Abstract
Leachates generated in methanogenic landfills contain high strength of ammonium nitrogen which removal is hard to be accomplished by means of conventional techniques. The chemical precipitation of struvite, which is a mineral that could be reused as a slow-release fertilizer, is an effective process in the removal and recovery of NH4 amount of high-concentrated wastewaters. In this paper, a struvite precipitation process using unconventional reagents is proposed for a sustainable recovery of nitrogen content. In particular, seawater bittern, a by-product of marine salt manufacturing, and bone meal, a by-product of the thermal treatment of meat waste, have been used as low-cost sources of magnesium and phosphorus, respectively. The process enables the removal of more than 98 % ammonia load, the recovery about 99 and 95 % of phosphorus and magnesium, respectively, and the production of a precipitate containing struvite crystals. Heavy metals concentrations of produced precipitate were below the threshold values specified by the EC Directive for use of sewage sludges as fertilizers. Specific agronomic tests were conducted to investigate the fertilizing value of precipitate recovered from landfill leachate. The fertilizing effect of struvite deposit in cultivating Spinacia oleracea was compared with that of vegetable soil and commercial fertilizer. The growth of selected vegetable in the pots with struvite precipitate resulted significantly greater in both than those in the control pots and in the pots with the complex fertilizer. Furthermore, the struvite application as fertilizer did not result in more heavy metals in the vegetables respect those from soil and model fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill leachate treatment is an important issue of the waste management system in municipal areas (He et al. 2007; Kabdaşli et al. 2008; Kim et al. 2007). Their quantity and quality depends on a number of factors: the type of deposited wastes as well as the age of the landfill and the phase of waste decomposition. The leachates withdrawn from landfill in methanogenic phase (methanogenic leachates) are characterized by high nitrogen load and large amount of refractory organic compounds with a high chemical oxygen demand/biochemical oxygen demand (COD/BOD) ratio (Kim et al. 2007). Many physicochemical techniques have been proposed for the removal of low biodegradable compounds, among which advanced oxidation processes are very effective (De Rosa and Siciliano 2010; Kumar et al. 2015; Kurniawan et al. 2006; Siciliano et al. 2015, 2016). However, the major issue in landfill leachate treatment is the development of effective and sustainable processes for ammonium removal. The ammonia stripping requires high pH and air flowrates, and it is difficult to operate (Kurniawan et al. 2006). The conventional nitrification–denitrification biological process, widely used for NH4 removal from municipal wastewaters, is not very suitable for treating leachates (He et al. 2007; Siciliano and De Rosa 2015). In fact, the biological treatments require large reaction volumes in order to reduce to a low level the toxicity due to ammonia (Di Iaconi et al. 2010). Furthermore, the high salinity and lack of sufficient electron donors, especially in stabilized leachates, are additional obstacles to nitrification–denitrification biological treatment (Di Iaconi et al. 2010). New biological techniques, such as shortcut nitrification/denitrification and anaerobic ammonium oxidization, have demonstrated potential in solving the above problems, but they are still under study and their control and management are very complex (He et al. 2007). Moreover, conventional processes do not recycle nitrogen compounds as truly sustainable products. The recovery of nitrogen and phosphorus from wastewater in the form of crystalline struvite is found to be a sustainable option because struvite is considered a potential fertilizer (Iqbal et al. 2008; Latifian et al. 2012; Uludag-Demirer et al. 2005; Uysal et al. 2010,2014; Wu and Zhou 2012). Struvite is a white orthorhombic crystalline structure, which is composed of magnesium, ammonium, and phosphate (MgNH4PO4•6H2O) in equal molar concentrations (Karabegovic et al. 2013; Korchef et al. 2011; Saidou et al. 2009). Struvite precipitation occurs when the combination of NH4, Mg, and PO4 concentration exceeds the solubility product under alkaline conditions (Fattah et al. 2008). Because of the low amounts of magnesium and phosphorus relative to ammonia concentrations, large amounts of the reagents are required for the treatment of landfill leachate, which results in an expensive process. Different alternative magnesium compounds, such as the by-products of magnesium oxide (Chimenos et al. 2003; Quintana et al. 2004), magnesite mineral (Gunay et al. 2008), magnesite pyrolysate (Huang et al. 2011) and seawater (Lee et al. 2003), have proved their effectiveness for struvite precipitation. However, the primary cost of the whole treatment is generally represented by the consumption of reactants of phosphorus, which is rarer and much more expensive than magnesium (Di Iaconi et al. 2010). To overcome this issue, the author has identified the bone meal as a low-cost source of phosphorus for struvite precipitation. This compound is a by-product of the thermal treatment of meat waste, and it is usually disposed in landfills because, in Europe, the use of it as a fertilizer is restricted (Coutand et al. 2008; Deydier et al. 2005). The exploitation of bone meal for the struvite precipitation process is particularly meaningful because it would allow recovery of its phosphorus content with the production of a more valuable fertilizer containing both phosphorus and nitrogen. The process developed by the author, together with bone meal, exploits the seawater bittern, a by-product of marine salt manufacturing (Siciliano et al. 2013; Siciliano and De Rosa 2014). However, an examination of the potential agronomic benefits of N recovered as struvite from the landfill leachates using low-cost reactants is still needed. Indeed, the applicability as fertilizer of struvite, generated from the treatment of high polluting wastewaters, is related to its characteristics and purity. In order to assess the fertilizer potential of struvite, a first set of experiments were planned to indentify the operating conditions for the treatment of leachate by exploiting unconventional reagents, whereupon, a series of germination tests were conducted on Spinacia oleracea to evaluate the agronomic potential of produced struvite as compared with that of a commercial fertilizer.
