beaker was kept under continuous shaking for 2.5 h at 23 ± 2 °C ambient room temperature. Finally, after 2.5 h, into the reaction mixture, the acetone was added to stop the polymerization reaction. A black color powdered product was acquired by filtration process and washed with deionized distilled water till the filtrate turn out to be colorless and finally washed twice with analytical grade acetone. The obtained PPy/Fe3O4 nanoparticles were vacuumed dried at 80 °C for 5 h, and analyzed its weight gravimetrically, which was found as 0.51 g. The weight ratio of Fe3O4 and PPy was obtained as 1:4. The magnetic property of the PPy/Fe3O4 nanoparticle was analyzed before the recovery of cyanotoxins and after the recovery of PPy/Fe3O4 from cyanotoxins using vibrating sample magnetometer (VSM).
The Brunauer, Emmett, and Teller (BET) surface area of the PPy/Fe3O4 nanoparticle was determined by the low-temperature N2 adsorption–desorption technique using Micrometrics. SEM analysis of PPy/Fe3O4 nanoparticles was carried out on a field emission JEOL-6500 F scanning electron microscope at 200 kV. The structure of PPy/Fe3O4 nanoparticle was characterized using a JEOL-2010 transmission electron microscope (TEM) at 200 kV. The X-ray photoelectron spectroscopy (XPS) measurement was taken on a Kratos Axis Ultra DLD with monochromatic Al Kα X-ray source (1486.6 eV of photons) to determine the N atoms present in the PPy coating and their oxidation states.
Zeta potential
The zeta (ζ) potential of PPy/Fe3O4 nanoparticle was analyzed by Zeta Potential Analyzer (Beckman Coulter Inc.) at 25 °C. All samples were measured three times, and presented results are mean values.
Batch adsorption studies
Batch studies were carried out at 200 rpm on temperature-controlled shaker with a fixed adsorbent dosage of 10 mg/L using 100 mL of cyanotoxin solution. MCs and CYN were analyzed at different time intervals (0–30 min). Isotherm studies were conducted at different initial concentrations of cyanotoxins (50–200 μg/L) in a series of 100-mL Erlenmeyer flasks, and the pH of the water were adjusted at pH 7.0 (for MC-LR, YR, and LA) and pH 9.0 (for CYN and MC-RR). The studies of cyanotoxin adsorption onto PPy/Fe3O4 were carried out in the temperature range of 303–323 K to determine the equilibrium adsorption isotherms of each targeted cyanotoxins separately. The equilibrium adsorption capacities were calculated using Eq. (1) as shown below
where q e (μg/mg) was the equilibrium adsorption capacity. C 0 and C e were the initial and equilibrium concentrations (μg/L) of cyanotoxins in their respective solution. V (L) was the volume and M (mg) was the weight of adsorbent.
Desorption studies
The PPy/Fe3O4 nanoparticles were regenerated by treating exhausted adsorbent with 2 M HCl or NaOH ranging from pH 1 to 12 for 20 min. After recovery, the abilities of the regenerated PPy/Fe3O4 nanoparticles were re-tested for cyanotoxin removal using similar procedure as described in “Batch adsorption studies” section.
Results and discussions
Characteristics of the PPy/Fe3O4 nanoparticle
In the present study, the sizes of the spherical particles of Fe3O4 and PPy/Fe3O4 nanoparticles were reported as 10 and 50–100 nm, respectively. The TEM images (Supplementary Fig. 1a) of two different magnifications suggest that the precipitating PPy moieties encapsulate the suspended Fe3O4 nanoparticles, where Fe3O4 nanoparticles were used as scaffold for PPy layers (Bhaumik et al. 2011b). The dark central core and light-colored outer layer were due to the different electron penetrabilities (Supplementary Fig. 1b) in Fe3O4 and PPy layers, respectively. These features indicate that the nanodimensional Fe3O4 particles were embedded in the PPy matrix, forming a core–shell magnetic structure. The SEM image (Supplementary Fig. 1c) reveals the spherical shape of PPy/Fe3O4 nanoparticle. The BET surface area of the PPy/Fe3O4 magnetic nanoparticle was determined as 13,169.27 m2/g using low-temperature N2 adsorption–desorption method.
