Abstract
Lead (Pb) intoxication is a worldwide health problem which frequently affects the liver. This study was carried out to investigate the potential protective effect of thymoquinone (TQ), the major active ingredient of volatile oil of Nigella sativa seeds, against Pb-induced liver damage. Adult male rats were randomized into four groups: Control group received no treatment, Pb group was exposed to 2000 ppm Pb acetate in drinking water, Pb-TQ group was cotreated with Pb plus TQ (5 mg/kg/day, per orally), and TQ group receiving only TQ. All treatments were applied for 5 weeks. Results indicated that Pb exposure increased hepatic Pb content, damaged hepatic histological structure (necrotic foci, hepatic strands disorganization, hypertrophied hepatocytes, cytoplasmic vacuolization, cytoplasmic loss, chromatin condensation, mononuclear cell infiltration, congestion, centrilobular swelling), and changed liver function investigated by plasma biochemical parameters (AST, ALT, ALP, γ-GT, LDH). Pb treatment also decreased total antioxidant status level and increased lipid peroxidation in the liver. Supplementation with TQ remarkably improved the Pb-induced adverse effects without significantly reducing the metal accumulation in the liver. In conclusion, our results indicate, for the first time, a protective effect of TQ against Pb-induced hepatotoxicity and suggest that this component might be clinically useful in Pb intoxication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is one of the well-known ubiquitous nonessential metal poisons that occur naturally in the environment. The levels of Pb in the environment are constantly increasing due to industrial activities. Pb is present in plastic, paints, ceramics, glass, water pipes, insecticides, and leaded gasoline. Pb poisoning has been reported since the discovery of Pb thousands of years ago, and it remains a major health problem issue worldwide (Karrari et al. 2012). The manifestations of Pb poisoning in humans are nonspecific. They may include neurotoxicity (Sharma et al. 2015), hepatotoxicity (Soliman et al. 2015), and nephropathy (Ng et al. 2013). Air, water, soil, food, and consumer products are the major routes of human exposure to Pb. It is considered to be an abnormal trace element in humans and animals and tends to accumulate in the body with the passage of time (Schroeder and Balassa 1961). A safe level of Pb exposure has not been defined, and today, it is widely accepted that even small quantities are harmful to humans and other organisms.
Absorbed Pb is conjugated in the liver and passed to the kidney, where a small quantity is excreted in urine and the rest accumulates in various body organs. Autopsy studies of Pb-exposed humans indicated that liver tissue is the largest repository (33 %) of Pb among soft tissues followed by kidney (Mudipalli 2007). The accumulated Pb affects many biological activities at the molecular, cellular, and intercellular levels, which may result in morphological alterations that can remain even after Pb levels have fallen (Flora et al. 2006). Pb-induced hepatotoxicity was reported to be associated with the impairments of liver structure and function (Aziz 2012).
Pb-induced oxidative stress or disruption of prooxidant/antioxidant balance in blood and other soft tissues has been postulated to be the major mechanism of Pb-associated tissue injury (Flora et al. 2012). Bolin et al. (2006) reported that Pb-exposed mammals showed not only generation of reactive oxygen species (ROS), a highly reactive oxidizing agents such as superoxide anion radical (O2 ·−), hydroxyl radical (OH·), and hydrogen peroxide (H2O2), but also inhibition of antioxidant defense system and stimulation of lipid peroxidation (LPO), a deleterious process only achieved by free radicals. Accumulated Pb in liver not only inhibited the activities of antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), glutathione reductase (GR), and glutathione S-transferase (GST) but also depleted the nonantioxidant defense system, such as reduced glutathione (GSH), which will increase the susceptibility of hepatic tissue to free radical-induced oxidative damage (Adikwu et al. 2013).
It is essential to find an appropriate approach to prevent Pb toxicity. The current approved treatment for Pb poisoning is to administer chelating agents (thiol chelators and other complex ions) that form an insoluble complex with the Pb and remove it from tissue, but these chelating agents have no effect on low levels of exposure and are incapable of removing metal from intracellular sites, as well as they cause many side effects, such as redistribution of the toxic metal, essential metal loss, and liver or renal dysfunction (Flora and Pachauri 2010). The fact that Pb exposure induces an excessive increase of ROS suggests that antioxidants could be used as an alternative therapy (Wang et al. 2006).
Medicinal plants nowadays are an important source of drug synthesis, and at least a third of current drugs are derived from plants (Bent 2008). Thymoquinone (TQ) (2-isopropyl-5-methyl-1,4-benzoquinone), the main active component of the essential oil of Nigella sativa seeds, has various pharmacological effects such as anti-diabetic (Abdelmeguid et al. 2010), anti-cancer (Woo et al. 2012), anti-inflammatory (Taka et al. 2014), and analgesic properties (Çelik et al. 2014). TQ is reported to possess strong antioxidant properties (Rifaioglu et al. 2013). TQ supplementation considerably protected several organs against oxidative damage induced by a variety of free radical generating agents including ethanol-induced gastric mucosal injury (Kanter et al. 2006), nephropathy produced by gentamicin (Yaman and Balikci 2010), aflatoxin B1 evoked hepatotoxicity (Nili-Ahmadabadi et al. 2011), and testicular injury induced by arsenic (Fouad et al. 2014). The high potency and low systemic toxicity of TQ make it a promising alternative to conventional therapeutic drugs (Lupidi et al. 2010).