Description of experiments
Materials
The experiments for struvite precipitation were conducted on a leachate sample collected from a municipal solid waste landfill site near Cosenza, in southern Italy. The sample was withdrawn from a storage tank and kept in a 25 L container at 4 °C. The parameters concentrations were typical of leachates withdrawn from a landfill in methanogenic phase (Table 1). In fact, a pH of about 8.2 and high values of alkalinity and conductivity were monitored. The COD amount was about of 4 g/L, while the ammonium nitrogen resulted in approximately 2.35 g/L. Compared with this value, the Mg and P-PO4 contents were extremely lower. Obviously, remarkable concentrations of alkaline elements, especially Na and K, and lower amounts of some metals, such as Fe and Al, were measured. The leachate sample contained trace quantities of other heavy metals, including Zn, Mn, Pb, and Cu, while Cd, Cr, Co, Hg, and As concentrations were below the detection limits.
The struvite precipitation process was conducted using bone meal and seawater bittern as sources of magnesium and phosphorus, respectively (Siciliano et al. 2013, 2015). The samples of bittern were supplied by a small enterprise, sited on Ionian coast of Cosenza, which produces seawater NaCl. The bone meal was obtained from an incineration plant equipped with a rotary furnace for the treatment of beef wastes. Magnesium and phosphate were properly solubilized before carrying out the experimental runs. The bittern was simply dissolved by mixing 50 g into 50 mL of tap water. To dissolve the phosphorus content of bone meal, 38 g of powder were mixed with 100 mL of 3 N sulphuric acid for 2 h. After this period, the mixture was centrifuged at 4000 rpm for 15 min to separate the remaining solids particles. The characteristics of solutions obtained from the solubilization of low-cost reactants are reported in Table 2. Particularly high concentrations of about 69 g Mg/L and 80 g P-PO4/L were reached for bittern and bone meal solutions, respectively. Remarkable amounts of Ca, Na, and K, especially from bittern solubilization, were also detected. For bone meal, the solubilized Ca was much lower than the calcium content of powder. Indeed, by using sulphuric acid as solubilizing reactant, the calcium amount remains mainly in insoluble form as calcium sulfate. A certain quantity of iron and zinc ions were also transferred into the solution of bone meal.
The agronomic tests were conducted on Spinacia o. using both the struvite produced trough the treatment of landfill leachate and a solid commercial fertilizer. Soil for vegetables was used as raw media for germination and growth of selected plant. The characteristics of soil and commercial fertilizer declared by suppliers are reported in Tables 3 and 4.
