Abstract
Low-cost water defluoridation technique is one of the most important issues throughout the world. In the present study, shale, a coal mine waste, is employed as novel and low-cost adsorbent to abate fluoride from simulated solution. Shale samples were collected from Mahabir colliery (MBS) and Sonepur Bazari colliery (SBS) of Raniganj coalfield in West Bengal, India, and used to remove fluoride. To increase the adsorption efficiency, shale samples were heat activated at a higher temperature and samples obtained at 550 °C are denoted as heat-activated Mahabir colliery shale (HAMBS550) and heat-activated Sonepur Bazari colliery shale (HASBS550), respectively. To prove the fluoride adsorption onto different shale samples and ascertain its mechanism, natural shale samples, heat-activated shale samples, and their fluoride-loaded forms were characterized using scanning electron microscopy, energy dispersive X-ray analysis, X-ray diffraction study, and Fourier transform infrared spectroscopy. The effect of different parameters such as pH, adsorbent dose, size of particles, and initial concentration of fluoride was investigated during fluoride removal in a batch contactor. Lower pH shows better adsorption in batch study, but it is acidic in nature and not suitable for direct consumption. However, increase of pH of the solution from 3.2 to 6.8 and 7.2 during fluoride removal process with HAMBS550 and HASBS550, respectively, confirms the applicability of the treated water for domestic purposes. HAMBS550 and HASBS550 show maximum removal of 88.3 and 88.5 %, respectively, at initial fluoride concentration of 10 mg/L, pH 3, and adsorbent dose of 70 g/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Occurrence of fluoride in groundwater has drawn a worldwide impact. Globally, it is estimated that more than 70 million people are affected from fluorosis, notably in USA, Africa, and Asia (Latha et al. 1998; Amini et al. 2008). Fluoride contamination in water can be considered as a double-edged sword (Piddennavar and Krishnappa 2013). Concentration of fluoride in drinking water below 0.7 mg/L has a considerable impact on human health (Pollick 2004). Again, chronic ingestion of excessive consumption of fluoride has been linked to neurological manifestations, teeth mottling, dental and skeletal fluorosis, and structural changes in DNA (British Fluoridation Society 2012). According to the Bureau of Indian Standards (BIS) and WHO standards for drinking water, desirable limits are 0.6–1.2 and 1.5 mg/L, respectively (World Health Organization 2004; BIS 2012). Different defluoridation methods, like electro-dialysis (Adhikari et al. 1989; Lahnid et al. 2008), ion-exchange (Meenakshi and Viswanathan, 2007), nano-filtration (Liu et al. 2007), adsorption (Daifullah et al. 2007; Hanumantharao et al. 2011), etc., are already in use. However, among all these methods, adsorption has been found to be more effective and economical, especially for small communities (Dhagat et al. 2013).
Gondwana coal is abundantly available in West Bengal, a state situated in the eastern part of India. During mining of coal, shale, a kind of sedimentary rock, is obtained as mine waste. Furthermore, shale is separated from raw coal during coal washing process and considered as a waste material (Zhao and Xiang 2004). Shale is composed of ∼3.9 μ-sized particle (Sadhu et al. 2013). It comprises both inorganic and organic components with different clay minerals like illite, kaolinite, smectite, etc., which are responsible for its adsorption characteristics (Hamdi and Sarsa 2009). Highly charged cations like aluminum, silicon, iron, etc. are also present in shale minerals (Sadhu et al. 2013). The abundance of shale in earth’s crust makes it a no-cost raw material to be used as an adsorbent for removal of pollutants. Though different research works have been carried out to remove fluoride using untreated and treated clay minerals (Bower and Hatcher 1967; Karthikeyan et al. 2005; Tang et al. 2010; Guo and Reardon 2012), as far the knowledge of the present research group is concerned, the utilization of shale for adsorption of fluoride has not yet been explored. Again, literature showed that after heat activation, calcined adsorbent can show better effectiveness in fluoride removal in comparison to its natural form (Das et al. 2005). Therefore, in the present work, both natural and heat-activated shale samples have been used to remove fluoride from simulated fluoride solution. The usage of shale in adsorption of fluoride from contaminated water serves two purposes. First, the removal of excess fluoride from contaminated water using shale ensures supply of safe drinking water to rural people who live on groundwater only, in a cost-effective manner. Secondly, the utilization of shale, a solid mine waste, as an adsorbent solves the problem of solid waste disposal in a fruitful manner in a colliery region.
Two collieries, namely Mahabir colliery and Sonepur Bazari colliery, West Bengal, India, have been chosen for collection of shale samples, and they have been termed as Mahabir colliery shale (MBS) and Sonepur Bazari colliery shale (SBS). To enhance the fluoride removal efficacy, natural shale samples have been heat activated at a higher temperature and used for abatement of fluoride. The kinetics of removal of fluoride from aqueous solution have been studied with natural shale samples and their heat-activated forms in batch contactor. The effect of different parameters, such as size of adsorbent particle, pH, adsorbent dose, initial fluoride concentration, and the presence of competing ions have been studied in a judicial manner. An attempt has been made to assess the mechanism of fluoride adsorption onto shale by characterizing shale samples with and without fluoride loading with the help of various analytical instruments such as scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDAX), X-ray diffraction (XRD) study, and Fourier transform infrared spectroscopy (FTIR).