The influence of TQ on Pb-induced liver injury has not been studied until now. Therefore, the present study was designed to investigate, for the first time, the possible beneficial impact of oral supplementation with TQ on Pb-induced hepatotoxicity in rats.
Materials and methods
Chemicals
Pb acetate trihydrate ((C2H3O2)2Pb. 3H2O) and TQ (2-isopropyl-5-methyl-1,4-benzoquinone) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, Missouri, USA). All other chemicals used were of the best analytical grade.
Animals
Healthy adult (4 months old) male Wistar rats, weighing 200–230 g, obtained from the Tunisian Society of Pharmaceutical Industries, were used in this study. The animals were housed in plastic cages (free from any source of chemical contamination) with free access to tap water (free from Pb) and standard diet. The rats were kept at 22 ± 3 °C, in natural light/dark cycle, with 55 % humidity and under ventilation system. Experiments were started after the animals were allowed to adapt to the laboratory conditions for a week. All experimental procedures in this study were in full compliance with The European Council Directive (86/609/EEC) and approved by the institutional bioethics committee.
Experimental design
After an acclimation period, the rats were randomly divided into four groups of eight animals each and were treated for 5 weeks as follows: Control group received tap water, Pb group received an aqueous solution containing 2000 ppm of Pb acetate (0.2 %, w/v) (Çaylak and Halifeoğlu 2007; Çaylak et al. 2007; Sivaprasad et al. 2004), Pb-TQ group was cotreated with Pb (as in Pb group) plus TQ (5 mg/kg body weight/day, gastric gavage) (Alenzi et al. 2010; Alsaif 2007; El-Sayed 2011), and TQ group received tap water and was given TQ (5 mg/kg body weight/day). TQ was administered by gastric tube daily between 8:00 and 9:00 a.m. Throughout the experiment, rats were weighed every five days. At the end of the treatment period, the animals were weighed and then euthanized by exsanguination through cardiac puncture under diethyl ether anesthesia.
Preparation of plasma and tissue collection
The blood samples were collected in heparinized tubes. Plasma was obtained by centrifugation (3000×g, for 15 min and at 4 °C) and stored at −80 °C until analysis. The liver was removed quickly from rats, cleared of the adhesive tissues, washed in ice-cold 0.9 % (w/v) NaCl solution, blotted, weighed, and divided into three parts. One was immediately fixed in 10 % neutral buffered formalin for a week (for the histopathological study), and the two others were frozen at −80 °C until metal estimation and oxidative stress evaluation.
Pb determination
A frozen piece of liver was digested with concentrated nitric acid, and the digests were diluted to a constant volume with ultrapure water. Pb concentrations were then determined from the resulting solutions by atomic absorption spectrophotometry (AAS), and the values were expressed in parts per million (micrograms per gram of wet hepatic tissue).
Histopathological analysis
After proper fixation, the tissue was rinsed with water and dehydrated in ascending grades of alcohols, cleared in toluene, and embedded in paraffin. Tissue blocks were cut into thin sections (5 μm) and stained with hematoxylin and eosin. Then, the histological slides were examined by light microscopy.
Biochemical analysis of liver integrity
Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyltransferase (γ-GT), and lactate dehydrogenase (LDH) activities were measured using the commercially available diagnostic kits supplied by Randox Laboratories (Ardmore, Northern Ireland, UK).
Liver extracts preparation, measurement of total antioxidant status level, and estimation of lipid peroxidation
Liver was homogenized in 10 volumes of ice-cold phosphate-buffered saline (PBS: 136.75 mM NaCl, 2.68 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4, pH = 7.4), and the homogenates were centrifuged at 3500×g for 15 min at 4 °C. The supernatant fractions were collected and used for biochemical analysis.
Total antioxidant status (TAS) level was measured by the commercial kit TAS (Randox laboratories Ltd., Crumlin, UK) based on the method of Miller et al. (1993). Manufacturer’s instructions were followed to determine the level. In this method, ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]) was incubated with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS·+. This product has a relatively stable blue-green color which is measured at 600 nm. The antioxidants in the added liver sample cause suppression of this color production to a degree which is proportional to their concentration. The results were expressed as micromoles per gram of wet liver tissue.