Struvite precipitation tests
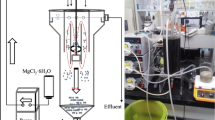
The tests were performed to establish the required dosages of reagents for maximizing the ammonia removal and the struvite production from the landfill leachate samples used in this study. In particular, R MN (Mg/N molar ratio) and R PN (P/N molar ratio) values of 1, 1.1, and 1.3 were tested. Each test was conducted in batch mode, at room temperature (20–22 °C) and in a 0.5 L glass flask, equipped with a thermometer and a pH meter. The reaction mixture was prepared by adding volumes of concentrated solutions of magnesium and phosphorus to the leachate samples to obtain the stoichiometric ratios established for the experiments (Table 5). After the dosage of reactants, the pH was set to 9, considered as optimal for struvite production (Kabdaşli et al. 2009, Kim et al. 2007, Gunay et al. 2008), using NaOH 10 N (Table 5). Each test was performed on a leachate volume of 0.25 L, magnetically stirred at 300 rpm for 15 min. After this period, the mixture was left to settle for 15 min to allow the precipitation of the insoluble compounds. The supernatant was withdrawn and filtered through a 0.45-μm filter before its chemical characterization. Once the optimal operating conditions were identified, a further test was conducted on a greater volume of landfill leachate, in order to obtain enough amount of struvite to be used in agronomic tests. In particular, 4.8 L of leachate were treated in a 10 L PVC container that was mixed by a vertical mechanical stirrer. The precipitate produced by leachate treatment was washed with about 2 L of deionized water, filtered at 0.45 μm, and dried at room temperature. This procedure prevented struvite decomposition during the drying phase.
Plant growth tests
The fertility of struvite produced from landfill leachate was investigated in a set of greenhouse experiments conducted on Spinacia o.. Rectangular plastic pots (height 10 cm, upper width 10.5 cm, bottom width 8 cm, upper length 28 cm, bottom length 23 cm) were used for these tests. The growth of plant was tested in three different conditions: (1) vegetable soil, without fertilizer, as control; (2) vegetable soil with addition of struvite; and (3) vegetable soil with model fertilizer. For each condition, three samples were prepared. Therefore, a total of nine pots were set up for the present plant growth tests. In each test, a total amount of 0.3 g of spinach seeds were provided. The dosages of struvite and commercial fertilizer were established to achieve an equivalent concentration of 12 gN/m2. In the cultivation of plants, following steps were implemented as reported in the literature (Li and Zhao 2003; Yetilmezsoy and Sapci-Zengin 2009) : (1) each plastic pot was filled with the vegetable soil media (Table 3); (2) about 2 cm of top layer was removed from the top; (3) for samples with fertilizers, pre-weighted chemicals (struvite or commercial fertilizer) were mixed with the removed soil media; (4) pre-weighted plant seeds (0.3 g) were laid evenly on the excavated surface; (5) plant seeds were covered with the removed solid media, eventually mixed with fertilizers; (6) 200 mL of water were evenly added; and (7) finally the pots were placed outdoor. With the exception of rainy periods, each pot has been irrigated twice a week with 200 mL of tap water. Length of leaves was periodically recorded in each pot. After 46 days, the plants were harvested and weighed before and after drying at 105 and 550 °C to determine the fresh, the dry, the inert, and volatile weights. As reported in the literature (Li and Zhao 2003), the spinach plants were sprayed with distilled water to wash out the dust prior to harvesting. The level of nutrient and metals in fresh and dry vegetables were determined. Moreover, also the nutrient amount into the soil, after the plants harvesting, was carried out.
Analytical methods
On liquid samples, the salinity and pH were measured by a multiparametric analyzer; chemical oxygen demand (COD) and alkalinity were estimated by titration methods, ammonium nitrogen was determined by Kjeldahl procedures, and reactive phosphorus was measured by colorimetric method using an UV spectrophotometer. Atomic adsorption spectrophotometry was used for determination of metallic elements (APHA 1998).
The analysis on solid samples was carried out after acid digestion. In a typical procedure, 1 g of sample was placed into a Pyrex tube provided with water-refrigerating coil, containing 50 mL of 1 N HCl. The mixture was heated at 70 °C for 1 h. After the temperature cooled down naturally, 10 mL of HNO3 1 N was added into the tube and manually mixed. The mixture was heated again for 1 h and then left cooled at room temperature. The digested mixture was centrifuged at 4000 rpm for 15 min, then the supernatant was filtered at 45 mm prior to analyze. The inert and volatile solids were measured by drying the sample at 105 and 550 °C. The precipitate produced through the treatment of landfill leachate was analyzed by X-ray diffraction and scanning electron microscopy (SEM) to analyze crystal morphology.
Presentation of the results
The analysis was repeated four times, and the data was expressed as mean value and standard deviation. The results of abatements were representative of the actual removal of pollutant amounts; thus, the values of the reported efficiencies were not affected by dilution due to reactant addition in the various processes.
