Abstract
Lead (Pb) poisoning is one of the pivotal environmental issues and prompts liver dysfunction by elevating oxidative stress and inflammation. Nicotinamide (NA) deficiency enhances sensitivity to Pb toxicity. So, we investigated the effect of nicotinamide (NA) on the rat’s liver histopathological and biochemical profiles in a rat model of Pb toxicity. Thirty-six rats were divided into four groups (nine rats at each): normal (N), lead toxicity (Pbt), and NA-treated N and Pbt groups. Treated groups took NA (180 mg/L in drinking water for one month). Pb intoxication was motivated in rats by acquiring 50 mg/L lead acetate in drinking water. Oxidative stress markers (advanced oxidation protein products and malondialdehyde), antioxidant markers (total glutathione, reduced glutathione to oxidized glutathione ratio, ferric ion reducing power, catalase, and paraoxonase-1), and inflammatory markers (hepatic nuclear factor-kβ expression, interleukin 1β level, and myeloperoxidase activity) in sera and liver homogenates were determined. In addition, the biochemical parameters of the liver function were measured. Finally, the liver of rats was evaluated by histopathological observation. NA corrected lead-persuaded biochemical and histopathological changes in the rat’s liver. In addition, treatment decreased Pb, oxidative stress, and inflammatory markers in the sera and liver homogenates of N and Pbt groups. In addition, it elevated antioxidant markers (p < 0.001). NA prevented Pb-induced liver histopathological alternations and reduced liver dysfunction by reducing Pb, oxidative stress, and inflammation. Moreover, raising GSH/GSSG and diminishing the hepatic NF-kβ pathway are cardinal mechanisms of the treatment against Pb-motivated hepatotoxicity in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) poisoning is one of the axial environmental issues. The common ways of Pb exposure are air, drinking water, food, and industrial compounds. The industrial progress globally has been correlated with elevating the effect of xenobiotics such as lead (Pb) on humans and animals [1]. Pb accumulation in the liver leads to tissue injury via effect on membranes and antioxidant defense systems. Pb prompts liver dysfunction by elevating inflammation following oxidative stress. Moreover, it can interfere with biological functions in lipids, proteins, DNA, and di-valance ions.
The chelating agents are a prevalent treatment for Pb intoxication. However, these compounds cannot remove the intracellular Pb [2] and cannot correct organ (liver or renal) dysfunction or compensate for oxidative stress [3]. Pb exposure often follows pathological conditions that are reciprocal with intracellular oxidative damage. Thus, the use of some antioxidants versus Pb-related hepatoxicity has recently increased [4,5,6,7,8]. Vitamins are drastic nutrients that have significant protective impacts on the liver. The deficits of C, B1, B6 [9], and B3 vitamins [10] enhance sensitivity to Pb toxicity. Nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) are co-enzymes of vitamin B3 or nicotinamide (NA) that are scavengers of free radicals and guard tissues versus oxidative damage [11]. The critical mechanism of Pb-motivated hepatotoxicity is oxidative stress. Thus, we investigated the effect of NA on the rat’s liver histopathological and biochemical profiles in a rat model of Pb toxicity.
Materials and Methods
Materials
NA (product no.: 1.06828), lead acetate (product no.: 215902), perchlorate sodium (product no.: 931950), sodium azide (product no.: 71289), thiobarbituric acid (TBA, product no.: T5500), trichloroacetic acid (TCA, product no.: t0699), CaCl2 (product no.: 746455), NaCl (product no.: 85810), ethylenediaminetetraacetic acid (EDTA, product no.: 798681), H2O2, citric acid (product no.: C1909), reduced (product no.: G4251) and oxidized (product no.: G4376) glutathione (GSH and GSSG, respectively), paraoxon (product no.: P7832), Trizol reagent (product no.: 50175111), and 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ, product no.: T1253) were purchased from Sigma-Aldrich Chemical Co. (USA). Sodium mono (product no.: 106345) and dihydrogen phosphate (product no.: 106346) were bought from Merck Company (Germany). Biochemical kits were received from Pars Azmoon Company (Tehran, Iran). SYBER Green qPCR Master Mix 2x and cDNA synthesis kit were provided from Yekta Tajhiz Azma Company (Iran).