The magnetic property of the PPy/Fe3O4 particle was explored using vibrating sample magnetometer at the temperature of 300 K (Supplementary Fig. 2a). The magnetic hysteresis loops (magnetization (M) versus applied field (H) curves) was obtained with the saturation magnetization (M s ) value 70 emu g−1; however, the value was slightly smaller than that of bulk magnetite (Fe3O4; 82 emu g−1) discussed by Dunlop and Ozdemir (1997). The value of M s and exhibiting hysteresis loop while subjected to cyclic magnetic field confirmed the ferrimagnetism behavior of PPy/Fe3O4. Supplementary Fig. 2b confirmed that the values of saturation magnetization (M s ), magnetic remanence (M r ), and coercivity (H c ) of the recovered PPy/Fe3O4 nanoparticles do not change (Supplementary Fig. 2c), indicating the persisted magnetic property of the PPy/Fe3O4 nanoparticles even after the adsorption-desorption process of cyanotoxins from water.
With reference of XPS spectrum in Supplementary Fig. 3, it was observed that 39.5 % of positively charged nitrogen atoms (N+) contributed the polypyrrole structure while rest of the nitrogen was amine (−NH–); however, no signal for imine was observed.
Effect of initial concentration of adsorbates and contact time
The trends of adsorption of cyanotoxins onto PPy/Fe3O4 nanoparticles with different initial concentration (50–200 μg/L) are shown in Fig. 1. The adsorption of all cyanotoxins onto PPy/Fe3O4 nanoparticles increased with time and quickly attained equilibrium state in between 8 and 15 min. The rate of equilibrium attained was found in order of MC-LA < LR = YR < CYN < RR with respect of time, where MC-LA attained its equilibrium at 8 min while MC-RR attained at 15 min. Figure 1 also reveals that the amount of cyanotoxin adsorption also depends upon the concentration of adsorbates present initially in water samples, which increased with increase of initial adsorbate concentration. Additionally, the time curves show that the removals of cyanotoxins are quite rapid till 5 and 6 min but slowly lower down till acquire the equilibrium. At low concentrations of the adsorbates, the ratios of the adsorption sites to the initial toxins are adequate which is concluded as high removal efficiency of adsorbent. Nevertheless, at higher concentrations, the available surface area becomes less compare to the moles of adsorbates present (Sathishkumar et al. 2008; Hena 2010); consequently, the amount adsorb increases with increase in initial concentration from 50 to 200 mg/L, with final different values of toxin removal in different time periods, whereas the removal efficiency decreases as mentioned in Table 1. The removal efficiencies were calculated by using Eq. (2) as below, where C i and C e were the initial and equilibrium concentrations of adsorbates (μg/mg), respectively, in the solution.
Zeta (ζ) potential of PPy/Fe3O4 and effect of pH
The pH changes the surface properties of the adsorbent as well as the ionic forms of cyanotoxins, and thus, it influences the adsorption capacity of PPy/Fe3O4 nanoparticles. The ionic forms of cyanotoxins at pH 7 are list down in Table 2. In order to further understand the impact of pH on PPy/Fe3O4 nanoparticles and its interactions with cyanotoxins, zeta potential measurements of the PPy/Fe3O4 nanoparticles were carried out (Fig. 2). The zeta potentials of the PPy/Fe3O4 nanoparticles maintain predominantly positive charge over a wide pH range (2–10) with an isoelectric point at pH 10.4 (Fig. 2). Maximum adsorptions of MC-YR, LR, and LA were found to occur at pH 7, since they are essentially in anionic form above pH 6, and thus, these cyanotoxins can be removed from the drinking water without compromising the quality of water. However, the adsorption decreases at higher pH 9–11, due to the presence of excess OH− ions competing with MC-YR, LR, and LA, while at lower than pH 4, they possess cationic form which does not favor the adsorption onto positively charged PPy matrix due to electrostatic repulsion.