Results and discussion
Struvite precipitation tests
To verify the optimal amounts of chemical reagents for ammonia removal by struvite precipitation, the R MN and R PN molar ratios were changed between 1 and 1.3. The ammonia abatement was close to 77 % in the test conducted with the theoretical stoichiometric ratio N:P:Mg = 1:1:1 and a remarkable enhancement, up to yields about 95 %, was obtained by increasing the dosages of phosphorus and magnesium up to 1.1 (Table 6). The need for greater availability of magnesium and phosphorus than the theoretical stoichiometric requirement is attributable to the possible formation, in addition to struvite crystals, of other magnesium and phosphorus insoluble compounds. Indeed, these compounds subtract Mg and PO4 ions from the solutions; thus, an excess of reactants must be dosed to avoid the reduction of struvite nucleation. Other works stated the positive effect on treatment efficiency of R MN and R PN values higher than the theoretical ratio (Çelen and Türker 2001; El Diwani et al. 2007; Lee et al. 2003). However, ammonia abatements of about 90 % were also reached in studies where analytical-grade reagents amounts with N:P:Mg of 1:1:1 were used (Kabdaşli et al. 2008; Li and Zhao 2003). These differences depend by several factors, such as the composition of the investigated wastewater, the type of reagents used, and the pH and ionic strength. The experimental results showed, anyhow, the negligible augmentation in N-NH4 removal with R MN and R PN values higher than 1.1 (Table 6). The recovery of added PO4 was about 96 % using R PN = 1, while it resulted close to 99 % during the test conducted with high amounts of reactants. The higher recovery efficiency for phosphorus, compared to that detected for ammonia removal, confirms the precipitation of some other types of phosphates (Le Corre et al. 2005; Pastor et al. 2010). The highest recovered amount of magnesium was close to 94 % (Table 6) in the tests conducted with R MN ratio of 1.1, and it decreased by increasing the magnesium dosage. In particular, it was detected the lower recovery efficiency for Mg when it was dosed in excess respect the dosage of phosphorus. This is due to the fact that, at the pH = 9, the mainly insoluble compounds of Mg are formed in conjunction with phosphates (Lee et al. 2003); thus, an excess of Mg ions respect the PO4 availability is hardly recoverable. Anyhow, if compared with the percentage of phosphorus recovery, the lower efficiencies for magnesium, detected in each operating conditions, are indicative, probably, of the formation also of some complexes able to slightly reduce the precipitation of Mg. On the basis of these results, the ratio N:P:Mg = 1:1.1:1.1 was considered as optimal for struvite production from the leachate treated in this work.
By using this ratio, a further test was carried out by treating a higher leachate volume of 4.8 L. The yields observed in this test were even slightly higher than those obtained by treating a lower volume of leachate. In fact, it was detected an ammonium removal of about 98 %, and the recovery yields for phosphorus and magnesium resulted close to 99 and 95 %, respectively. This could be attributable to the more efficient mixing conditions guaranteed by the mechanical stirring adopted for this experiment that, probably, promotes the struvite crystallization. A total quantity of dried precipitate close to 303 g was produced from this treatment. The characterization of precipitate and supernatant permitted to verify the substantial matching between the initial N-NH4 mass on leachate (Table 7, Mi column) and the overall amounts detected after the treatment on residual liquid and solid phase (Table 7, Mf column). In fact, only a small ammonia volatilization, of about 2 %, occurred during the treatment. This percentage, in agreement with other works (Uysal et al. 2010), proves the occurrence only of extremely low stripping effect during the treatment and confirms the effectiveness of the operating conditions identified.
The SEM images of recovered precipitate showed mainly orthorhombic crystals, typical of struvite, with size between 10 and 40 μm (Fig. 1). The X-ray diffractogram (Fig. 2) confirmed the production of struvite crystals. In fact, the position and intensity of many peaks matched those of the standard diffractogram of pure struvite. Anyhow, some strong peaks, such as those at 2θ of 11.2, 13.2, and 26.6, suggest the presence of other crystalline compounds. In order to identify these elements, the diffractogram of produced precipitate has been compared, using the JCPDS data (1988), to the standard of several compounds. This analysis allowed to identify, as possible further crystalline compounds, the magnesium phosphate (Mg3(PO4)2 ⋅8H2O) and potassium metaphosphate (KPO3). Indeed, some of the peaks of these elements match with those above mentioned (Fig. 2). No significant correspondences have been detected with the standard of other compounds potentially present in the precipitate, such as calcium salts or metal hydroxides.
