Abstract
The study monitored the contamination of fish muscle tissue by elements Al, As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn at 17 sampling sites, in order to assess the pollution status of the main rivers in Serbia. Of the six commercially important fish species included in the study (pikeperch Sander lucioperca, catfish Silurus glanis, bream Abramis brama, barbel Barbus barbus, chub Squalius cephalus, nase Chondrostoma nasus), the bioconcentration factor (BCF) indicated that benthivore bream and barbel and predatory catfish have the highest tendency toward the accumulation of elements. This study achieved its primary objective and produced a contamination map of Serbia as a basis for further research. The estimated metal pollution index (MPI) showed the Tisa River to be unaffected by direct pollution (with an MPI value of 0.31) and the West Morava and Pek rivers to be affected (with MPI values of 1.92 and 0.73 for the WM1 and WM2 sampling sites and 0.65 for the Pek sampling site). Over the past two decades, Serbia has not expanded its industrial activity, which has resulted in the barely noticeable anthropogenic input of heavy metals in the rivers close to industry, and the main rivers are mostly unaffected and slightly affected. We assumed that pollution by heavy metals in the 1990s was trapped in the sediment, thus showing an increased concentration of elements in the species that live and feed on the bottom. Hg concentrations exceeded the maximum permitted concentrations (MPCs) only in catfish samples (0.62 mg kg−1) from the Danube (D3 sampling site) and barbel (0.78 mg kg−1) from the West Morava (WM1 sampling site), while Cd concentrations exceeded the MPC in catfish samples (0.09 mg kg−1) from the Danube (D1 sampling site) and chub samples (0.1 mg kg−1) from the South Morava (SM2 sampling site). The average concentrations of Pb exceeded the MPC in chub and barbel samples (0.32 and 0.82 mg kg−1, respectively) from the West Morava (WM1 sampling site); chub, barbel, and nase samples (0.35, 0.32, 0.31 mg kg−1, respectively) from the West Morava (WM2 sampling site); chub and barbel samples (0.35 and 0.3 mg kg−1, respectively) from the Ibar; chub samples (0.39 mg kg−1) from the Drina; chub and barbel samples (0.59 and 0.4 mg kg−1, respectively) from the Great Timok; and nase samples (0.33 mg kg−1) from the Pek. These results demonstrate that there is a need for future studies that would involve similar analyses and focus especially on smaller rivers that have been neglected so far.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in rivers has become a matter of great concern, not only because of the threat it poses to public water supplies but also because of the hazard to the human consumption of fishery resources (Terra et al. 2008). Water and/or sediment analysis may be inefficient at identifying the inherent variability of the flow and contaminant concentrations of metal inputs to fluvial systems (Ricart et al. 2010). Traditional toxicity tests and chemical-specific sensors cannot provide comprehensive real-time information relating to toxic events in an aquatic system. Aquatic organisms must be included as a major component of the assessment processes (van der Schalie et al. 2001), thereby providing a more effective diagnosis of environmental conditions. Fish are susceptible to toxins present in the water and are useful bioindicators for the determination of metal pollution in aquatic ecosystems (Chovanec et al. 2003; Alibabić et al. 2007; Lamas et al. 2007) because they occupy various trophic levels, they are the key species in trophic chains, they concentrate large amounts of some metals, and some are widely consumed by humans (Uysal et al. 2009; Burger et al. 2002). Since the discovery of Minamata disease caused by severe mercury poisoning, human health concerns related to consumption of fish polluted with heavy metals have long been recognized and studied (Uchida et al. 1961; FAO/WHO 1972; Hutton 1987).
Many biotic factors have been recognized to affect the bioaccumulation of metals in fish, such as variability in age/size, the size-specific metabolic rate of individuals (Phillips 1980), life cycle, life history, and the diet differences of species (Hardisty et al. 1974; Prosi 1981).
Dallinger and Kautzky (1985) stated that in low-level, chronic diffuse pollution, metal uptake in fish is predominantly from food. Studies from field and laboratory experiments have shown that the accumulation of heavy metals in fish tissue is mainly dependent upon concentrations of the metals in the surrounding water, in addition to the exposure period (Yi and Zhang 2012; Liu et al. 2011). The season of capture can also be one of the factors influencing metal tissue concentrations (McCoy et al. 1995). Independently of the season and how the exposure occurred, some fish organs have a greater affinity for accumulating some specific metals than others. The lipid content of tissues is an important variable affecting the concentration of metals accumulated in fish (Braune et al. 1999), especially for the bioaccumulation of Hg since this metal deposited mainly in the lipid fraction of fish (as methylmercury) has a very low elimination rate, and therefore, even in slightly polluted environments, the Hg concentration in fish continuously increases throughout the life span of the fish. Since Hg can accumulate more easily in muscle than the other organs (Čelechovská et al. 2007; Has-Schön et al. 2008) and thus put biota and, according to the food chain, humans at risk, edible muscle tissue should be analyzed.
Each fish species has a particular way of accumulating and/or eliminating metals when exposed to such contaminants. Hence, more than one fish species should be analyzed in comparative environmental studies due to substantial variations in pollution processes (Burger et al. 2002). Multispecies comparisons covering different feeding habits of fish and a wide range of age categories have the potential to distinguish recent exposure from long-term load (Dušek et al. 2005).
Some elements such as iron, copper, zinc, and manganese are essential for biological systems such as enzymatic activities; others like nickel and aluminum are nonessential, whereas arsenic, lead, and cadmium have no known important role in living organs and are toxic even in trace amounts (Fernandes et al. 2008). Accordingly, screening all elements which could possibly occur in food is important because they simultaneously play biological and toxicological roles.