The maximum adsorptions for MC-RR and CYN were found to occur at pH 9, as shown in Fig. 3. The 99.9 % removal of MC-RR and CYN costs a little compromise in quality of drinking water, since the pH of the water become slightly alkaline. However at pH 7, more than 85 % of both MC-RR and CYN were removed. At higher pH 10.4 and above, lower adsorptions of MC-RR and CYN had happen due to the presence of excess OH− ions competing with MC-RR and CYN, while at low pH 2–6, the reason for lower adsorption is same as stated above.
Effect of temperature
The effects of temperature on removal of cyanotoxins with the initial concentration of 50 μg/L using PPy/Fe3O4 were studied. The adsorption capacity of cyanotoxin MC-LA, LR, and YR onto PPy matrix increased with rise in temperature of the system from 293 to 323 K at pH 7. Generally, adsorption is an exothermic process because the adsorption of a relatively hydrophobic MC-LA, LR, and YR by a hydrophobic adsorbent PPy/Fe3O4 nanoparticle from an aqueous solution should be exothermic, but in present study, it is observed that the adsorption process is endothermic in nature. The enhancement in the adsorption capacity with increase of temperature may be due to the heat liberated during adsorption of cyanotoxins that were insufficient to compensate for the heat required to displace the solvent (aqueous) (Pendleton et al. 2001). For the adsorption of cyanotoxins at initial concentration of 50 μg/L, the thermodynamic parameters such as changes in entropy (ΔS°), enthalpy (ΔH°), and standard Gibbs free energy (ΔG°) were determined under the influence of temperature range of 293–323 K by using Eqs. (3) and (4), at pH 7 for MC-LA, LR, and YR and pH 9 for MC-RR and CYN by the PPy/Fe3O4 (Fig. 4).
where R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature, and K C is the equilibrium constant.
ΔS° and ΔH° were calculated from the intercept and slope of the graph plotted ln K C versus 1/T as shown in Fig. 5. The adsorption processes were found endothermic in nature for all the cyanotoxins since the ΔH° possessed positive values, while ΔG° increased in its negative magnitude with increase in temperature for every cyanotoxins. The negative magnitudes of ΔG° revealed the viability and spontaneous nature of the adsorption process (Table 3), while higher values of ln K C refer as adsorption affinity; the affinities were in the sequence of MC-RR > CYN > MC-YR > MC-LR > MC-LA at 323 K.
The adsorption capacity of cyanotoxin mixture (CTM) onto PPy matrix also reflected the same trend, and it increased with rise in temperature of the system from 293 to 323 K for the initial concentration of total 50 μg/L (mixture of 10 μg/L each of MC-LA, LR, YR, RR, and CYN) at pH 7. The most remarkable finding was that at 323 K, the percent removal of MC-RR and CYN significantly increased from 85.63 and 86.21 % to 92.12 and 94.89 % (Fig. 6), respectively, at pH 7. The adsorption capacity of MC-RR and CYN increased with temperature due to containing less hydrophobicity than others, which might need extra heat energy to displace aqueous molecules. The cyanotoxin removals at pH 8 and 9 for 323 K were analyzed, and removals were found as high as 99 %, since the main aim of this study was simultaneous removal of cyanotoxins at neutral pH from drinking water; thus, the data have not mentioned here for comparisons.
Adsorption kinetics
The adsorption kinetic explains the rate of adsorption of uptake of cyanotoxins onto the PPy matrix which controls the equilibrium time. In order to determine the adsorption kinetics of cyanotoxins, the pseudo-first-order and pseudo-second-order models were analyzed to fit the kinetic data.
Pseudo-first-order model
The pseudo-first-order rate model of Lagergren (1898) is based on solid capacity and as described by Eq. (5).
where k 1 (min−1) is the first-order rate constant, q t (μg/mg) represents residual concentration of solute at time t in solution, and q e (μg/mg) is the equilibrium concentration of cyanotoxins present in solutions. In Table 4, the low values of correlation coefficient R 2 show that the adsorption of none of the studied cyanotoxins onto PPy/Fe3O4 follows first-order kinetics.