LPO was estimated indirectly by measuring the malondialdehyde (MDA), an end product of LPO. The MDA level was determined spectrophotometrically by using the method of thiobarbituric acid which measures MDA-reactive products (Placer et al. 1966), as described by Todorova et al. (2005). In brief, the liver samples were mixed with 0.9 % (w/v) NaCl and 25 % (w/v) trichloroacetic acid, and centrifuged at 2000×g for 15 min. The supernatant was then mixed with 0.5 % (w/v) thiobarbituric acid and heated in a water bath at 95 °C for 1 h. After cooling, the absorbance of the colored complex formed (MDA-TBA) was measured at 532 nm against an appropriate blank without sample. The concentration of MDA was calculated by using the molar extinction coefficient of thiobarbituric acid reactants (TBARS; 1.56 × 105/mol/cm). LPO was expressed as nanomoles MDA per gram of wet liver tissue.
Statistical analysis
The results were expressed as mean ± SEM. Comparisons between the groups were performed by Student’s t test. Differences were considered statistically significant at p < 0.05.
Results
General rat health
During experiments, death was not observed during the experimental period. Rats treated with Pb and/or TQ exhibited a normal behavior without obvious signs of toxicity, in comparison to the control group.
Body weight gain and relative weight of the liver
No significant differences (p > 0.05) have been observed for the body weight gain among the four groups of animals, during the experiment (Fig. 1). The absence of effect (p > 0.05) was also noted for the relative liver weight (Table 1).
Liver Pb
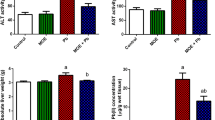
We remarked a similar Pb liver (p > 0.05) level in the control and TQ groups. After 5 weeks of heavy metal treatment, the Pb liver content was significantly increased (p < 0.05) by 1023.52 % compared with the value of control rats. Supplementation with TQ had no important effect (p > 0.05) on liver Pb accumulation (Fig. 2).
Biochemical indicator of liver integrity
Results presented in Table 2 indicated that the administration of TQ alone had no significant effect (p > 0.05) on plasma ALT, AST, LDH, ALP, and γ-GT activities compared to control rats. In contrast, Pb exposure induced a significant elevation (p < 0.05) in these biochemical liver markers in relation to control animals. Liver enzymes plasmatic levels of rats cotreated with Pb and TQ were found to be significantly lower (p < 0.05) compared to those treated with Pb only.
TAS and MDA levels
As shown in Fig. 3, the liver TAS level was comparable (p > 0.05) in control and TQ groups, while following Pb treatment, the overall antioxidative status was significantly decreased (p < 0.05) by 26.51 %. Interestingly, cotreatment with Pb and TQ increased significantly (p < 0.05) the level of this antioxidant parameter by about 25.4 % in comparison with Pb group.
Liver MDA concentration remained unchanged (p > 0.05) in TQ-treated rats, while it significantly increased (p < 0.05) by 119.41 % after metal treatment compared to control group. TQ supplementation perfectly inhibited (p < 0.05) (by 42.11 %) the liver LPO induced by Pb administration. In fact, rats cotreated with Pb and TQ showed similar MDA level than control animals (Fig. 4).
Histopathological changes
Light microscopic examination of the liver of control (Fig. 5a) and TQ (Fig. 5b) groups showed normal hexagonadal or pentagonadal lobules with central veins and peripheral hepatic triads or tetrads embedded in connective tissue. Hepatocytes are arranged in strands running radially from the central vein and are separated by sinusoids containing Kupffer cells. They are regular and contain a large spheroidal nucleus. In contrast, Pb exposure (Fig. 5d–i, and Table 3) induced marked liver injury, among other, by hepatic strands disorganization, zonal necrosis (N A), mononuclear cell infiltrations (L I), centrilobular swelling (C S), and sinusoidal and centrilobular congestions (Cg). The liver parenchyma also showed hepatocytes with cytoplasmic vacuolization (Vac), cytoplasm loss (Ct L), and/or condensed nuclear chromatin (C Chr). In most animals, the hypertrophy of hepatocytes remarkably reduced the sinusoidal diameter. However, coadministration of TQ prevented the degenerative changes induced by Pb in hepatic structure. In fact, in Pb-TQ group (Fig. 5c and Table 3), we noticed only the presence of mild hypertrophy and Ct L in the hepatocytes of some animals.