Many studies have been carried out into the trace metal accumulation patterns in aquatic biota, along with the bioconcentration and biomagnification processes. In Serbia, various fish species have been effectively used for the bioindication of contamination (Visnjic-Jeftic et al. 2010; Poleksić et al. 2010; Jarić et al. 2011; Lenhardt et al. 2012; Subotić et al. 2013a, b; Jovičić et al. 2015). The cited references reveal diverse approaches to choosing the most appropriate fish group, species, and tissue for these kinds of monitoring studies. The main objective of any biomonitoring program is to assess the health of the aquatic ecosystem, so there is a need to establish standardized methods. The abovementioned studies were generally limited to various tissues of individual species in particular sections of rivers (mainly the Danube and Sava), so there is still no study that would give a comprehensive picture of the spatial pollution of the inland waters in Serbia. In this study, we recorded the Al, As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn concentrations in the muscle tissue of six commercial fish species chosen as potentially sensitive species for monitoring studies and estimated the pollution status of the main rivers in Serbia. Based on this, the main objectives of this study were to highlight the importance of a multispecies approach in contaminant studies and to present a preliminary contamination map as a baseline for Serbia. Since in recent years fish consumption in Serbia has increased due to a public campaign on its health benefits through popular media, studying the potential impact of contaminated fish consumption on human health was also one of the study goals.
Materials and methods
Study area and sampling locations
The largest and the most important rivers that flow through Serbia are the Danube, Sava, Tisa, Great Morava, West Morava, South Morava, Ibar, Drina, and Timok. The Danube River is the second largest river in Europe, and as such, it is subjected to large amounts of wastewater input (Pawellek et al. 2002; Teodorović et al. 2000). Metals are considered to be among the major pollutants in the Danube in Serbia, especially in the area of the cities of Belgrade and Novi Sad (Teodorović 2009). The Sava River, as a typical lowland and the Danube’s largest tributary, was affected until the 1990s by heavy pollution from the metallurgical, chemical, leather, textile, food, cellulose, and paper industries, as well as agricultural activities. Also, the Sava is the main recipient of wastewater from many cities, and it is impacted by the polluted water from its tributaries. The largest tributary of the Sava, the Drina, as a mainly mountainous river with great power purification, used to be of high quality. However, in recent years, heavy metals (mainly iron, arsenic, manganese, nickel, and lead) have arrived in the Drina River from damaged tailings. The Kolubara, the right tributary of the Sava, is one of the flows with a higher degree of degradation of water as it flows through towns and villages, where it receives effluent and sewage from industry such as surface mines and a thermal power plant. The Tisa River, the second largest Danube tributary, is also contaminated through numerous industrial accidents from the Carpathian mountain region in Romania, which has a long tradition of mining, especially of gold (Au), silver (Ag), lead (Pb), zinc (Zn), copper (Cu), cadmium (Cd), and manganese (Mn) (Triebskorn et al. 2008). The Great Morava River, the largest Serbian river and also a significant right tributary of the Danube River, is created by the confluence of the South Morava and the West Morava rivers. As a typical lowland river, the Great Morava River flows through the most densely populated area of Central Serbia, the Morava River valley, receiving untreated or incompletely treated wastewater from urban areas and animal farms, which leads to serious degradation of the water quality. As the greatest right tributary of the West Morava, the Ibar River is affected with lead and zinc pollution, since in this area are a vast number of production and manufacturing plants of the mining-metallurgical system, Trepča-nine lead and zinc mines, three flotations, two of metallurgy, the chemical industry, and a battery factory. The right Danube tributaries, the Great Timok, Mlava, and Pek are the most important in East Serbia. The Great Timok and the Pek rivers partly drain the Bor and Majdanpek copper ore districts, respectively. Large average concentrations of calcium, manganese, and arsenic have been found in the Mlava river water (Djinovic and Popovic 2007).
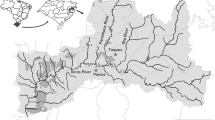
The study region includes the main and the most important rivers in Serbia. The sampling locations are given in Fig. 1 for the Danube: Novi Sad (D1), Zemun (D2), and Radujevac (D3); the Sava: Ostružnica (S); the Tisa: Novi Bečej (T); the Great Morava: Ljubičevski most (GM1) and the Bagrdan (GM2); the West Morava: Kraljevo (WM1) and Jasika (WM2); the South Morava: Niš (SM1) and Ristovac (SM2); the Ibar: Kraljevo (I); the Drina: Badovinci (DR); the Kolubara: Mislođin (K); the Great Timok: Vražogrnac (GT); the Mlava: Bratinac (M); and the Pek: Neresnica (P).