Pseudo-second-order model
The kinetic data were further analyzed using the pseudo-second-order model, which can be expressed as Eq. (6)
where k 2 (mg/μg min) is the second-order rate constant and q t is the amount adsorbed at time t. From Table 4, the values (R 2 ≈ 0.989–0.998) of the correlation coefficients R 2 of the pseudo-second-order model gave better description of the cyanotoxin adsorption compared to pseudo-first-order model (R 2 ≈ 0.802–0.942).
The pseudo-second-order model has been successfully applied to the adsorption of pollutants from aqueous solutions mainly, where chemisorptions occur involving valency forces through sharing or the exchange of electrons between adsorbent and adsorbate (Ho 2006); in present study, the chloride ions of PPy were replaced by the negative charge carrying cyanotoxins. From Table 4, high-correlation values and proximity of data of adsorption capacities with experimental values reflected better fit for pseudo-second-order model in adsorption of cyanotoxins onto PPy/Fe3O4 from aqueous media.
Intraparticle diffusion model
The rate of adsorbate diffusion within the adsorbent pores controlled the process of intraparticle diffusion. The intraparticle diffusion changes with square root of time given by Weber and Morris (1963) as Eq. (7)
q t (μg/mg) is the adsorbed amount onto the PPy/Fe3O4 at a time t (min), whereas k id (μg/mg min0.5) is the rate constant of intraparticle diffusion, which was evaluated from the slope of the linear plot of q t versus t 1/2. If the regression of q t versus t 1/2 is linear and passes through the origin, then intraparticle diffusion is the sole rate-limiting step. In present study, the regressions were linear for all the adsorbates and the plot did not pass through the origin (Fig. 7), thereby suggesting that intraparticle diffusions were related to the adsorption but not as a sole rate-controlling step. The rate constants of intraparticle diffusion at varying temperatures (303–323 K) are shown in Table 5. In order to understand the mechanism of adsorption of cyanotoxins onto PPy/Fe3O4, it has been divided into three stages. The first stage was called as an instantaneous adsorption, which happened because of electrostatic bond between cyanotoxins and the positive charged nitrogen (N+) present on the surface of PPy/Fe3O4. The second stage was recognized as intraparticle diffusion of cyanotoxins within the PPy matrix through the pores present on the surface of conducting polymer which would have been formed during the processes of polymerization. The intraparticle diffusion process is considered as a gradual adsorption stage, since it might need adsorbates to change their molecular orientation depending upon the size of the pores, as discussed by Pendleton et al. (2001). The final stage corresponds to the equilibrium adsorption when adsorbates occupy all active or possible sites of the adsorbent. Among all the studied, cyanotoxin MC-RR, YR, and CYN have shown highest R 2 values at temperature 313 K, which demonstrated that high temperature favors intraparticle diffusion in PPy/Fe3O4 adsorbent.
Adsorption isotherms
To quantify the adsorption capacity of PPy/Fe3O4 for the removal of cyanotoxins from drinking water, the Langmuir and Freundlich isotherm models were used at various temperatures from 303 to 323 K. The cyanotoxins MC-LA, LR, and YR were analyzed at pH 7, while MC-RR and CYN at pH 9; however, MC-RR and CYN were analyzed at pH 7 also as for comparison since the prime aim of this study was to remove cyanotoxins from drinking water without alteration in pH.