Impact of thymoquinone (TQ) supplementation on lead (Pb)-induced changes in liver histology. Liver of a control (a, ×400) and TQ (b, ×400)-treated rats, showing normal histological pattern, contained normal hepatocyte architecture with distinct hepatic strands, sinusoids, and centrilobular veins. Liver of Pb-treated rats (d–g, ×400; h, i, ×1000), showing hepatic strands disorganization, sinusoidal and centrilobular congestions, mononuclear cell infiltrations, necrotic foci, centrilobular swelling, hypertrophied (marked by reduction of sinusoidal diameter) and vacuolized hepatocytes, cytoplasm loss, and condensed nuclear chromatin. The liver of Pb and TQ-cotreated rats (c, ×400), showing almost normal histological structure. Sections were stained with hematoxylin and eosin. S sinusoid, C V centrilobular vein, K C Kupffer cell, Hp hepatocyte, H Sr hepatic strands, C S centrilobular swelling, Cg congestion, N A necrotic area, L I leukocyte infiltrations, C Chr condensed chromatin, Vac vacuolization, Ct L cytoplasm loss. The color image is available in the online version of the article
Discussion
Exposure to Pb acetate for 5 weeks was not found to significantly alter the body weight gain when compared with the control group, indicating the absence of overt general toxicity in rats. Similar results were also observed in rats by Anjum et al. (2011) and Ng et al. (2013). In the present study, no significant change in the relative liver weight was observed in Pb-treated animals. Similarly, Pb intoxication during pregnancy and lactation did not show significant effect on absolute and relative liver weights in rat’s pups (Massó-González and Antonio-García 2009). The same result was also found in adult male rats receiving an aqueous solution containing 1500 ppm of Pb acetate for 70 days (Sainath et al. 2011). Alabbassi et al. (2008) have detected a remarkable change in relative liver weight, but for a tissue Pb concentration much higher than that found in our work.
Only one hour after intestinal absorption, Pb accumulates in all the organs of the body with maximum concentration per gram weight of tissue being recorded in liver and kidneys (Gerhardsson et al. 1995; Roggeman et al. 2014), which could be because these two organs are primarily responsible for its excretion (Goyer and Cherian 1979). Another important factor responsible for the privileged hepatic and renal Pb distribution is the existence of high-affinity Pb-binding nonenzyme proteins like calcium-sensing receptor, thymosin β4, acyl-CoA binding protein, and metallothionein in the liver and the kidneys (ATSDR Agency for Toxic Substances and Disease Registry 2005; Handlogten et al. 2000). In agreement with the findings of Dewanjee et al. (2013) and Jovanović et al. (2013), our investigation indicated a significant deposition of Pb in liver of treated rats, which may target structure and function of the organ.
Our results indicated that daily Pb treatment resulted in evident liver structural changes. Previous investigators (Ait Hamadouche et al. 2012; Aziz 2012) have noted similar or more pronounced changes in rat liver tissue under Pb effect.
According to Adikwu et al. (2013) and García-Niño and Pedraza-Chaverrí (2014), Pb affects the liver primarily by oxidative damage especially LPO. Membrane LPO, via a free-radical chain reaction, affects the membrane integrity, permeability, and function, finally leading to necrosis (Porter et al. 1995). The rupture of lysosome membranes, following an oxidative attack, leads to the release of hydrolysis enzymes which will cause cell death (Fong et al. 1973). By modifying the fatty acids composition of plasma and organelles membranes, Pb increases the susceptibility of cells to LPO (Donaldson and Knowles 1993). The reduction in the hepatocyte cytoplasmic content noted in treated rats is most probably the consequence of membrane integrity loss.
Our results showed aggregation of mononuclear inflammatory cells in the liver tissue after Pb treatment. It has been shown that the Pb overproduction of ROS may induce an inflammatory response characterized by the release of pro-inflammatory cytokines (TNF-α, IL-1, IL-6) and the recruitment of circulating immunocompetent cells in injured site (Schreck and Baeuerle, 1991). In addition, tissue damage and the LPO products induced an inflammatory response (Attia et al. 2010).
It is known that Pb, through hydropic degeneration, can lead to massive dilated mitochondria that appear as cytoplasmic vacuoles (Buchheim et al. 1998). Moreover, the hepatocyte vacuolation could be triglyceride inclusions, because Pb was reported capable of disrupting lipid metabolism in rat (Ademuyiwa et al. 2009). The hepatocyte hypertrophy noted in our study could be explained by Pb-induced biochemical changes (Columbano et al. 1983). Cells with condensed chromatin could be at the beginning of apoptotic process (Chattopadhyay and Wahi 2009).
In accordance with previous data (Azoz and Raafat 2012; Ibrahim et al. 2012), our results indicated that Pb exposure produced significant increase in the plasma activity of liver enzymes ALT, AST, LDH, GGT, and PAL, indicating a damaged structural and functional hepatic integrity.
The plasma elevation of liver enzymes level may be due to Pb-induced hepatocellular damage which causes leakage of these enzymes into blood circulation. Enzymatic leakage could be attributable to alteration of hepatocyte membrane permeability after LPO mediated by Pb-overproduced free radicals (Ashry et al. 2010; El-Sokkary et al. 2005). Cell membrane LPO leads to various membrane damage (fluidity loss, potential change, and permeability increase), which all lead to a leakage of liver enzymes (Nehru and Anand 2005). The competition of Pb with calcium (Kaminsky et al. 1993), a mineral that is necessary for cell membrane integrity and stability, may also increase the hepatocyte membrane permeability and thus resulting in release of hepatocyte enzymes into the bloodstream. Necrosis and cytoplasmic loss observed within the liver parenchyma of intoxicated rats, in the present work, may explain at least in part the plasma biochemical changes. Moreover, Pb-induced synthesis increase or a catabolism decrease of liver enzymes cannot be excluded (Nduka 1999).