Sampling locations: the Danube: Novi Sad (D1), Zemun (D2), and Radujevac (D3); the Sava: Ostružnica (S); the Tisa: Novi Bečej (T); the Great Morava: Ljubičevski most (GM1) and the Bagrdan (GM2); the West Morava: Kraljevo (WM1) and Jasika (WM2); the South Morava: Niš (SM1) and Ristovac (SM2); the Ibar: Kraljevo (I); the Drina: Badovinci (DR); the Kolubara: Mislođin (K); the Great Timok: Vražogrnac (GT); the Mlava: Bratinac (M); and the Pek: Neresnica (P)
Sample collection, preparation, and determination of element concentrations by inductively coupled plasma optical emission spectrometry
A total of 361 fish belonging to six species were analyzed for the content of Al, As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn in their muscle tissue. The most important commercial fish species were studied, namely pikeperch Sander lucioperca (Linnaeus 1758), catfish Silurus glanis (Linnaeus 1758), bream Abramis brama (Linnaeus 1758), barbel Barbus barbus (Linnaeus 1758), nase Chondrostoma nasus (Linnaeus 1758), and chub Squalius cephalus (Linnaeus 1758). In the Danube, Sava, and Tisa rivers, pikeperch, catfish, and bream were sampled because the majority of first-grade quality fish species (e.g., catfish, pikeperch) and second-grade quality fish species (bream species Abramis spp.) are harvested by commercial fishermen in these rivers (Smederevac-Lalić et al. 2012). The number of commercial fishermen has decreased over recent years, while the number of recreational fisherman has increased (Smederevac-Lalić et al. 2011). Therefore, barbel, nase, and chub were included in this study and sampled in the Great Morava, West Morava, South Morava, Drina, Ibar, Great Timok, Kolubara, Mlava, and Pek, as fish caught substantially for recreational purposes. Thus, the term commercial fish species, in this study, applies not only to species harvested for profit purposes but also to species significant for recreational fishing.
Our field surveys were carried out between 2011 and 2013, during the same season (June–October). The electrofishing procedure was conducted at nine sampling stations: GM1, GM2, SM1, SM2, WM1, WM2, I, M, and P using the Aquatech DC electrofisher IG 1300 (2.6 kW, 80–470 V). Professional fishing nets of different lengths, widths, and mesh diameters were used for sampling at four sampling stations: D2, D3, S, and T, while at the other four sampling stations: D1, DR, K, and GT, fish were collected from local fishermen. Fish were randomly selected for analysis in relation to their commercial consumption sizes, killed with a quick blow to the head, washed with distilled water, and transported on ice (in individual zipper bags) to the laboratory.
In the laboratory, the fish were measured for their total body length (to the nearest cm), weighed (to the nearest g), and subsequently dissected. The right dorsal muscle, below the dorsal fin (±5 g), of each specimen was dissected with a decontaminated ceramic knife. After dissection, the muscle tissue was weighed using an electronic balance (±0.1 g) and stored at −20 °C prior to analysis.
Muscle samples were submitted to the Analytical Chemistry Laboratory within the Institute of Chemistry at the Faculty of Chemistry, University of Belgrade, Serbia, for chemical analysis. The element concentrations (Al, As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn) were determined by inductively coupled plasma optical emission spectrometry (ICP-OES), using a Thermo Fisher Scientific iCAP 6500 Duo ICP (Cambridge, UK) equipped with a RACID86 Charge Injector Device (CID) detector, concentric type nebulizer, quartz torch, and alumina injector.
At first, the fish samples (∼1.5 g) were dried in a lyophilizer (Christ Alpha 2-4 LD, Harz, Germany) and then digested in an Advanced Microwave Digestion System (ETHOS 1, Milestone, Italy) using a mixture of 65 % nitric acid and 30 % hydrogen peroxide (Suprapur®, Merck, Darmstadt, Germany, 10:2, v/v) at 200 °C for 20 min. After cooling to room temperature and without filtration, the solution was diluted to a fixed volume (volumetric flask, 25 mL) with ultra-pure water with a conductivity of 0.055 μS cm−1 (Barnstead™ GenPure™ Pro, Thermo Scientific, Germany), before being analyzed by ICP-OES.
Blanks with no fish tissue were run with each batch of samples to monitor contamination by the reagents used. The standards for the instrument calibration were prepared on the basis of the multielement (SS-Low Level Elements ICV Stock, 10 mg L−1) and mono-element (Hg Calibration Stock, 10 mg L−1 Hg) certified reference solutions ICP Standard (VHG Labs, Inc-Part of LGC Standards, Manchester, NH 03103, USA) and analyzed to support quality assurance and control. The muscle standard reference material (DORM-4; National Research Council of Canada) was digested in triplicate and analyzed to support quality assurance and control.
The results of analyzing the elements in the water were provided by the Agency for Environmental Protection of Serbia and were measured in the same months as our study during routine monitoring at the selected sampling sites.
The bioconcentration factor and metal pollution index
The bioconcentration factor is defined as the net result of the absorption, distribution, and elimination of a substance in any organism after exposure via water (Lau et al. 1998). It was calculated as the ratio of the mean element level in the muscle tissue (C fish), expressed as mg kg−1 wet weight (ww), and the element concentration in water (C water), expressed as mg L−1:
The metal pollution index (MPI) was calculated to compare the total metal content in the different fish species, as well as in the sampling sites using the following equation (Usero et al. 1997):
where Cf n is the concentration of the metal n in the sample
Statistics
The mean values and standard deviations were calculated for each element in each species. Prior to interpreting the heavy metal measurement data, the analysis of covariance (ANCOVA) was used separately for each element to determine if the element concentrations differed between species, with element concentrations as the dependent variable, species as the independent variable, and sampling sites as the covariate (interaction was significant at p ≤ 0.05). The homogeneity of regression slopes for metal concentrations-fish species and sampling sites was tested before ANCOVA. The least significant difference (LSD) post hoc tests were conducted to pair-wise comparisons. In order to assess any significant differences between the BCF and MPI values of fish species, the nonparametric Kruskal-Wallis test was applied. Post hoc inter-group comparisons of BCF and MPI (between pairs of species) were performed by the nonparametric Mann-Whitney test for two independent values. The statistical analyses were processed by the SPSS version 16.0 software (SPSS, Inc., Chicago, Illinois).