Langmuir model
The data of the equilibrium studies for adsorption of cyanotoxins onto PPy/Fe3O4 particles follow the following form of Langmuir model (Eq. (8)).
where C e was the equilibrium concentration (μg/L) of cyanotoxin in water and Q e (μg/mg) was the amount adsorbed onto the surface of adsorbent at equilibrium. The value of b (L/μg) relates the heat of adsorption, while the Langmuir constants Q 0 (μg/mg) represent the adsorption capacity of monolayer adsorbent. The isotherm data Q 0 and b were calculated and reported in Table 6. It was observed that the uptake of cyanotoxins increased onto PPy/Fe3O4 with rise in temperature. High temperature increases the thermal energy of the adsorbing species which favors to higher adsorption capacity. However, the increases in Q 0 with increase in temperature were highest for MC-RR and CYN, in comparison to m-YR, LR, and LA, because heat required displacing the solvent (aqueous) are higher since they are less hydrophobic (Table 2). The high values of R 2 (0.9991–0.9999) shown in Table 6 advocate better a concurrence between the parameters of adsorption isotherm and confirmed the energetically equivalent units of monolayer adsorption of cyanotoxins onto Fe3O4 coated with polypyrrole surface.
Freundlich model
Freundlich model hints towards the conclusion that the adsorbent deals with the multilayer adsorption process due to the presence of energetically heterogeneous adsorption sites which is shown in linear form as in Eq. (9).
where C e is the equilibrium concentration of cyanotoxins in water sample (μg/L) and the amount of toxins adsorbed on the matrix of conducting polymer (μg/mg), K f is the Freundlich equilibrium constant, and b f is an experimental parameter which describe the intensity of adsorption related with heterogeneity of the adsorbent surface. The adsorption is considered as favorable if the value of b f lies in between 0.1 and 1. The related experimental parameters were determined and listed in Table 6. The closer the values of b f towards 1, the better the favorability of adsorption is.
Sips model
The Sips model is combination of the Langmuir and Freundlich isotherm models that can be used to represent equilibrium adsorption data. The Sips model takes the following form for representing adsorbate equilibrium data as shown in Eq. (10):
where C e (μg/L) is the equilibrium concentration of cyanotoxins in water sample and Q e (μg/mg) is the amount of toxins adsorbed on the matrix of conducting polymer, where Q 0 (μg/mg) and n are the Sips constant characteristics of the system, indicating the maximum cyanotoxin uptake and adsorption intensity, respectively. The Sips model provided slightly lower values of Q 0 for all the cyanotoxins studied than those obtained with the Langmuir model. However, the value of R 2 in Sips model that varies in between 0.9891 and 0.9019 as shown in Table 6 indicates inferior accord between the parameters of adsorption isotherm, comparing to Langmuir model.
In Table 6, the data revealed that the Langmuir adsorption isotherm model yielded the best fit as indicated by the highest R 2 values compared to the other models for all cyanotoxins, showing that the adsorptions of them onto the PPy surface were homogeneous and monolayer in nature. Previous researches (Pendleton et al. 2001; Sathishkumar et al. 2010) also reported that Langmuir isotherm model is suitable for cyanotoxin evaluation.
From Table 6, it was evident that the Langmuir monolayer adsorption capacity increased by 6.71, 6.66, 5.33, 50.74, and 26.86 %, for MC-LA, LR, YR, RR, and CYN, respectively, with an increase in temperature from 303 to 323 K. The effects of temperature changes were highest in case of MC-RR and followed by CYN, while MC-LA, LR, and YR showed almost equal and low percent of increase in adsorption capacity. The Sips model produced the second best fitting isotherm parameter values for all the cases studied and low error values. The magnitude of b that increased with temperature confirmed the endothermic nature of adsorption, while increase in Freundlich constant b f with temperature shows the favorability of cyanotoxin adsorption onto PPy surface at higher temperatures. Nevertheless, the comparative studies of adsorption isotherms for MC-RR and CYN confirmed that the adsorption capacities decreased at pH 7 comparing with the values obtained at pH 9 almost by 11and 7.8 % at 303 K, 5.5 and 5.2 % at 313 K, and 5 and 4.7 % at 323 K, respectively.