TAS measurement has been used to evaluate the overall performance of the antioxidant system. In the present work, Pb treatment resulted in a decrease in the liver TAS level. Attri et al. (2003) and Marchlewicz et al. (2007) have also reported a decline in TAS after Pb exposure. It is known that Pb, through inhibition of functional sulfhydryl groups (SH) by irreversible binding, impairs both the enzymatic (mainly SOD, GPX, and CAT) and nonenzymatic (primarily GSH) antioxidant systems in the rat’s liver (Newairy and Abdou 2009; Sivaprasad et al. 2004), thus inducing tissue oxidative stress.
LPO is oxidative degradation of lipid carried out solely by free radicals. In the present investigation, LPO as measured by the amount of MDA was significantly elevated in the liver of Pb-treated rats. Jovanović et al. (2013) and Lakshmi et al. (2013) reported the same finding in Pb-intoxicated rat’s liver. An increased generation of highly ROS was observed in the liver after Pb exposure (Liu et al. 2011, 2012). However, Pb does not readily undergo the redox reaction characteristic of transition metals and directly is not able to enhance the ROS formation, but it stimulates the LPO indirectly through depletion of cell’s antioxidant defense system (Gordon et al. 2002; Patra et al. 2001) as evidenced, in the current study, by the significant decline in the TAS level.
Our results show that Pb may destroy the antioxidant defense system of the liver, which can cause an elevation of oxidative status regarding the overproduction of ROS. At high levels, the ROS might be responsible for cellular injuries and oxidative damages to critical biomolecules, such as membrane lipids, proteins, and nucleic acids (Franco et al. 2009), which resulted in liver injury. LPO occurs readily in the hepatocytes due to the presence of membrane phospholipids rich in polyunsaturated highly oxidizable fatty acids. Peroxidative decomposition of membrane lipids leads to structural and functional liver damages.
Despite extensive research that is now focusing on herbal products as an alternative medicine, no evidence has been reported in the literature regarding the role of TQ against Pb liver toxicity. In the present study, cotreatment of Pb-exposed rats with TQ on one side did not significantly affect the liver Pb burden but, on the other side, improved the histopathological changes, ameliorated the impaired hepatic function, enhanced the reduced TAS, and inhibited the elevated LPO level in the liver.
Our results are consistent with literature data. Oral supplementation of TQ (3 × 15 mg/kg in an 18-h time interval) lowers the liver injury scores, restores the elevated serum ALT and AST activities, and corrects the stimulated hepatic LPO in male rat treated with acetaminophen, one of the most commonly used analgesic and antipyretic drugs (Aycan et al. 2014). Jaswal et al. (2013) assessed the therapeutic effect of TQ (10, 20, and 40 mg/kg/day, 3 days/week, 8 weeks; per orally) against anti-tuberculosis drug-induced liver damage. The TQ supplementation ameliorated the structural damages, normalized the high values of liver function serum biomarkers (ALT, AST, ALP), inhibited the stimulated LPO, and enhanced the reduced antioxidant enzyme activities (SOD, CAT). Also, Abdel-Wahab (2013) reported, under the TQ effect, a significant correction not only of hepatic LPO and antioxidative status (SOD, GPX, CAT, GST, GSH) but also of liver function serum parameters (AST, ALT, ALP, LDH) in sodium fluoride-treated rats.
To our knowledge, TQ has not yet been investigated as a metal chelator, because its chemical structure does not seem to have this property. The beneficial effects of TQ, obtained in the current work, are very likely due to its strong antioxidant properties. The mechanisms of TQ antioxidant action are not yet clear. Nevertheless, TQ is known to reduce oxidative stress not only through direct antioxidant effect but also indirectly. Mansour et al. (2002) and Badary et al. (2003) reported that TQ acts as strong ROS scavenger. Recent studies have demonstrated that TQ supplementation increases the expression of antioxidant genes of SOD, GPX, CAT, and GST in rat liver (Ismail et al. 2010; Sayed-Ahmed et al. 2010). The direct antioxidant effect of TQ may be related to its quinone structure. TQ, as a quinone, is known to undergo one or two electron reduction by cellular reductases (El-Najjar et al. 2010). One electron reduction results in the formation of semiquinones that are converted to ROS when reacting with molecular oxygen (Monks and Jones 2002), while the two electron reductions produce the antioxidant hydroquinones by quinone reductase (Nagi and Almakki 2009). This obligatory two electron reduction competes with the one electron reduction of quinones and protects cells against oxidative stress (Guo et al. 2008). Both TQ and dihydrothymoquinone (DHTQ), a TQ metabolite, were found to be potent O2 ·− scavengers and as general free radical scavengers (Khalife and Lupidi 2007). Interestingly, the in vitro antioxidant protection by DHTQ may be greater than TQ at low concentrations less than 1 μM (Nagi et al. 1999).