The concentrations in the fish muscle samples were also compared with the maximum permitted concentrations (MPCs) in fish meat for utilization in the human diet, established by the European Union (EU) and national legislation. According to EU legislation (European Commission Regulation 2006), the MPCs for Cd, Hg, and Pb are 0.05, 0.50, and 0.30 mg kg−1 ww, respectively. The MPCs for As, Cd, Hg, Pb, Cu, Fe, and Zn in fish meat are 2.0, 0.1, 0.5, 1.0, 30.0, 30.0, and 100.0 mg kg−1 ww, respectively, as prescribed by national legislation (Official Gazzette of RS 2011).
Results
A replicate analysis of the reference material showed good accuracy during analysis, with recovery ranging from 85.80 to 106.48 %. All of the fish species examined were above the minimum length permitted for fishing prescribed by Fisheries law (Official Gazette of RS 2005). The biological data for the specimens per sampling site (number of examined fish samples, average length, and weight) are shown in Table 1. The element concentrations in water and the muscle samples for each examined fish species are presented in Table 2. The element concentrations in the water were different at each sampling site, and there was no unique pattern of distribution of the elements in the fish species observed, but the average element concentration generally displayed a similar order at all sampling sites for each of fish species. In the muscles of all fish species, Zn, Fe, and Al concentrations were the highest, while Cd, Co, and Ni concentrations were the lowest.
The ANCOVA results revealed that pikeperch, bream, and catfish significantly differed in terms of the As (F = 9.599, p = 0.00, ƞ 2 = 0.319), Cd (F = 4.011, p = 0.026, ƞ 2 = 0.164), Hg (F = 7.645, p = 0.002, ƞ 2 = 0.272), Mn (F = 7.657, p = 0.001, ƞ 2 = 0.272), and Zn (F = 3.803, p = 0.031, ƞ 2 = 0.156) (Fig. 2a). In addition, Hg (F = 11.194, p = 0.000, ƞ 2 = 0.177) and Mn (F = 4.267, p = 0.017, ƞ 2 = 0.076) as well as Pb (F = 3.286, p = 0.041, ƞ 2 = 0.059) concentrations significantly varied between chub, barbel, and nase (Fig. 2b). The spatial variability of element concentrations had a significant effect (sampling sites as a covariate in ANCOVA) on the elements Cd, Co, and Ni (Fig. 3a, b). According to adjusted means from ANCOVA, with no spatial variability, the highest concentration of As, Cr, Cu, Mn, and Pb and concentrations of Cu, Fe, Hg, Mn, Pb, and Zn were recorded for bream and barbel, respectively.
The BCF results are given in Table 3. The estimated BCF values in the first group of fish species examined (pikeperch, bream, and catfish) confirmed that bream and catfish have a similar affinity for the elements Cd, Co, Ni, and Zn, which is distinctly higher than that observed for pikeperch. On the other hand, in the second group of species examined (chub, barbel, and nase), the dominant species for the bioconcentration of elements was barbel.
Since the ANCOVA showed that only the concentrations of Cd, Co, and Ni showed significant spatial variation, we decided to exclude those metals from any further calculations of MPI. The MPI values observed in this study were different between fish species (Fig. 4). The Kruskal-Wallis test revealed no significant differences between the MPI values calculated for pikeperch, bream, and catfish (p < 0.05). On the other hand, the MPI calculated for chub, barbel, and nase significantly differed (p < 0.05). The post hoc Mann-Whitney test showed that the level of MPI calculated for barbel was significantly higher at all sites (p < 0.05), except for site P. Having all of this in mind, since there were no statistically significant differences in the MPIs in the first group of fish species examined, the highest MPIs can be considered as relevant for comparison of the sampling sites. In the second group, according to the MPIs, barbel proved to have significantly higher MPI values for almost all of the sampling sites (except sampling site P), so the values for this fish species were used for comparison.
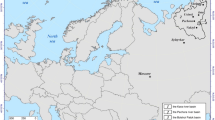
According to the computed MPI data, one site on the Morava River is highly polluted (WM1, MPI = 1.92), the majority of sites reveal intermediate pollution status (MPI 0.36–0.73), and the section designated on the Tisa River is minimally affected by metal pollution (MPI = 0.31).
The As, Fe, Cu, and Zn concentrations detected in all of the fish muscle were below the MPC established by both the EU (EC 2006) and the Republic of Serbia (Official Gazette of RS 2011). Hg concentrations exceeded the MPC only in catfish samples (0.62 mg kg−1) from D3 and barbel (0.78 mg kg−1) from WM1 sampling sites, while Cd concentrations exceeded the MPC in catfish samples (0.09 mg kg−1) from D1 and chub samples (0.1 mg kg−1) from SM2 sampling sites. We would emphasize that the Pb level exceeded the MPC at most sampling sites (WM1, WM2, I, DR, and GT) with the highest concentration of Pb in barbel from WM1 sampling site (0.82 mg kg−1).
Discussion
The chosen species—pikeperch, bream, and catfish—are keystone populations of commercial fish species from large lowland rivers, the Danube, Sava, and Tisa, while chub, barbel, and nase are keystone populations from other rivers researched in Serbia (Simić et al. 2014). In our study, muscle was chosen as a target tissue since EU limits are fixed to heavy metal concentrations in fish muscle, which are most relevant in the context of fish consumption. Another reason we focused on muscle concentrations was to make the results relevant for comparable and sustainable management.