Study Design
Thirty-six male Wistar rats weighing 235 ± 15 g were obtained from the Pasteur Institute of Iran, Karaj. The rats were kept in restrained conditions with free access to food and water. After two weeks, rats were haphazardly distributed into four groups (nine rats in each): normal rats (N), lead toxicity (Pbt), and two similar groups under NA treatment, respectively, N (NA) and Pbt (NA). Pb poisoning was motivated in rats with 50 mg/L lead acetate in drinking water for one month. Treated groups received 180 mg/L of NA in drinking water daily for one month [12]. The groups’ total weight was equal. In addition, rat taking water level depends on its weight. Thus, receiving Pb and NA in drinking water not only did not cause a problem in the design process but also prevented stress [13]. The groups fed a standard chow diet (carbohydrate 48.8%, protein 21%, fat 3%, calcium 0.8%, phosphorus 0.4%, fiber 5%, moisture 13%, and ash 8%) that was provided from Faradam Zarin KHavarmianeh Company, Isfahan, Iran. The study was permitted by the Ethics Committee of Ardabil University of Medical Sciences (IR.ARUMS.REC.1401.044). After 16 h of fasting and anesthetizing [14] with an intraperitoneal (I.P) injection of ketamine and xylosine (respectively, 90 and 10 mg/kg body mass), blood samples were collected from their heart [15] and transferred into the test tubes with EDTA (4 mL) and without EDTA (1 mL). The blood was permitted to clot for 30 min and centrifuged at 2000 g for 15 min. Liver tissue was taken away and weighed readily. 200 mg of liver slices was homogenized in 2 mL of homogenization buffer (50 mmol/L phosphate buffered saline, pH 7.4). The homogenized tissues were centrifuged at 4 °C and 12,000 rpm for 10 min to obtain the supernatants. Then, 75% of the supernatant was removed for analysis of oxidative stress and inflammatory markers except total, reduced (GSH), and oxidized glutathione (GSSG). Finally, equivalent to the remaining volume of the supernatant, ice-cold MPA (5% w/v) was added and centrifuged at 4 °C and 8000 rpm for 25 min. Then, supernatant was removed for measuring total glutathione, GSH, and GSSG [16].
Determination of Biochemical Parameters
Alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), total serum protein, albumin, and total bilirubin (TB) were determined through using commercial kits (Pars Azmoon, Tehran, Iran). The total globulin fractions were computed by subtracting the albumin from the total protein. Liver index of the rat was estimated (liver weight/rat weight × 100%). Lead content in sera and liver homogenates was detected with the flame atomic absorption spectrophotometer (PerkinElmer, 3100) at 283.3 nm.
Measuring Oxidative Stress and Inflammatory Markers
The malondialdehyde (MDA) level of samples was assessed by adding 100 μL of sample to a mixture (1 mL of TBA 0.67% and 500μL of TCA 20%), boiling for 30 min, and fast cooling. Then, MDA was determined from the absorbance at 535 nm [17]. Advanced oxidation protein products (AOPP) were detected by measuring absorbance in wavelength 340 nm in diluted serum. Briefly, 40 μL of sample diluted 1 : 5 with citrate solution (200 mmol/L). Chloramine T (0–100 μmol/L) was used for calibration. Then, 10 μL of potassium iodide (1160 mM) and 20μL of glacial acetic acid were added and the absorbance of the solution was instantly detected at 340 nm [18]. The ferric ion-reducing antioxidant power (FRAP) was detected at 593 nm. Concisely, 50 μL of sample was added to a reaction mixture (acetate buffer 300 mmol/L with pH 3.6 containing 10 mmol/L of TPTZ and FeCl3 20 mmol/L) [19]. Reverse phase-HPLC at 210 nm was used for determining reduced glutathione (GSH). Moreover, the mobile phase was sodium perchlorate 100 mmol/L [16]. The paraoxonase-I (PON1) activity was assessed by determining one minute the absorbance of p-nitrophenol at 412 nm. P-nitrophenol was acquired by paraoxon hydrolysis. 5 μL of sample added to a 0.800-mL mixture of 1.0 mmol/l paraoxon and 1.0 mmol/l CaCl2, in 0.05 mole glycine buffer, pH 10.5. The release of p-nitrophenol was succeeded at 412 nm [20]. Catalase (CAT) activity was detected with a modified Abi method [21]. Briefly, a 5 μL sample was added to one mL of phosphate buffered saline (buffer 50 mmol/l, pH = 7 and containing 10 mm of H2O2) and read absorbance at 240 nm to 20 s.