Conclusions
Our study indicates that Pb, in its acetate form, damaged histological structure, impaired function, inhibited endogenous antioxidant defense system, and stimulated LPO in the rat liver tissue. Besides, our results demonstrate, for the first time, that TQ oral supplementation, at a safe dose, has remarkable protective effect against Pb-induced hepatoxicity in rats. This protection makes TQ as a promising agent in a variety of conditions where cellular damage is a consequence of oxidative stress. In the present work, TQ supplementation was found to be ineffective in decreasing tissue Pb content; thus, further studies exploring the combined effect of TQ with chelating agent on treating Pb poisoning are needed.
References
Abdelmeguid NE, Fakhoury R, Kamal SM, Al Wafai RJ (2010) Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β-cells of streptozotocin-induced diabetic rats. J Diabetes 2:256–266
Abdel-Wahab WM (2013) Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J Basic Appl Zool 66:263–270
Ademuyiwa O, Agarwal R, Chandra R, Behari JR (2009) Lead-induced phospholipidosis and cholesterogenesis in rat tissues. Chem Biol Interact 179:314–320
Adikwu E, Deo O, Geoffrey OP, Enimeya DA (2013) Lead organ and tissue toxicity: roles of mitigating agents (Part 1). British J Pharmacol Toxicol 4:232–240
Ait Hamadouche N, Slimani M, Aoues A (2012) Beneficial effect administration of vitamin C in amelioration of lead hepatotoxicity. Not Sci Biol 4:7–13
Alabbassi MG, Hussain SA, Ali SH (2008) Therapeutic effects of melatonin in lead-induced toxicity in rats. Iraqi J Pharm Sci 17:47–54
Alenzi FQ, El-Bolkiny Y-S, Salem ML (2010) Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br J Biomed Sci 67:20–28
Alsaif MA (2007) Effect of thymoquinone on ethanol-induced hepatotoxicity in Wistar rats. J Med Sci 7:1164–1170
Anjum MR, Sainath SB, Suneetha Y, Reddy PS (2011) Lead acetate induced reproductive and paternal mediated developmental toxicity in rats. Ecotoxicol Environ Saf 74:793–799
Ashry KM, El-Sayed YS, Khamiss RM, El-Ashmawy IM (2010) Oxidative stress and immunotoxic effects of lead and their amelioration with myrrh (Commiphora molmol) emulsion. Food Chem Toxicol 48:236–241
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxicological profile for lead. Department of Health and Human Services, Public Health Service, Atlanta, Georgia, U.S
Attia A, Ragheb A, Sylwestrowicz T, Shoker A (2010) Attenuation of high cholesterol-induced oxidative stress in rabbit liver by thymoquinone. Eur J Gastroenterol Hepatol 22:826–834
Attri J, Dhawan V, Mahmood S, Pandhi P, Parwana HK, Nath R (2003) Effect of vitamin C supplementation on oxidative DNA damage in an experimental model of lead-induced hypertension. Ann Nutr Metab 47:294–301
Aycan IÖ, Tüfek A, Tokgöz O, Evliyaoğlu O, Fırat U, Kavak GÖ, Turgut H, Yüksel MU (2014) Thymoquinone treatment against acetaminophen-induced hepatotoxicity in rats. Int J Surg 12:213–218
Aziz FM (2012) Protective effects of latex of Ficus carica L. against lead acetate-induced hepatotoxicity in rats. JJBS 5:175–182
Azoz HA, Raafat RM (2012) Effect of lead toxicity on cytogenisity, biochemical constituents and tissue residue with protective role of activated charcoal and casein in male rats. Aust J Basic Appl Sci 6:497–509
Badary OA, Taha RA, AM G e-D, Abdel-Wahab MH (2003) Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 26:87–98
Bent S (2008) Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med 23:854–859
Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, Cardozo-Pelaez F (2006) Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J 20:788–790
Buchheim K, Stoltenburg-Didinger G, Lilienthal H, Winneke G (1998) Myopathy: a possible effect of chronic low level lead exposure. Neurotoxicology 19:539–545
Çaylak E, Halifeoğlu İ (2007) Effects of sulfur-containing antioxidants on malondialdehyde and catalase levels of liver, kidney and brain in lead-exposed rats. Turkiye Klinikleri J Med Sci 27:1–8
Çaylak E, Halifeoğlu İ, Aydin S, Telo S, Bulmuş Ö, Çelik H (2007) The effects of sulfur-containing compounds on total antioxidant capacity levels of liver, kidney and brain in lead-exposed rats. Turkiye Klinikleri J Med Sci 27:823–828
Çelik F, Göçmez C, Karaman H, Kamaşak K, Kaplan İ, Akıl E, Tufek A, Guzel A, Uzar E (2014) Therapeutic effects of thymoquinone in a model of neuropathic pain. Curr Ther Res 76:11–16
Chattopadhyay P, Wahi AK (2009) Hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver transplantation model. Trends Biomater Artif Organs 23:1–5