We hypothesized that the concentrations of heavy metals in the water would affect the heavy metal concentrations in the fish muscle. Poleksić et al. (2010) pointed out that elemental concentrations in fish organs depend on the actual level of pollution in the water body they inhabit, and studies of elemental accumulation in fish tissues are able to indicate the actual elemental levels in their environment. On the other hand, Andres et al. (2000) and Yi and Zhang (2012) stated that direct proportionality does not necessarily exist between water concentrations and bioaccumulation levels in aquatic organisms. The results of ANCOVA in our study showed that the concentrations of several elements varied markedly for the fish species examined. According to Dušek et al. (2005) and Zrnčić et al. (2013), predator species revealed important indicator potential even when young for the maximum content of Hg accumulated in muscle tissue, which is also seen in this study. Subotić et al. (2013b) stated that there was no difference in the concentration of any element examined (As, Cu, Fe, Hg, Mn, and Zn) between the pikeperch and catfish muscle for the fish of the same length as in our study, which was not the case here. Our study showed that, as a predatory fish, situated at the top of the food chain, catfish (S. glanis) can adequately reflect ambient metal concentrations as seen in the research of Squadrone et al. (2013). In biomonitoring studies of variations in heavy metal pollution, bream (A. brama) proved to be a useful bioindicator species (Farkas et al. 2003). Generally, our findings support the thesis of Zrnčić et al. (2013) that it is not necessarily the case that predatory fish are the best indicator species. Poleksić et al. (2010) and Sunjog et al. (2012) confirmed the value of barbel as an indicator species for the status of water bodies. The best sentinel species should be the most abundant and ubiquitous ones, such as the species studied here. Additionally, our results concerning the bioaccumulation of elements and the MPI index pointed toward bream and barbel as good candidates for the further establishment of pollution monitoring. Since the element load of the water column from the sampling sites proved to be relatively low, the results for metal concentrations in bream and barbel are another confirmation that metal uptake from food is predominant (Dallinger and Kautzky 1985). It can be seen from the results that fish that feed on invertebrates which have absorbed elements from the water, and additionally from the sediment, have the highest tendency for the uptake of those elements.
A survey of the existing published data focusing on element concentrations in fish species in Serbia indicated that many fish species, tissues, and organs have been studied, so far. Visnjic-Jeftic et al. (2010) assessed Al, Cd, Cu, B, Ba, Fe, Mg, Sr, Zn, Li, Co, Cr, Mn, Mo, Ni, and P in the muscle, liver, and gills of pontic shad (Alosa immaculata Benet 1835) and found the highest As concentration in the muscle, and Cd and As above the MPC. Sterlet (Acipenser ruthenus) tissue liver, gills, intestines, and muscle were used in a study by Jarić et al. (2011), in which Ag, Al, As, B, Ba, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Se, Sr, Zn, and Li were analyzed, and the muscle showed the lowest tendency for element accumulation, although the level of Cd partly exceeded the MPC. According to Subotić et al. (2013a, b), only Hg and Zn concentrations were above the MPC in the muscle tissue of pikeperch (S. lucioperca), catfish (S. glanis), burbot (Lota lota), common carp (Cyprinus carpio), and gobies (Neogobius gymnotrachelus and Neogobius melanostomus). Compared with the same species in our study, those authors found higher concentrations for almost all of the elements (Al, As, Cd, Fe, Hg, Mn, Zn in pikeperch samples and Al, As, Cd, Fe, Hg, Mn, Zn in catfish samples). Jovičić et al. (2015) also used 14 tissues from catfish (S. glanis) of the same lengths as in our study for element estimations (As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Se, and Zn), but all of the element concentrations were below the MPC. The concentrations of As, Co, Cu, Fe, Hg, Mn, Ni, and Zn were higher; the concentrations of Cd and Pb were lower, while the concentration of Cr was approximate to the concentration in our study. The widest range of fish species, silver carp (Hypophtalmichtys molitrix), freshwater bream (A. brama), white bream (Blicca bjoerkna), common carp (C. carpio), and catfish (S. glanis), were used in a study by Lenhardt et al. (2012) in which the accumulation of Al, As, B, Ba, Cd, Co, Cr, Cu, Fe, Li, Mn, Mo, Ni, Pb, Se, Sr, and Zn was analyzed in the liver, muscle, gills, and gonads, and only Fe and Zn concentrations were above the MPC. The levels of Al, Fe, Mn, and Zn in bream muscle and Cu, Fe, Mn, and Zn in catfish muscle were higher than in our study. This is very important since the length of the fish examined in the abovementioned study was lower than in our study. All of the studies mentioned here were conducted in the Danube River, mostly near the capital city of Belgrade.
The type of chemical, metabolic properties of the tissues and the degree of environmental pollution affect the bioaccumulation levels of fish (Prosi 1981). As expected, this study indicates the highest BCFs for Hg (Table 3), which was also confirmed in the studies by Subotić et al. (2013a, b), Dušek et al. (2005), Has-Schön et al. (2008), and Nabavi et al. (2012), who also stated that Hg is accumulated in the highest concentrations in muscle tissue. The research presented here has also shown that water was a good source of Fe in the West Morava River and Zn in the South Morava and rivers from East Serbia such as the Great Timok, Mlava, and Pek. Generally, predatory species had the highest BCF for Fe, as can be seen in a study by Subotić et al. (2013a).