The amounts of ammonium, phosphorus, and magnesium into the dried precipitate were equal to 35.8 mgN/g, 89.6 mgP/g, and 66.1 mgMg/g, respectively (Table 7). Thus, the N:P:Mg molar ratio was about 1:1.13:1.07. This value, if compared with the theoretical ratio for struvite (1:1:1), proves the production of other insoluble compounds of phosphorus and magnesium. Moreover, the amount of potassium that was equal to 2.3 mg/g (0.058 n/g) also confirms the presence of potassium compounds (Table 7). The formation of these insoluble salts was probably promoted by the substantial initial amount of potassium in the treating wastewater (molar ratio = 0.35 nK/nN), caused by the remarkable quantities of K ions of raw leachate and of reactants solutions. Some authors hypothesized the presence of MgKPO4 like potassium compound into the precipitate of struvite precipitation process (Huang et al. 2006; Di Iaconi et al. 2010). However, other works stated that the production of MgKPO4 could occur only in the case of low ammonium concentrations (Marti et al. 2010; Pastor et al. 2010). The XR diffractogram discussed above indicates the possible presence only of crystals of some phosphates of magnesium (Mg3(PO4)2 ⋅8H2O) and potassium (KPO3). Therefore, other eventual compounds, such as MgKPO4, could be into the precipitate in amorphous form.
In agreement with many works (Pastor et al. 2010; Le Corre et al. 2005), the analysis of produced precipitate also showed a certain quantity of calcium that resulted equal to 4.1 mg/g (0.102 n/g). Indeed, if Ca ions are available into wastewater to be treated, the insoluble salts of calcium, e.g., hydroapatite (Ca5(PO4)3OH), are commonly detected into the solid produced from struvite precipitation process. Moreover, as reported by Le Corre et al. (2005), when the Ca/Mg molar ratio is higher than 0.5, the formation of struvite crystals could be completely hindered. During the treatment conducted in the present paper, although Ca ions were supplied through the solutions of bittern and bone meal, the Ca/Mg was extremely low (0.04 nCa/nMg). Therefore, the struvite crystallization occurred without interferences and, as underlined by the amount of detected elements, the calcium compounds represent only a small quantity of recovered precipitate (Table 7).
Furthermore, a low quantity of sodium was detected. This suggests that also restricted amount of MgNaPO4 or other insoluble sodium phosphates (Na2HPO4 · 12H2O) can be formed during the struvite precipitation process. Anyhow, by comparing the recovered sodium with the very high initial amount in the treating mixture, it was confirmed the poor production effectiveness of Na salts. Moreover, as aforementioned, the insoluble compounds both of calcium and sodium are formed in amorphous forms in agreement with the findings of other works (Le Corre et al. 2005). By considering the fertilizer potential, in addition to struvite, a certain quantity of Ca, K, and Na into the produced precipitate is a positive aspect. In particular, it is important the presence of potassium compounds, being this element considered a main nutrient for many vegetables. In the application as agricultural fertilizer of precipitate acquired from struvite precipitation, the heavy metal content is, clearly, an important factor to be investigated. Indeed, during the struvite separation, the coprecipitation of heavy metals could occur. In particular, the metals can be incorporated into the crystal lattice or sorbed to the struvite surface (Uysal et al. 2010). The results of these experiments confirm that struvite precipitation process reduces the heavy metal content (Fe, Zn, Mn, Al, Pb, and Cu) of raw leachate (Table 7). However, the amounts detected into the precipitate were below the values reported by the EC Directives for the use of sewage sludge in agriculture (Council of the European Communities 1986). Certainly, the low amount transferred into the recovered solid is due to the characteristics of raw leachate used in this study. On the contrary, in the treatment of acid leachates, which have remarkable metals contents, the level of these elements in the precipitate could be significantly higher. In the present tests, the struvite precipitate has incorporated also a fraction of particulate organic matter that produced a COD reduction of about 15 % into treated leachate. As regards to the other characteristics of treated leachate, the pH dropped from the set value of 9 to 8.4 because of the acidification caused by struvite production. Due to the high precipitation efficiencies, only a slight increase in salinity was observed from the characteristic value of digestate about of 20.6 up to 22.1 mS/cm. This enhancement is attributable mainly to the additions of reactants. Thus, the treatment, using the proposed low-cost reagents for ammonium removal, significantly reduces the overall polluting load of raw wastewater and produces a precipitate with a high fertilizer potential. To verify this agronomic potential, several tests were conducted.