Interleukin-1β (IL-1β) was measured with the ELISA kit (ZellBio GmBH, Germany). The activity of myeloperoxidase (MPO) was determined via reading the absorbance of oxidized guaiacol at wavelength 470 nm. Slightly, 10 μL of the sample was added to a mixture (50 mM potassium phosphate buffer with 100 mM guaiacol and 0.0017% (w/w) hydrogen peroxide, pH 7.0 at 25 °C) and read absorbance at 470 nm until 4 min.
NF-κB Expression in the Rat Liver
RNA from hepatic cells was separated with a TRIzol reagent (Invitrogen, USA). Its mass and grade were identified by a NanoDrop at 260 nm and 260/280 nm ratios, distinctly. Reverse transcription (MBI Ferments, Lithuania) was applied for cDNA generation. qRT-PCR was accomplished with a high-quality SYBR-Green PCR kit (Toyobo, Japan). The ABI 7300 (Applied Biosystems, Germany) was used for gene-specific PCR amplification. Β-Actin (ACTB) normalized the gene expression data. RT-PCR primer sequences were as comes behind: NF-kβ: 5′-CCTGTCTGCACCTGTTCCAA-3′ (forward) and 3′ACTCCTGGGTCTGTGTTGTT-5′ (reverse) as well as ACTB: 5′-GGAGAA GATTTGGCACCACACT-3′ (forward) and 3′-CGGTTGGCCTTAGGGTTCAGA-5′ (reverse). After the normalization, the 2- ΔΔCT method determined the correlative gene expression levels. The first ΔCT is the alternation in the threshold cycle between the NF-kβ and ACTB: ΔCT = CT (NF-kβ)-CT (ACTB).
Pathological Study
The liver’s sections were fixed in a buffer solution containing 10% formalin and processed for paraffin embedding. Then, the sections were stained with hematoxylin-eosin (H&E) and observed under light microscopy for histopathological parameters.
Statistical Analysis
All data were expressed as mean ± S.D (standard deviations). The Kolmogorov–Smirnov test assessed the normal distribution of the results. The comparison of variables in all four groups was done with an analysis of variance (ANOVA-Tukey) test using SPSS version 16. Statistical significance was accepted as p < 0.05.
Results
Table 1 compares liver function parameters, LWI, and body weight in all rat groups. The enzyme activities (ALT, AST, ALP, and GGT), TB, and LWI in the Pbt group were higher than in other groups. In addition, total protein, Alb, globulins, and body weight were lower in Pbt. Here, nicotinamide compensated for the cited changes. There was no difference in the enzyme activities, bodyweight, and BWI between N and N (NA) groups. However, TB was lower in N (NA) than N (p < 0.001).
Table 2 represents the effect of NA on Pb, total glutathione, GSH/GSSG, FRAP, CAT, MDA, AOPP, and MPO in both sera and liver homogenates of all rat groups. Figure 1 shows the hepatic NF-kβ/BACT in rat gropes. The hepatic NF-kβ/BACT, MPO, MDA, and AOPP were less, and total glutathione, GSH/GSSG, CAT, PON-1, and FRAP were more in treated groups ones. However, the Pb level only was lower in Pbt (NA) (p < 0.001).