Possible adsorption mechanism
Below pH 10.5, the zeta potentials of PPy/Fe3O4 were positive, and over the range of pH 4 to 10, it remained relatively constant (approximately 37.4 mV). All targeted cyanotoxins possessed net negative charge except MC-RR and CYN up to pH 8.5, whereas MC-RR and CYN possessed negative charge at and above pH 9. At pH 7, the positive sites on PPy surface and net negative charges of MC-LA, LR, and YR and their high hydrophobicity favor strong electrostatic attraction between adsorbent and adsorbates, without any alteration in quality of drinking water. However, comparatively low hydrophobicity of MC-RR and CYN have shown better adsorption at higher temperature; this might had happen due to the utilization of heat energy in displacement of solvent molecules (Pendleton et al. 2001). Thus, it can be concluded that adsorption of cyanotoxins onto Fe3O4-coated PPy layer govern by two major factors: (i) electrostatic force of attraction between pair ions and (ii) solubility of adsorbates. Highly soluble adsorbate showed less adsorption onto adsorbent, which can be increased by increase in temperature, since high temperature favors adsorption as well as intraparticle diffusion.
Desorption studies
The ability of regeneration of used PPy/Fe3O4 nanoparticle material after removal of cyanotoxins is an important factor to evaluate the cost-effectiveness and environmental friendliness of an adsorbent. The results of cyanotoxin desorption from PPy/Fe3O4 nanoparticle are compiled in Fig. 8; shown pH 2 is the best condition to desorb almost 99 % of all the adsorbed cyanotoxins. Since the percent desorption at pH 1 was same as pH 2, thus to avoid the extreme condition, pH 2 was selected as the most favorable pH for desorption process. The percent desorption decreased with the increase of pH for MC-LR, LA, and YR and reached to their least desorption values at pH 7, which again start to increase with increase of pH. Up to pH 3, MC-LR, LA, and YR show net positive charge which obviously caused repulsion with dominantly positively charged adsorbent and lead to desorption. Minimum desorption occurred in between pH 6 and 8, where MC-LR, LA, and YR showed dominantly net negative charges. The same trend has been reported with MC-RR and CYN, where these two cyanotoxins reached to their least desorption values at pH 10, which again started to increase with increase of pH. Increments in percent desorption of cyanotoxins MC-RR and CYN had happened because of acquiring negative zeta potential on PPy particles above pH 10.8. The adsorption capacities of PPy/Fe3O4 nanoparticles decreased from 6.6 to 8.9 % depending upon cyanotoxins, after using five times as shown in Table 7. Reductions in adsorption capacities were reported as 30–40 % after using the same PPy/Fe3O4 nanoparticles for eight times. Even after using it for eight times, the adsorption capacities were found better than many reported adsorbent as listed in Table 8. Therefore, the proposed adsorbent can be successfully reused for multiple adsorption-desorption cycles with gradual loss of its original adsorption capacity. The quality of PPy/Fe3O4 nanoparticles deteriorated or efficiency of adsorbent decreased little faster with MC-LA, representing almost 40 % loss in efficiency after eight cycles. The efficiency losses of PPy/Fe3O4 nanoparticles were in the sequence of MC-LA > LR > YR > RR ≈ CYN.
Comparison of Fe3O4-coated PPy with other adsorbents
In order to prove the superiority of the proposed adsorbent over others, the comparison has been done as shown in Table 8, on the basis of adsorption capacity, time required to achieve equilibrium, and alteration in quality of water. It can be observed that at low pH, the adsorptions of cyanotoxins were high regardless the type of toxins and adsorbents. This is accepted with the fact that most of the adsorbents used for the removal of cyanotoxins were activated carbons from different origin. However, the activated carbons are good adsorbents for the removal of cyanotoxins since they contain negative charges, and to exploit the maximum advantages of activated carbon for removal of cyanotoxins, the pH of the water had been kept as acidic to acquire positive net charge on cyanotoxins. This leads to compromise the quality of drinking water and added extra cost to neutralize it before supply. It was also noticed that generally, removal at neutral pH or close range of neutral pH, either the time of adsorption equilibrium were high (300 min to 7 days) or the adsorption capacities were low. However, here, the proposed adsorbent Fe3O4 coated with polypyrrole was found to have a relatively large adsorption capacity, which proved to be a promising material for the removal of cyanotoxins from drinking water safely without altering the pH of water.