Fertilizing potential of the MAP precipitate
During the greenhouse tests, the plants sprouted for about 7 days. The growth of edible leaves started from the 24th day. After this period, for each pot, the longest leaves representative of the highest plants (Li and Zhao 2003; Yetilmezsoy and Sapci-Zengin 2009) were monitored until the 46th day. The trends reported in Fig. 3 showed how the high sizes of vegetables were obtained in the experiments conducted using struvite as fertilizer. In fact, the leaves reached lengths between 11.1 and 15.5 cm. Instead, using the commercial fertilizer, maximum lengths between 10.4 and 13.9 cm were measured, while, in the control pots, the plants have grown up to lengths between 10.4 and 12.1 cm.
The positive effect of struvite for spinach growth was also confirmed by the wet and dry biomass amounts detected after the plants harvesting. Indeed, in each pot with struvite, the average biomass quantity resulted about twice the value obtained for the control pots (Table 8). This is approximately representative of a doubling of growing rate in the case of struvite utilization. Moreover, greater amounts of biomass, around 30 %, were detected respect that harvested in the pots with the complex fertilizer. The remarkable increase in biomass production detected using struvite is due both to the higher size of plants and to a greater number of vegetables that have grown during the experiments (Fig. 4). According to many authors, the greater biomass yield in MAP-treated plants could be attribute to several factors, such as the long-last availability of nutrients and the reaction with the binding sorbing sites (El Diwani et al. 2007; Ryu et al. 2012). In comparison with the complex fertilizer tested in this study, the struvite produced from the leachate treatment had a greater amount of both phosphorus and magnesium. This higher presence of Mg might also have an important effect on plants synthesis, since the Mg is a component of chlorophyll that acts as an activating agent of enzyme production and P transfer (Li and Zhao 2003).
To evaluate the actual possibility for the exploitation of struvite as fertilizer, the heavy metal levels in vegetables tissue were also analyzed. The data reported in Table 9 show similar values for every sample in the different pots. Therefore, the application of struvite, as a soil fertilizer, does not increase the metals content into vegetables respect the amounts in the plants grown with vegetables soil and commercial fertilizer. Instead, as expected, the analytical results indicate that the vegetables, in the struvite pots, had the greatest amount of phosphorus and magnesium, which represents two valuable constituents of vegetables biomass. The results of plants characterization showed nitrate nitrogen lower than 0.9 g/kg for spinach fertilized with struvite. This value is largely below the threshold value fixed in the EU Regulations (European Commission 2011). Low amounts of nitrate nitrogen were detected also in the upper soil (2 cm from the top) and bottom soil after the vegetables harvesting (Table 10). These restricted values suggest that the slow release of nitrogen content of struvite prevents the occurrence of excessive nitrification phenomena. This is clearly a positive aspect because the NO3 can be easily drained through the soil.
The ammonia nitrogen in the pots with struvite was extremely low and similar to the values detected in the other samples. The low residual ammonium quantity confirms the effective nitrogen uptake by vegetables using struvite as fertilizer.
Due to its phosphorus content, the struvite precipitate caused the higher increase of residual P levels into the soil. Anyhow, the detected values are of the same order of magnitude than the concentrations detected on pots with commercial fertilizer. Based on the results of these experiments, struvite recovered from methanogenic landfill leachate might be an effective fertilizer for spinach cultures. Anyhow, its utilization, likewise of commercial fertilizers, must be accurately planned to avoid land spreading of nutrient, mainly phosphorus compounds.
Analysis of chemical costs
The economic evaluation of the costs for chemicals necessary in the struvite production from treatment of the leachate used in this study (Table 11) was done by analyzing the Italian market (2014 market prices). The price of seawater bittern and bone meal is very difficult to estimate because both are by-products of a limited market. However, being the bittern a waste of marine NaCl production, whose industrial price is around 50 €/ton, for this analysis it was considered plausible that the cost of by-product cannot be higher than 15 €/ton (Table 11). Similarly it was assumed a threshold price of 70 €/ton for bone meal equal to about of 30 % of industrial price of its main constituent which is calcium phosphate dibasic (Deydier et al. 2005). The prices of H2SO4 (96 % w/v) and NaOH (10 N) were considered equal to 185 and 235 €/m3, respectively.
Carrying out the treatment using the defined operating conditions (pH = 9, N:P:Mg = 1:1.1:1.1), a total cost of about 7.98 €/m3 leachate is estimated for the added chemicals (Table 11). This value is significantly lower than the expenses required if pure reagents were to be used. In fact, Di Iaconi et al. (2010) estimated a total expense of about 24 €/m3 using H3PO4 and MgO for the treatment of a leachate comparable with that used in this study. By considering that the amount of recovered struvite is around to 63 kg/m3 of treated leachate, the production cost results was equal to 0.127 €/kg, therefore, 127 €/ton. This cost is about half the price of commercial fertilizers composed both of ammonium and phosphorus such as (NH4)3PO4.