The MPI is more reliable for expressing the contamination status of fish than the accumulation patterns since it considers all metals synchronously, unlike the BCF. The estimated MPI values for the muscle of pikeperch and catfish in the present study (Fig. 4) were lower than those reported by Subotić et al. (2013b) for the same species of the same length as in our study (0.840 and 0.907, respectively), which were estimated based on the analysis of six elements (As, Cu, Fe, Hg, Mn, and Zn). In a study by Jovičić et al. (2015), the MPI values for the muscle of catfish, of the same length as in our study, calculated for 11 elements (As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Zn), were lower than 0.1. Considering that the MPI is influenced by the elements that are used for its calculation, this might explain the differences between ours and the two studies mentioned above. One more of the factor affecting the MPI of fish is weight (Subotić et al. 2013a, b).
Using the MPI index, we proposed a water quality classification for the main rivers in Serbia (Table 4). The classification showed spatial patterns of the elements, which led to the identification of “site unaffected by direct pollution,” “slightly affected,” or “affected” sites among the main rivers in Serbia. According to this classification, the Tisa River can be categorized as site unaffected by direct pollution while the West Morava and Pek can be categorized as affected. Other rivers are slightly affected. As a result of this water quality classification, a contamination map of Serbia was formed (Fig. 5). Based on previous studies by Teodorović (2009) who stated that heavy metals are considered to be among the major pollutants in the Danube River in Serbia, especially pronounced between Novi Sad and Belgrade (the two largest cities), and Sakan et al. (2013) who demonstrated the high ecological risk and need for environmental monitoring in the Tisa River, supporting the development of an efficient strategy to reduce local pollution and contamination, we expected a high level of fish contamination in our study. Surprisingly, this was not the case, and those sites were slightly or moderately affected with metal pollution. Sakan et al. (2015) found that the most polluted sediment samples from the West Morava which originated from industrial plants and mines, and agricultural products, were mainly due to the use of manganese-containing products, such as fertilizer and fungicide. This has probably led to the high MPI values of the barbel studied in this river, and it is another proof that the accumulation of elements in fish is mainly derived from sediment acting as an adsorptive sink with metal concentrations often being many times greater than in the water column (Salomons and Förstner 1980) and sediment-dwelling organisms, because they can scavenge some elements from sediment.
Our study showed that Hg concentrations exceeded the prescribed MPC in the Danube (sampling site D3), which is in accordance with Subotić et al. (2013b) who stated the same for catfish of a similar size to those in our study. Elevated concentrations of Cd were also found in the Danube (sampling site D1). Jovičić et al. (2014) stated that fish with excess concentrations of Hg and Cd from the Danube can be utilized in the human diet only in limited quantities and that it is necessary to establish permanent monitoring of heavy metal concentrations in this area. Since Hg and Cd concentrations were above the MPC in West Morava (sampling site WM1) and South Morava (sampling site SM2), respectively, as were Pb concentrations in the West Morava (sampling sites WM1 and WM2), Ibar, Drina, and Great Timok, we suggest that fishing in these rivers should be limited, because of the potential health risk. Our findings also suggest that the current lack of legal limits for a number of metals in European and Serbian legislation should be tackled as soon as possible, in order to enable more efficient control of contaminants in fish products. In the future, it will be important to focus efforts on defining the appropriate quantities of fish meat to be used in the human diet.
To conclude, this study clearly indicates that the MPI is a reliable index for expressing the contamination status of fish, giving a clear picture of pollution. Therefore, our opinion is that it is possible for MPI to be included in complex freshwater monitoring programs. Also, more than one fish species should be analyzed in comparative environmental studies due to substantial variations in pollution loads. The benthophagous species bream and barbel, preying close to the bottom, were recognized as susceptible indicators of the environmental gradient of element pollution. The predator species catfish revealed important indicator potential because it resides in muddy littoral. This study achieved its primary objective and produced a contamination map of Serbia as a basis for further research. Over the past two decades, Serbia has not expanded its industrial activity, which has resulted in the barely noticeable anthropogenic input of heavy metals in the rivers close to industry, and the main rivers are mostly slightly affected. On the other hand, the West Morava River and Pek can be marked as polluted when it comes to heavy metals and elements. Also, we assumed that pollution by heavy metals in the 1990s was trapped in the sediment, resulting in an increased concentration of elements in the species that live and feed on the bottom. Therefore, the proposed contamination map presents the current state of the entire ecosystem. Future studies should involve similar analyses that would include more fish species and focus especially on smaller rivers that have been neglected so far.