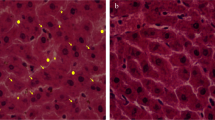
Figure 2a–d presents the liver histopathological regard of rat groups. Further, Table 3 displays their semi-quantitative assessment. Figure 2a, b, d exhibits the histopathological views of N, N (NA), and Pbt (NA) groups. Hepatocytes of the cited groups were arranged in plates that connected together, and the cells were polygonal in shape with round shape nuclei. One month of Pb exposure ruined the liver structures of the Pbt group. Many hepatocytes are destroyed and lost their shape. In addition, the accumulation of Pb residue in the Kupffer cells, hyperplasia of Kupffer cells, a high number of bi-nucleated hepatocytes, and congested sinusoids was seen (Fig. 2c and Table 3). NA inhibited the cited changes by reducing the collection of Pb in hepatocytes of the Pbt (NA) group.
Histopathologic observations (stained by H&E & original magnification ×400) of the liver in the lead toxicity (Pbt) and nicotinamide treated one (Pbt-NA). a Normal liver architecture in N group, hepatocytes of the cited groups arranged in plates that connected together, and the cells were polygonal in shape with round shape nuclei. b Normal liver structure in N (NA) group alike N group. c Arrows, circles, and stars respectively represent the hyperplasia of Kupffer cells, binucleated hepatocytes, congested sinusoids, and autolytic cytoplasm in the liver of Pb group. d NA inhibited the histopathological modifications of the liver in Pbt (NA) group that was induced with lead acetate
Discussion
Thirty-seven years ago, evidence reported that nicotinamide deficiency elevated the Pb absorption in young rats [10]. Lead acetate participated in the liver damage and dysfunction following the modification of the liver histopathological and biochemical profiles. Protective effect of nicotinamide versus Pb simulated hepatotoxicity is satisfied by diminishing on Pb, oxidative stress, and inflammation along with raising of GSH/GSSG in the sera and liver homogenate.
Pb via the accumulation in the liver, free radical generation, endogenous antioxidant reduction, and bio-membrane injury participates in liver damage and dysfunction [8, 22]. Pb intoxication modified the rat’s liver histopathological and biochemical profiles. Pb participated in the liver damage and dysfunction following simulation of notable inflammation and necrosis that confirms through hyperplasia of Kupffer cells, a high number of bi-nucleated hepatocytes, congested sinusoids, and autolytic cytoplasm (Fig. 2d and Table 3). In addition, raising ALT, AST, ALP, and GGT activities and TB quantity and elevating total protein, Alb, and globulins levels (Table 1) in the Pbt group satisfies liver damage and dysfunction. The hepatic NF-kβ is a principal coordinator in liver physiology and diseases [23]. A decrease in NF-kβ signaling is a potential target for the hepatoprotective agents proceeding [13]. Pb conduced to liver poisoning by upping the hepatic NF-kβ signaling following an increase on oxidative stress [8] and IL-1β [24]. In addition, elevating MPO activity in liver tissue results in liver injury due to raising MDA [25] and AOPP [8] as pointers of lipid peroxidation and protein oxidation, separately. Pbt group had the highest Pb, oxidative stress markers (AOPP and MDA), and inflammatory markers (NF-kβ, IL-1β, and MPO) along with the lowest antioxidant markers (total glutathione, GSH/GSSG, FRAP, CAT, and PON) in the sera and the liver homogenates. The advantageous effect of the treatment against the Pb-motivated hepatotoxicity was through a diminish on Pb residue, oxidative stress, and inflammatory markers, as well as an elevation on antioxidant markers (Table 2) that confirms chelating, antioxidant, and anti-inflammatory properties. The treatment had a hepatoprotective effect by reducing NF-kβ signaling (Fig. 2), its stimulators, and MPO activity (Table 2). Reduced glutathione powerfully retards the Pb reactive toxic metabolites [26]. Hence, lifting GSH/GSSG is the profitable strategy versus lead-induced hepatotoxicity. PON-1 has an antioxidant effect against lipid peroxidation in the cell membranes and lipoproteins [27]. In this study, NA raised total glutathione and GSH/GSSG in N and Pbt groups (Table 2) due to elevating glutathione synthesis and glutathione reductase activity in N (NA) and Pbt (NA). An increase in GSH level causes a decrease on Pb levels in the sera and liver homogenates of the cited groups by elevating Pb excretion [8, 28]. An elevation on endogenous antioxidant (GSH, CAT, and PON-1) decreases lipid peroxidation [29] and protein oxidation [8]. The signs of liver histopathological alternations in this research are alike to previous studies [8, 30]. We reported for the first time the effect of NA on the cited liver histopathological and biochemical parameters in the lead intoxication rat model. Lately, the hepaprotective effect of nicotinic acid in a rat model of zinc and nicotinic acid deficiency via its raising effect on antioxidant enzyme activities (superoxide dismutase, glutathione peroxidase, and CAT) was represented [31]. In addition, the ameliorating effect of vitamin B3 on renal failure in rats via its diminishing renal NF-kβ signaling and lipid peroxidation was reported [32].