Conclusion
Magnetic nanoparticle coated with electro-conducting polypyrrole comprises a remarkable potentiality to remove cyanotoxins from water. PPy/Fe3O4 nanoparticle provides a cost-effective and environmental friendly process for removal of cyanotoxins. High adsorption capacity and short time requirement for equilibrium establishment are significant advantages of PPy/Fe3O4 nanoparticle over many other frequently used adsorbents. The cyanotoxin adsorption equilibrium data fitted very well to Langmuir adsorption isotherm model, and the mechanism was considered to be mainly due to the electrostatic attraction between the nanoparticles and cyanotoxins. The adsorption capacities that increased with rise in temperature revealed that the adsorptions were endothermic, which were also confirmed by the evaluated thermodynamic parameters. The ability of regeneration of PPy/Fe3O4 nanoparticle material after removal of cyanotoxins confirmed the reusability of the nanoparticles for next cycle of cyanotoxin adsorption.
References
Ansari R, Fahim NK (2007) Application of polypyrrole coated on wood sawdust for removal of Cr(VI) ion from aqueous solutions. React Funct Polym 67:367–374
Ansari R, Fahim NK, Dellavar AF (2009) Removal of thiocyanate ions from aqueous solutions using polypyrrole and polyaniline conducting electroactive polymers. J Iran Chem Res 2:163–171
Bhaumik M, Maity A, Srinivasu VV, Onyango MS (2011a) Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J Hazard Mater 190:381–390
Bhaumik M, Leswifi TY, Maity A, Srinivasu VV, Onyango MS (2011b) Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic nanocomposite. J Hazard Mater 186:150–159
Cook D, Newcombe G (2008) Comparison and modeling of the adsorption of two microcystin analogues onto powdered activated carbon. Environ Technol 29:525–534
de Albuquerque Júnior EC, Méndez MOA, dos Reis Coutinho A, Franco TT (2008) Removal of cyanobacteria toxins from drinking water by adsorption on activated carbon fibers. Mater Res 11:371–380
Deng Y, Qi D, Deng C, Zhang X, Zhao D (2008) Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J Am Chem Soc 130:28–29
Dixon MB, Falconet C, Ho L, Chow CWK, O’Neill BK, Newcombe G (2010) Nanofiltration for the removal of algal metabolites and the effects of fouling. Water Sci Technol 61:1189–1199
Dixon MB, Falconet C, Ho L, Chow CWK, O’Neill BK, Newcombe G (2011) Removal of cyanobacterial metabolites by nanofiltration from two treated waters. J Hazard Mater 188:288–295
Donati C, Drikas M, Hayes R, Newcombe G (1994) Microcystin-LR adsorption by powdered activated carbon. Water Res 28:1735–1742
Dunlop DJ, Ozdemir O (1997) Rock magnetism: fundamentals and frontiers. Cambridge University Press, Cambridge
Eisazadeh H (2008) Removal of arsenic in water using polypyrrole and its composites. World Appl Sci J 3:10–13
El-Sheikh SM, Zhang G, El-Hosainy HM, Ismail AA, O’Shea KE, Falaras P, Kontos AG, Dionysiou DD (2014) High performance sulfur, nitrogen and carbon doped mesoporous anatase-brookite TiO2 photocatalyst for the removal of microcystin-LR under visible light irradiation. J Hazard Mater 280:723–733
Falconer IR (1989) Effects on human health of some toxic cyanobacteria (blue-green algae) in reservoirs, lakes, and rivers. Toxicity Assess 4:175–184
Falconer IR (2005) Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. CRC Press, Boca Raton
Fotiou T, Triantis T, Kaloudis T, Hiskia A (2015) Photocatalytic degradation of cylindrospermopsin under UV-A, solar and visible light using TiO2. Mineralization and intermediate products. Chemosphere 119:S89–S94
Gurbuz F, Codd GA (2008) Microcystin removal by a naturally-occurring substance: pumice. Bull Environ Contam Toxicol 81:323–327