References
Alibabić V, Vahcić N, Bajramović M (2007) Bioaccumulation of metals in fish of Salmonidae family and the impact of fish meat quality. Environ Monit Assess 131(1–3):349–364
Andres S, Ribeyre F, Tourencq J-N, Boudou A (2000) Interspecific comparison of cadmium and zinc contamination in the organs of four fish species along a polymetallic pollution gradient (Lot River, France). Sci Total Environ 248(1):11–25
Braune B, Muir D, DeMarch B, Gamberg W, Eamer J, Elkin B, Evans M, Grundy S, Hebert C, Johnstone R, Kidd K, Koenig B, Lockhart L, Marshall H, Reimer K, Sanderson J, Shutt L (1999) Spatial and temporal trends of contaminants in Canadian Arctic freshwater and terrestrial ecosystems: a review. Sci Total Environ 230:145–207
Burger J, Gaines KF, Boring CS, Stephens WL, Snodgrass J, Dixon C, McMahon M, Shukla S, Shukla T, Gochfeld M (2002) Metal levels in fish from the Savannah River: potential hazards to fish and other receptors. Environ Res 89(1):85–97
Čelechovská O, Svobodová Z, Žlábek V, Macharáčková B (2007) Distribution of metals in tissues of the common carp (Cyprinus carpio L.). Acta Vet Brno 76:S93–S100
Chovanec A, Hofer R, Schiemer F (2003) Fish as bioindicators. Trace metals and other contaminants in the environment 6. In: Markert BA, Breure AM, Zechmeister HG (eds) Bioindicators and biomonitors. Elsevier, Amsterdam, pp 639–677
Dallinger R, Kautzky H (1985) The importance of contaminated food and uptake of heavy metals by rainbow trout (Salmo gairdneri): a field study. Oecologia 67:82–89
Djinovic JM, Popovic AR (2007) In situ influence of coal ash dump on the quality of neighboring surface and ground waters by applying correlation statistic analysis. Fuel 86(1–2):218–226
Dušek L, Svobodová Z, Janoušková D, Vykusová B, Jarkovský J, Šmíd R, Pavliš P (2005) Bioaccumulation of mercury in muscle tissue of fish in the Elbe River (Czech Republic): multispecies monitoring study 1991–1996. Ecotoxicol Environ Saf 6(2):256–267
European Commission Regulation (2006) Setting maximum levels for certain contaminants in foodstuffs, Official Journal of the European Union No 1881/2006
Farkas A, Salánki J, Specziár A (2003) Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res 37(5):959–964
Fernandes C, Fontaínhas-Fernandes A, Cabral D, Salgado MA (2008) Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-Paramos lagoon, Portugal. Environ Monit Assess 136(1–3):267–275
Food and Agriculture/World Health Organisation (FAO/WHO) (1972) Evaluation of certain food additives and the contaminants mercury, cadmium and lead, WHO Technical Report Series No. 505. WHO, Geneva
Hardisty MW, Karta S, Sainsbury M (1974) Dietary habits and heavy metal concentrations in fish from Severn Estuary and Bristol Channel. Mar Pollut Bull 5:61–63
Has-Schön E, Bogut I, Kralik G, Bogut S, Horvatić J, Cacić I (2008) Heavy metal concentration in fish tissues inhabiting waters of “Buško Blato” reservoir (Bosna and Herzegovina). Environ Monit Assess 144(1–3):15–22
Hutton M (1987) Human health concerns of lead, mercury, cadmium and arsenic. In: Hutchinson TC, Meema KM (eds) Lead, mercury, cadmium and arsenic in the environment. Wiley, Chichester
Jarić I, Višnjić-Jevtić Z, Cvijanović G, Lj J, Skorić S, Lenhardt M (2011) Determination of differential heavy metal and trace element accumulation in liver, gills, intestine and muscle of sterlet (Acipenser ruthenus) from Danube River in Serbia by ICP-OES. Microchem J 98(1):77–81
Jovičić K, Lenhardt M, Višnjić-Jeftić Ž, Đikanović V, Skorić S, Smederevac-Lalić M, Jaćimović M, Gačić Z, Jarić I, Hegediš A (2014) Assessment of fish stocks and elemental pollution in the Danube, Sava and Kolubara Rivers on the Territory of the City of Belgrade. Serbia Acta zool bulg 7:179–184
Jovičić K, Nikolić DM, Višnjić-Jeftić Ž, Đikanović V, Skorić S, Stefanović SM, Lenhardt M, Hegediš A, Krpo-Ćetković J, Jarić I (2015) Mapping differential elemental accumulation in fish tissues: assessment of metal and trace element concentrations in wels catfish (Silurus glanis) from the Danube River by ICP-MS. Environ Sci Pollut Res 22(5):3820–3827
Lamas S, Fernández JA, Aboal JR, Carballeira A (2007) Testing the use of juvenile Salmo trutta L. as biomonitors of heavy metal pollution in freshwater. Chemosphere 67(2):221–228
Lau S, Mohammed MA, Yen TC, Su'ut S (1998) Accumulation of heavy metals in fresh water molluscs. Sci Total Environ 214:113-121
Lenhardt M, Jarić I, Višnjić-Jevtić Ž, Skorić S, Gačić Z, Pucar M, Hegediš A (2012) Concentrations of 17 elements in muscle, gills, liver and gonads of five economically important fish species from the Danube River. Knowl Managt Aquatic Ecosyst 407:02
Liu J, Zhang XH, Tran H, Wang DQ, Zhu YN (2011) Heavy metal contamination and risk assessment in water, paddy soil, and rice around an electroplating plant. Environ Sci Pollut Res 18(9):1623–1632
McCoy CP, O’Hara TM, Bentt LW, Boyle CR (1995) Liver and kidney concentrations of zinc, copper and cadmium in Channel catfish (Ictalurus punctatus): variations due to size, season and health status. Vet Human Toxicol 37:11–15
Nabavi SF, Nabavi SM, Latifi AM, Eslami S, Ebrahimzadeh MA (2012) Determination of trace elements level of pikeperch collected from the Caspian Sea. Bull Environ Contam Toxicol 88(3):401–405
Official Gazette of RS (2005) Fisheries Law
Official Gazzette of RS (2011) Regulation on quantity of pesticides, metals, metalloids, and other toxic substances, chemotherapeutics, anabolics, and other substances which can be found in food. Official Gazzette of RS No 28/2011