Conclusion
Nicotinamide prevented Pb-induced liver histopathological alternations and reduced liver dysfunction by reducing Pb, oxidative stress, and inflammation in the sera and liver homogenates. Moreover, raising GSH/GSSG and diminishing the hepatic NF-kβ pathway are cardinal saving mechanisms of the treatment against Pb-motivated hepatotoxicity in rats.
References
Mahar FK, He L, Wei K, Mehdi M, Zhu M, Gu J, Zhang K, Khatri Z, Kim I (2019) Rapid adsorption of lead ions using porous carbon nanofibers. Chemosphere 225:360–367
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7(7):2745–2788
Mohamed RS, Fouda K, Akl EM (2020) Hepatorenal protective effect of flaxseed protein isolate incorporated in lemon juice against lead toxicity in rats. Toxicol Rep 7:30–35
Fehaid A, Al‐Ghamdi MS, Alzahrani KJ, Theyab A, Al-Amer OM, Al-Shehri SS, Algahtani M, AA AO, Alnfiai MM, Aly MH, Alsharif KF, Albrakati A, Kassab RB, Althagafi HA, Alharthi F, Abdel Moneim AE, Lokman MS (2023) Apigenin protects from hepatorenal damage caused by lead acetate in rats. J Biochem Mol Toxicol 37(3):e23275
Mansour LAH, Elshopakey GE, Abdelhamid FM, Albukhari TA, Almehmadi SJ, Refaat B, El-Boshy M, Risha EF (2023) Hepatoprotective and neuroprotective effects of naringenin against lead-induced oxidative stress, inflammation, and apoptosis in rats. Biomedicines 3(11):1080
Mesalam NM, Ibrahim MA, Mousa MR, Said NM (2023) Selenium and vitamin E ameliorate lead acetate-induced hepatotoxicity in rats via suppression of oxidative stress, mRNA of heat shock proteins, and NF-kB production. J Trace Elem Med Biol 79:127256
Shalan MG, Al-Bakry NA, Rashwan HM (2023) The ameliorative effects of lactoferrin against lead acetate toxicity in female albino rat. Egypt J Zoology 80:35–49
Mahdavifard S, Sekhavatmand N (2022) Glutamine is a superior protector against lead-induced hepatotoxicity in rats via antioxidant, anti-inflammatory, and chelating properties. Biol Trace Elem Res 200(11):4726–4732
Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT (1998) Effects of micronutrients on metal toxicity Marjorie Rael. Environ Health Perspect 106:203–216
Flora SJ, Tandon SK (1987) Influence of dietary deficiency of nicotinamide on lead toxicity in young rats. Biol Trace Elem Res 14:143–151
Lanska DJ (2010) Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Handb Clin Neurol 95:445–476
Brown AP, Dinger N, Levine BS (2000) Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci 39(1):17–21
Sun B, Karin M (2008) NF-kB signaling, liver disease and hepatoprotective agents. Oncogene 27:6228–6244
Akbudak İH, Kilic Erkek O, Tuzcu EB, Pakyurek H, Bor Kucukatay M (2021) Ketamine/xylazine anesthesia is safe in hemorheological point of view: a preliminary report. Pamukkale Tıp Dergisi 14(2):444–450
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1(2):87–93
Begic A et al (2017) The simple isocratic HPLC—UV method for the simultaneous determination of reduced and oxidized glutathione in animal tissue. Acta Chromatogr 29(1):67–84
D'souza D, Subhas BG, Shetty SR, Balan P (2012) Estimation of serum malondialdehyde in potentially malignant disorders and post-antioxidant treated patients: a biochemical study. Contemp Clin Dent 4:448–451