Conclusions
In this study, seawater bittern and bone meal have been used as low-cost sources of magnesium and phosphorus for struvite production from landfill leachates. The experiments carried out demonstrated that it is possible to achieve an ammonium abatement of about 98 % using a stoichiometric ratio of N:P:Mg = 1:1.1:1.1. With this dosage, moreover, the process enables to recover about 99 and 95 % of the dosed amounts of phosphorus and magnesium, respectively. Moreover, the experiments demonstrated that the produced precipitate actually contains struvite crystals. Some other valuable compounds, such as potassium and calcium salts, have been detected in small quantities. Furthermore, the amounts of heavy metals into the precipitate were widely below the limits established in the EC Directives. The agronomic tests conducted on Spinacia oleracea allowed to verify the fertilizing potential of precipitate produced. In fact, its use as slow-release fertilizer permitted to doubling the vegetables growth. Besides, the biomass production with struvite precipitate was higher of about 30 % than the amount detected with a complex fertilizer. Furthermore, the struvite application as fertilizer did not caused the increase of heavy metals content in the vegetables. Thus, the findings of the present work indicate that the struvite produced from landfill leachate, through the proposed treatment, could be effectively exploited for agricultural uses. This approach could have a remarkable environmental impact. In this way, in fact, using by-products of phosphorus and magnesium, it is possible to reduce the polluting load of a high-concentrated wastewater and to recover and reuse its nitrogen content. Obviously, further researches must be conducted to verify the applicability of struvite produced from different types of wastewaters as fertilizer for other vegetables species.
References
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association and Water Environment Federation, Washington DC, USA
Çelen I, Türker M (2001) Recovery of ammonia as struvite from anaerobic digester effluents. Environ Technol 22:1263–1272
Chimenos JM, Fernández AI, Villalba G, Segarra M, Urruticoechea A, Artazab B, Espiella F (2003) Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res 37:1601–1607
Council of the European Communities (1986) Directive 86/278/EEC, Use of sewage sludge in agriculture. Journal of the European Union, Brussels, BE
Coutand M, Cyr M, Deydier E, Guilet R, Clastres P (2008) Characteristics of industrial and laboratory meat and bone meal ashes and their potential applications. J Hazard Mater 150:522–532
De Rosa S, Siciliano A (2010) A catalytic oxidation process of olive oil mill wastewaters using hydrogen peroxide and copper. Desal and Water Treat 23:187–193
Deydier E, Guilet R, Sarda S, Sharrock P (2005) Physical and chemical characterisation of crude meat and bone meal combustion residue: “waste or raw material?”. J Hazard Mater B 121:141–148
Di Iaconi C, Pagano M, Ramadori R, Lopez A (2010) Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour Technol 101:1732–1736
El Diwani G, El Rafie S, El Ibiari NN, El-Aila HI (2007) Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 214:200–214
European Commission (2011) Commission Regulation (EU) N. 1258/2011, Amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs. Journal of the European Union, Brussels, BE
Fattah KP, Sabrina N, Mavinic DS, Koch FA (2008) Reducing operating costs for struvite formation with a carbon dioxide stripper. Water Sci Technol 58(4):957–962
Gunay A, Karadag D, Tosun I, Ozturk M (2008) Use of magnesit as a magnesium source for ammonium removal from leachate. J Hazard Mater 156:619–623
He S, Zhang Y, Yang M, Du W, Harada H (2007) Repeated use of MAP decomposition residues for the removal of high ammonium concentration from landfill leachate. Chemosphere 66:2233–2238
Huang H, Mavinic DS, Lo KV, Koch FA (2006) Production and basic morphology of struvite crystals from a pilot-scale crystallization process. Environ Technol 27:233–245
Huang H, Xu C, Zhang W (2011) Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour Technol 102:2523–2528
Iqbal M, Bhuiyan H, Mavinic DS (2008) Assessing struvite precipitation in a pilot-scale fluidized bed crystallizer. Environ Technol 29:1157–1167
JCPDS (1988) International centre for diffractogram data. Search manual and data cards. Park lane, Swarthmore, USA
Kabdaşli I, Şafak A, Tünay O (2008) Bench-scale evaluation of treatment schemes incorporating struvite precipitation for young landfill leachate. Waste Manag 28:2386–2392