Pawellek F, Frauenstein F, Veizer J (2002) Hydrochemistry and isotope geochemistry of the upper Danube River. Geochim et Cosmochim Acta 66(21):3839–3854
Phillips DJH (ed) (1980) Quantitative aquatic biological indicators. Applied Science Publishers, London
Poleksić V, Lenhardt M, Jarić I, Djordjević D, Gačić Z, Cvijanović G, Rašković B (2010) Liver, gills and skin histopathology and heavy metals content of the Danube sterlet (Acipenser ruthenus Linnaeus, 1758). Environ Toxicol Chem 29:515–521
Prosi F (1981) Heavy metals in aquatic organisms. In: Förstner U, Wittmann GTW (eds) Berlin. Springer, Heidelberg, New York, pp 271–323
Ricart M, Guasch H, Barcelό D, Brix R, Conceiçào MH, Geiszinger A, de Alda MJL, Lόpez-Doval JC, Muñoz I, Postigo C, Romani AM, Villagrasa M, Sabater S (2010) Primary and complex stressors in polluted Mediterranean rivers: pesticide effects on biological communities. J Hydrol 383(1–2):52–61
Sakan S, Sakan N, Đorđević D (2013) Trace element study in Tisa River and Danube alluvial sediment in Serbia. Int J Sediment Res 28(2):234–245
Sakan S, Dević G, Relić D, Anđelković I, Sakan N, Đorđević D (2015) Evaluation of sediment contamination with heavy metals: the importance of determining appropriate background content and suitable element for normalization. Environ Geochem Health 37(1):97–113
Salomons W, Förstner U (1980) Trace metal analysis on polluted sediments: part II: evaluation of environmental impact. Environmental Technology 1(11):506–517
Simić V, Simić S, Stojković Piperac M, Petrović A, Milošević D (2014) Commercial fish species of inland waters: a model for sustainability assessment and management. Sci Total Environ 497–498:642–650
Smederevac-Lalić M, Višnjić-Jeftić Ž, Pucar M, Mićković B, Skorić S, Nikčević M, Hegediš A (2011) Fishing circumstances on the Danube in Serbia. Water Research and Management 1(4):45–49
Smederevac-Lalić M, Pešić R, Cvejić S, Simonović P (2012) Socio-economic features of commercial fishery in the bordering upper Danube River area of Serbia. Environ Monit Assess 184(5):2633–2646
Squadrone S, Prearo M, Brizio P, Gavinelli S, Pellegrino M, Scanzio T, Guarise S, Benedetto A, Abete MC (2013) Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere 90(2):358–365
Subotić S, Spasić S, Višnjić-Jeftić Z, Hegediš A, Krpo-Ćetković J, Mićković B, Skorić S, Lenhardt M (2013a) Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotoxicol Environ Saf. 98:196–202
Subotić S, Višnjić Jeftić Ž, Spasić S, Hegediš A, Krpo-Ćetković J, Lenhardt M (2013b) Distribution and accumulation of elements (As, Cu, Fe, Hg, Mn, and Zn) in tissues of fish species from different trophic levels in the Danube River at the confluence with the Sava River (Serbia). Environ Sci Pollut Res 20(8):5309–5317
Sunjog K, Gačić Z, Kolarević S, Višnjić-Jeftić Z, Jarić I, Knežević-Vukčević J, Vuković-Gačić B, Lenhardt M (2012) Heavy metal accumulation and the genotoxicity in barbel (Barbus barbus) as indicators of the Danube River pollution. Sci World J 2012:351074
Teodorović I (2009) Ecotoxicological research and related legislation in Serbia. Environ Sci Pollut Res 16(1):S123–S129
Teodorović I, Đukić N, Maletin S, Miljanović B, Jugovac N (2000) Metal pollution index: proposal for freshwater monitoring based on trace metal accumulation in fish. Tiscia 32:55–60
Terra BF, Araújo FG, Calza CF, Lopes RT, Teixeira TP (2008) Heavy metal in tissues of three fish species from different trophic levels in a tropical Brazilian river. Water Air Soil Pollut 187:275–284
Triebskorn R, Telcean I, Casper H, Farkas A, Sandu C, Stan G, Colărescu O, Dori T, Köhler HR (2008) Monitoring pollution in River Mureş, Romania, part II: metal accumulation and histopathology in fish. Environ Monit Assess 141(1–3):177–188
Uchida M, Hirakawa Y, Inoue T (1961) Biochemical studies on Minamata disease IV. Isolation and chemical identification of the mercury compound in the toxic shellfish with special reference to the causal agent of the disease. Kumamoto Medical Journal 14:181–184
Usero J, González-Regalad E, Gracia I (1997) Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ Int 23(3):291–298
Uysal K, Köse E, Bülbül M, Dönmez M, Erdoğan Y, Koyun M, Ömeroğlu C, Özmal F (2009) The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ Monit Assess 157:355–362
van der Schalie WH, Shedd TR, Knechtges PL, Widder MW (2001) Using higher organisms in biologically early warning systems for real time toxicity detection. Biosens Bioelectron 16(7–8):457–465
Visnjic-Jeftic Z, Jaric I, Lj J, Skoric S, Smederevac-Lalic M, Nikcevic M, Lenhardt M (2010) Heavy metal and trace element accumulation in muscle, liver and gills of the Pontic shad (Alosa immaculate, Bennet 1835) from the Danube River (Serbia). Microchem J 95(2):341–344
Yi YJ, Zhang SH (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19(9):3989–3996
Zrnčić S, Oraić D, Ćaleta M, Mihaljević Ž, Zanella D, Bilandžić N (2013) Biomonitoring of heavy metals in fish from the Danube River. Environ Monit Assess 185(2):1189–1198
Acknowledgments
This study was supported by the Ministry of Education, Science, and Technological Development of the Republic of Serbia, Project No. TR31011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Milošković, A., Dojčinović, B., Kovačević, S. et al. Spatial monitoring of heavy metals in the inland waters of Serbia: a multispecies approach based on commercial fish. Environ Sci Pollut Res 23, 9918–9933 (2016). https://doi.org/10.1007/s11356-016-6207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6207-2