Columbano A, Ledda GM, Sirigu P, Perra T, Pani P (1983) Liver cell proliferation induced by a single dose of lead nitrate. Am J Pathol 110:83–88
Dewanjee S, Sahu R, Karmakar S, Gangopadhyay M (2013) Toxic effects of lead exposure in Wistar rats: involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves. Food Chem Toxicol 55:78–91
Donaldson WE, Knowles SO (1993) Is lead toxicosis a reflection of altered fatty acid composition of membranes? Comp Biochem Physiol C 104:377–379
El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H (2010) Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 15:183–195
El-Sayed WM (2011) Upregulation of chemoprotective enzymes and glutathione by Nigella sativa (black seed) and thymoquinone in CCl4-intoxicated rats. Int J Toxicol 30:707–714
El-Sokkary GH, Abdel-Rahman GH, Kamel ES (2005) Melatonin protects against lead-induced hepatic and renal toxicity in male rats. Toxicology 213:25–33
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Flora S, Flora G, Saxena G (2006) Environmental occurrence, health effects and management of lead poisoning. In: Casas JS, Sordo J (eds) Lead: chemistry, analytical aspects, environmental impact and health effects. Elsevier Science Publishers, Amsterdam, The Netherlands, pp 158–228
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7:2745–2788
Fong KL, McCay PB, Poyer JL, Keele BB, Misra H (1973) Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem 248:7792–7797
Fouad AA, Albuali WH, Jresat I (2014) Protective effect of thymoquinone against arsenic-induced testicular toxicity in rats. Int J Med Dent Pharm Health Sci Eng 8
Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: ménage à trois. Mutat Res 674:3–22
García-Niño WR, Pedraza-Chaverrí J (2014) Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 69:182–201
Gerhardsson L, Englyst V, Lundström NG, Nordberg G, Sandberg S, Steinvall F (1995) Lead in tissues of deceased lead smelter workers. J Trace Elem Med Biol 9:136–143
Gordon JN, Taylor A, Bennett PN (2002) Lead poisoning: case studies. Br J Clin Pharmacol 53:451–458
Goyer RA, Cherian MG (1979) Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci 24:433–438
Guo W, Reigan P, Siegel D, Ross D (2008) Enzymatic reduction and glutathione conjugation of benzoquinone ansamycin heat shock protein 90 inhibitors: relevance for toxicity and mechanism of action. Drug Metab Dispos 36:2050–2057
Handlogten ME, Shiraishi N, Awata H, Huang C, Miller RT (2000) Extracellular Ca(2+)-sensing receptor is a promiscuous divalent cation sensor that responds to lead. Am J Physiol Renal Physiol 279:F1083–F1091
Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel-Mobdy YE (2012) Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed 2:41–46
Ismail M, Al-Naqeep G, Chan KW (2010) Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med 48:664–672
Jaswal A, Sinha N, Bhadauria M, Shrivastava S, Shukla S (2013) Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage. Environ Toxicol Pharmacol 36:779–786
Jovanović JM, Nikolić RS, Kocić GM, Krstić NS, Krsmanović MM (2013) Glutathione protects liver and kidney tissue from cadmium and lead-provoked lipid peroxidation. J Serb Chem Soc 78:197–207
Kaminsky P, Klein M, Duc M (1993) Physiopathology of inorganic lead poisoning. Rev Med Interne 14:163–170 (in French)
Kanter M, Coskun O, Uysal H (2006) The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol 80:217–224
Karrari P, Mehrpour O, Abdollahi M (2012) A systematic review on status of lead pollution and toxicity in Iran; guidance for preventive measures. Daru 20:1–17
Khalife KH, Lupidi G (2007) Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic Res 41:153–161
Lakshmi BV, Sudhakar M, Aparna M (2013) Protective potential of Black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol 35:361–368
Liu CM, Ma JQ, Sun YZ (2011) Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol 49:3119–3127
Liu CM, Ma JQ, Sun YZ (2012) Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp Toxicol Pathol 64:575–582
Lupidi G, Scire A, Camaioni E, Khalife KH, De Sanctis G, Tanfani F, Damiani E (2010) Thymoquinone, a potential therapeutic agent of Nigella sativa, binds to site I of human serum albumin. Phytomedicine 17:714–720
Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM (2002) Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct 20:143–151
Marchlewicz M, Wiszniewska B, Gonet B, Baranowska-Bosiacka I, Safranow K, Kolasa A, Głabowski W, Kurzawa R, Jakubowska K, Rać ME (2007) Increased lipid peroxidation and ascorbic Acid utilization in testis and epididymis of rats chronically exposed to lead. Biometals 20:13–19