Kabdaşli I, Tünay O, Özcan P (2009) Application of struvite precipitation coupled with biological treatment to slaughterhouse wastewaters. Environ Technol 30:1095–1101
Karabegovic L, Uldal M, Werker A, Morgan-Sagastume F (2013) Phosphorus recovery potential from a waste stream with high organic and nutrient contents via struvite precipitation. Environ Technol 34:871–883
Kim D, Ryu HD, Kim MS, Kim J, Lee SI (2007) Enhancing struvite precipitation potential for ammonia nitrogen removal in municipal landfill leachate. J Hazard Mater 146:81–85
Korchef A, Saidou H, Ben Amor M (2011) Phosphate recovery through struvite precipitation by CO2 removal: effect of magnesium, phosphate and ammonium concentrations. J Hazard Mater 186:602–613
Kumar B, Chakrabortty S, Pal P (2015) Membrane-integrated physico-chemical treatment of coke-oven wastewater: transport modelling and economic evaluation. Environ Sci Pollut Res 22:6010–6023
Kurniawan TA, W-h L, Chan GYS (2006) Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J Hazard Mater B129:80–100
Latifian M, Liu J, Mattiasson B (2012) Struvite-based fertilizer and its physical and chemical properties. Environ Technol 33:2691–2697
Le Corre KS, Valsami-Jones E, Hobbs P, Parsons SA (2005) Impact of calcium on struvite crystal size, shape and purity. J Cryst Growth 283:514–522
Lee SI, Weon SY, Lee CW, Koopman B (2003) Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 51:265–271
Li XZ, Zhao QL (2003) Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer. Ecolog Eng 20:171–181
Marti N, Pastor L, Bouzas A, Ferrer J, Seco A (2010) Phosphorus recovery by struvite crystallization in WWTPs: influence of the sludge treatment line operation. Water Res 44:2371–2379
Pastor L, Mangin D, Ferrer J, Seco A (2010) Struvite formation from the supernatants of an anaerobic digestion pilot plant. Bioresour Technol 101:118–125
Quintana M, Colmenarejo MF, Barrera J, García G, García E, Bustos A (2004) Use of a byproduct of magnesium oxide production to precipitate phosphorus and nitrogen as struvite from wastewater treatment liquors. J Agric Food Chem 52:294–299
Ryu H-D, Lim C-S, Kang M-K, Lee S-I (2012) Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J Hazard Mater 221–222:248–255
Saidou H, Moussa B, Ben AM (2009) Influence of airflow rate and substrate nature on heterogeneous struvite precipitation. Environ Technol 30:75–83
Siciliano A, De Rosa S (2014) Recovery of ammonia in digestates of calf manure through a struvite precipitation process using unconventional reagents. Environ Technol 35:841–850
Siciliano A, De Rosa S (2015) Experimental formulation of a kinetic model describing the nitrification process in biological aerated filters filled with plastic elements. Environ Technol 36:293–301
Siciliano A, Ruggiero C, De Rosa S (2013) A new integrated treatment for the reduction of organic and nitrogen loads in methanogenic landfill leachates. Process Saf Environ Prot 91:311–320
Siciliano A, Stillitano MA, De Rosa S (2015) Increase of the anaerobic biodegradability of olive mill wastewaters through a pre-treatment with hydrogen peroxide in alkaline conditions. Desal and Water Treat 55:1735–1746
Siciliano A, Stillitano MA, De Rosa S (2016) Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew Ener 85:903–916
Uludag-Demirer S, Demirer GN, Chen S (2005) Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem 40:3667–3674
Uysal A, Yilmazel YD, Demirer GN (2010) The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J Hazard Mater 181:248–254
Uysal A, Demir S, Sayilgan E, Eraslan F, Kucukyumuk Z (2014) Optimization of struvite fertilizer formation from baker’s yeast wastewater: growth and nutrition of maize and tomato plants. Environ Sci Pollut Res 21:3264–3274
Wu Y, Zhou S (2012) Improving the prediction of ammonium nitrogen removal through struvite precipitation. Environ Sci Pollut Res 19:347–360
Yetilmezsoy K, Sapci-Zengin Z (2009) Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer. J Hazard Mater 166:260–269
Acknowledgments
The author thanks Eng. Camilo Haro Barroso and Eng. Maria Assuntina Stillitano for the analytical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Siciliano, A. Assessment of fertilizer potential of the struvite produced from the treatment of methanogenic landfill leachate using low-cost reagents. Environ Sci Pollut Res 23, 5949–5959 (2016). https://doi.org/10.1007/s11356-015-5846-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5846-z