He X, de la Cruz AA, O’Shea KE, Dionysiou DD (2014) Kinetics and mechanisms of cylindrospermopsin destruction by sulfate radical-based advanced oxidation processes. Water Res 63:168–178
Hena S (2010) Removal of chromium hexavalent ion from aqueous solutions using biopolymer chitosan coated with poly 3-methyl thiophene polymer. J Hazard Mater 181:474–479
Hena S, Ismail N, Isaam AM, Ahmad A, Bhawani SA (2014) Removal of microcystin-LR from aqueous solutions using % burn-off activated carbon of waste wood material. J Water Supply Res Technol AQUA 63:332–341
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ho L, Slyman N, Kaeding U, Newcombe G (2008) Optimizing PAC and chlorination practices for cylindrospermopsin removal. J Am Water Works Assoc 100:88–96
Ho L, Lambling P, Bustamante H, Duker P, Newcombe G (2011) Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Water Res 45:2954–2964
Huang WJ, Cheng BL, Cheng YL (2007) Adsorption of microcystin-LR by three types of activated carbon. J Hazard Mater 141:115–122
Jones GJ, Orr PT (1994) Release and degradation of microcystin following algicide treatment of a Microcystis aeruginisa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res 28:871–876
Lagergren S (1898) About the theory of so called adsorption of soluble substances, Kungliga Svenska Vetenskapsakademiens. Handlingar Band 24(04):1–39
Mohamed ZA, Carmichael WW, El-Sharouny J, An HM (1999) Activated carbon: removal efficiency of microcystins in an aqueous cell extract of Microcystis aeruginosa and Oscillatoria tenuis strains isolated from Egyptian freshwaters. Environ Toxicol 14:197–201
Pavagadhi S, Tang ALL, Sathishkumar M, Loh KP, Balasubramanian R (2013) Removal of microcystin-LR and microcystin-RR by graphene oxide: adsorption and kinetic experiments. Water Res 47:4621–4629
Pearl HW, Huisman J (2008) Climate: blooms like it hot. Science 320:57–58
Pendleton P, Schumann R, Wong SH (2001) Microcystin-LR adsorption by activated carbon. J Colloid Interface Sci 240:1–8
Sathishkumar M, Binupriya AR, Swaminathan K, Choi JG, Yun SE (2008) Arsenite sorption in liquid phase by Aspergillus fumigates: adsorption rates and isotherm studies. J Microbiol Biotechnol 24:1813–1820
Sathishkumar M, Pavagadhi S, Mahadevan A, Balasubramanian R (2010) Removal of a potent cyanobacterial hepatotoxin by peat. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng 45:1877–1884
Wang H, Ho L, Lewis DM, Brookes JD, Newcombe G (2007) Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res 41:4262–4270
Wang F, Wu Y, Gao Y, Chen Z (2014) Biodegradation of microcystin-LR by Burkholderia Vietnamiensis. Chin J Environ Eng 8:3837–3842
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Yan H, Gong A, He H, Zhou J, Wei Y, Lv L (2006) Adsorption of microcystins by carbon nanotubes. Chemosphere 62:42–148
Zhang X, Bai R (2003) Surface electric properties of polypyrrole in aqueous solutions. Langmuir 19:10703–10709
Zhang G, Zhang YC, Nadagouda M, Han C, O’Shea K, El-Sheikh SM, Ismail AA, Dionysiou DD (2014) Visible light-sensitized S, N and C co-doped polymorphic TiO2 for photocatalytic destruction of microcystin-LR. Appl Catal B Environ 144:614–621
Acknowledgment
This research was financially supported by Malaysian Department of Higher Education through disbursement of Fundamental Research Grant Scheme 203/PTEKIND/6711465.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOCX 133 kb)
Supplementary Fig. 2
(DOCX 52 kb)
Supplementary Fig. 3
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Hena, S., Rozi, R., Tabassum, S. et al. Simultaneous removal of potent cyanotoxins from water using magnetophoretic nanoparticle of polypyrrole: adsorption kinetic and isotherm study. Environ Sci Pollut Res 23, 14868–14880 (2016). https://doi.org/10.1007/s11356-016-6540-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6540-5