Massó-González EL, Antonio-García MT (2009) Natural antioxidants protect against lead-induced damage during pregnancy and lactation in rat's pups. Ecotoxicol Environ Saf 72:2137–2142
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 84:407–412
Monks TJ, Jones DC (2002) The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr Drug Metab 3:425–438
Mudipalli A (2007) Lead hepatotoxicity & potential health effects. Indian J Med Res 126:518–527
Nagi MN, Alam K, Badary OA, Al-Shabanah OA, Al-Sawaf HA, Al-Bekairi AM (1999) Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. Biochem Mol Biol Int 47:153–159
Nagi MN, Almakki HA (2009) Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: possible role in protection against chemical carcinogenesis and toxicity. Phytother Res 23:1295–1298
Nduka N (1999) Clinical biochemistry for students of pathology. Longman Nigeria PLC, Ikeja, pp 1–236
Nehru B, Anand P (2005) Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol 19:203–208
Newairy AS, Abdou HM (2009) Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem Toxicol 47:813–818
Ng HY, Tain YL, Lee YT, Hsu CY, Chiou TT, Huang PC, Lee CT (2013) Renin angiotensin system blockade ameliorates lead nephropathy. Biochem Biophys Res Commun 438:359–363
Nili-Ahmadabadi A, Tavakoli F, Hasanzadeh G, Rahimi H, Sabzevari O (2011) Protective effect of pretreatment with thymoquinone against aflatoxin B(1) induced liver toxicity in mice. Daru 19:282–287
Patra RC, Swarup D, Dwivedi SK (2001) Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162:81–88
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 6:359–364
Porter NA, Caldwell SE, Mills KA (1995) Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30:277–290
Rifaioglu MM, Nacar A, Yuksel R, Yonden Z, Karcioglu M, Zorba OU, Davarci I, Sefil NK (2013) Antioxidative and anti-inflammatory effect of thymoquinone in an acute Pseudomonas prostatitis rat model. Urol Int 91:474–481
Roggeman S, De Boeck G, De Cock H, Blust R, Bervoets L (2014) Accumulation and detoxification of metals and arsenic in tissues of cattle (Bos taurus), and the risks for human consumption. Sci Total Environ 466–467:175–184
Sainath SB, Meena R, Supriya CH, Reddy KP, Reddy PS (2011) Protective role of Centella asiatica on lead-induced oxidative stress and suppressed reproductive health in male rats. Environ Toxicol Pharmacol 32:146–154
Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah OA, Hafez MM, Nagi MN (2010) Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev 3:254–261
Schreck R, Baeuerle PA (1991) A role for oxygen radicals as second messengers. Trends Cell Biol 1:39–42
Schroeder HA, Balassa JJ (1961) Abnormal trace metals in man: lead. J Chronic Dis 14:408–425
Sharma P, Chambial S, Shukla KK (2015) Lead and neurotoxicity. Indian J Clin Biochem 30:1–2
Sivaprasad R, Nagaraj M, Varalakshmi P (2004) Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem 15:18–23
Soliman MM, Baiomy AA, Yassin MH (2015) Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in wistar rats. Biol Trace Elem Res 167:91–102
Taka E, Mazzio E, Goodman C, Reeams R, Soliman K (2014) Anti-inflammatory effects of thymoquinone in LPS-stimulated BV-2 murine microglia cells (730.2). FASEB J 28
Todorova I, Simeonova G, Kyuchukova D, Dinev D, Gadjeva V (2005) Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Pathol 13:190–194
Wang C, Zhang Y, Liang J, Shan G, Wang Y, Shi Q (2006) Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin Chim Acta 370:82–88
Woo CC, Kumar AP, Sethi G, Tan KH (2012) Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol 83:443–451
Yaman I, Balikci E (2010) Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol 62:183–190
Acknowledgments
This work was supported by funds allocated to the Research Unit of Genetic, Genotoxicity and Child Disease (UR 12 ES 10) by the Tunisian Ministry of Higher Education and Scientific Research. The authors thank to Prof. Abdelhedi Miled (Laboratory of Biochemistry, Farhat Hached University Hospital, Sousse, Tunisia), Prof. Mohsen Sakly (Laboratory of Integrative Physiology, Faculty of Sciences of Bizerte, Tunisia), and Prof. Zohra Haouas (Laboratory of Histology and Cytogenetic, Faculty of Medicine of Monastir, Tunisia) for their help.
Conflict of interest
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Mabrouk, A., Bel Hadj Salah, I., Chaieb, W. et al. Protective effect of thymoquinone against lead-induced hepatic toxicity in rats. Environ Sci Pollut Res 23, 12206–12215 (2016). https://doi.org/10.1007/s11356-016-6419-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6419-5