Taylor EL, Armstrong KR, Perrett D, Hattersley AT, Winyard PG (2015) Optimisation of an advanced oxidation protein products assay: its application to studies of oxidative stress in diabetes mellitus. Oxidative Med Cell Longev 2015:496271
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Ceron JJ et al (2014) Serum paraoxonase 1 (PON1) measurement: an update. BMC Vet Res 10:74
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–129
Chen C, Lin B, Qi S, He J, Zheng H (2019) Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in Sprague-Dawley rats. Biol Trace Elem Res 191:426–434
Gehrke N, Schattenberg JM (2020) Metabolic inflammation-a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 158(7):1929–1947.e6
Gao D, Madi M, Ding C, Fok M, Steele T, Ford C et al (2014) Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Phys Endocrinol Metab 307:289–304
Demir M et al (2007) Liver lipid peroxidation in experimental Escherichia coli peritonitis: the role of myeloperoxidase and nitric oxide inhibition. Med Sci Monit 13(10):Br225–Br229
Hsu JM (1981) Lead toxicity as related to glutathione metabolism. J Nutr 111(1):26–33
Yıldırım A, Aslan Ş, Ocak T, Yıldırım S, Kara F, Şahin YN (2007) Serum paraoxonase/arylesterase activities and malondialdehyde levels in trauma patients. EAJM 39:85–88
Sharma V, Kansal L, Sharma A (2010) Prophylactic efficacy of Coriandrum sativum (coriander) on testis of lead-exposed mice. Biol Trace Elem Res 36:337–354
Mohamed OI, El-Nahas AF, El-Sayed YS, Ashry KM (2016) Ginger extract modulates Pb-induced hepatic oxidative stress and expression of antioxidant gene transcripts in rat liver. Pharm Biol 54:1164–1172
Narjes Beheshti FG, Sepehri H (2015) Effect of vitamin C and quercetin treatment on the liver histopathologic profile in congenital lead exposed male rat pups. Physiol Pharmacol 10:46–52
Tupe RS, Tupe SG, Agte VV (2011) Dietary nicotinic acid supplementation improves hepatic zinc uptake and offers hepatoprotection against oxidative damage. Br J Nutr 105(12):1741–1749
Cho KH, Kim H, Rodriguez-Iturbe B, Vaziri ND (2009) Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Ren Physiol 297(1):106–113
Acknowledgements
The results explained in this paper were part of the student thesis.
Funding
The authors are thankful to Ardabil University of medical sciences for financial support.
Author information
Authors and Affiliations
Contributions
1) Study plan, evaluation and exegesis of data: Mahdavifard & Shahi
2) Manuscript writing and revising: Mahdavifard
3) Ultimate sanction of the version to be submitted and any revised version: Mahdavifard & Shahi
Corresponding author
Ethics declarations
Ethics Approval
Ethics assent and written informed consent have been attained.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The survey is a genuine study that has not been lately sent to or admitted by any other journal that has been ratified.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdavifard, S., Shahi, Z. Hepatoprotective Effect of Nicotinamide Versus Lead-Motivated Hepatotoxicity in Rats via Correcting Effect on Nuclear Factor-kβ Pathway and Glutathione Metabolism. Biol Trace Elem Res 202, 4047–4053 (2024). https://doi.org/10.1007/s12011-023-03980-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03980-x