Materials and methods
All chemicals were obtained from Merck (India) unless otherwise stated. Standard fluoride solution and TISUB-III buffer were obtained from Thermo Scientific Orion (USA). All experiments were performed thrice and arithmetic mean is represented.
Collection and preparation of shale samples
Shale samples were collected from two collieries, namely Mahabir colliery (latitude 23° 37′ 05″ N and longitude 87° 06′ 19″ E) and Sonepur Bazari colliery (latitude 23° 41′ 33″ N and longitude 87° 15′ 21″ E) in Raniganj Coalfield of West Bengal, India, and termed as Mahabir colliery shale (MBS) and Sonepur Bazari colliery shale (SBS). Shale rocks obtained from the collieries were first broken down to small pieces using a hammer. The small pieces of shale were then crushed in a jaw crusher and later ground to powdered form in a ball mill and sieved to get the desired particle size. Heat activation of shale was done by heating the powdered shale samples (particle size 90 μ), taken in heat-resistant crucibles, in a pre-heated muffle furnace (Servotronics DIC 9681) at three different temperatures such as 350, 450, and 550 °C, separately for 1 h. The adsorbents, thus prepared, were designated as HAXS350, HAXS450, and HAXS550, where “X” stands for “MBS” or “SBS.”
Characterization of shale samples
Both natural and heat-activated shale samples were characterized in terms of their specific gravity, bulk density, total organic carbon, and moisture content. Brunauer–Emmett–Teller (BET) surface area was determined using BET surface area analyzer (BET—Quantachrome make Nov. 4000E, USA). SEM images of shale samples, with and without fluoride loading, were taken using the scanning electron microscope (Hitachi–S-3000N, Japan) to acquire their topographical characteristics at a desired magnification. Analysis was done at working distance of 5.5 mm and acceleration voltage of 10 KV. EDAX study (Hitachi–S-3000N, Japan) was performed to get an elemental analysis of the samples. XRD patterns of the samples were recorded between 0° and 100° (2θ) at a scanning speed of 2°/min, using a PANalyticalX’Pert HighScore Plus Diffractometer (China) with mono-chromated Cu_kα1 radiation (30 mA and 40 kV). FTIR analysis of shale samples was obtained using a Perkin–Elmer FTIR Spectrometer (Malaysia) for determination of functional groups with their stretching frequencies. Spectra over the 4000–400 cm−1 range were studied.
Zero point charge (pHzpc) was determined by salt addition method following the standard protocol (Srivastav et al. 2014, Tentli-Sequeira et al. 2014). pHzpc is the point where the sum of the all the surface positive charges balance the sum of all the surface negative charges. The experiments were carried out with MBS and SBS. KNO3 solutions (0.1 M) were prepared with pH values adjusted between 2 and 12, with intervals of two units by adding 0.1 M HCl and NaOH solutions. KNO3 solutions (0.1 M) were then contacted with 1 g of adsorbent. After 24 h of contact, the slurry was centrifuged and supernatant was collected for measuring the final pH of the solution. pHzpc of MBS and SBS was determined from the relation between the pHinitial and ∆pH, i.e., (pHfinal − pHinitial).
Removal of fluoride using shale samples
Fluoride stock solution (1000 mg/L) was prepared by dissolving 2.211 g of sodium fluoride in 1 L de-ionized water. Desired concentrations of fluoride in a solution were obtained by diluting the stock solution with requisite amount of de-ionized water. Volume of solution was maintained 50 mL in each experimental run. To avoid chemical reaction between fluoride and borosilicate, a major component of glass material, 100 mL polyvinyl chloride (PVC - synthetic plastic) flasks were used for all experiments as suggested by Islam and Patel (2007). pH of the solution was adjusted using 1(N) HCl and 0.4 (N) NaOH solution. Simulated solution of fluoride was contacted with requisite amount of shale samples in batch contactor for definite time period. The solution was then centrifuged, and supernatant was used for estimation of residual fluoride concentration using ion-selective fluoride electrode (Ion Meter 4Star, B36531, Thermo Scientific, India) by adding TISUB-III buffer, following a standard protocol (Kennedy 1990).
Optimization of operating parameters for removal of fluoride using natural shale
Natural shale was used as an adsorbent to remove fluoride from simulated solution. Requisite amount of natural shale was contacted with the fluoride solution at the desired pH in a batch contactor. Initially, the contact time was 48 h. However, it is seen that after 24 h, there is a negligible increase in the removal of fluoride and the solution attains equilibrium. Therefore, experimental time was reduced to 24 h during optimization of operating parameters. Effect of three input parameters, viz., initial pH of solution (2–12), adsorbent dose (0.5–5 g), and size of adsorbent particle (53–300 μ) on adsorption of fluoride was examined by varying them individually in a prescribed manner. In order to make the process cost-effective, temperature was not considered as a parameter. Therefore, all the experiments were done at ambient temperature of 30 °C with shaking speed of 180 rpm.
Selection of heat activation temperature and optimization of operating parameter for removal of fluoride using heat-activated shale
Heat activation of natural shale was done at three different temperatures such as 350, 450, and 550 °C, separately. The samples obtained after heat activation are termed as HAX350, HAX450, and HAX550, where “X” denotes MBS and SBS. Simulated solution of fluoride having initial concentration of 10 mg/L was contacted with equal amount (3.5 g) of heat-activated shale samples, individually for 24 h. Size of heat-activated shale, pH, temperature, shaking speed, and volume of solution were maintained as 90 μ, 3.2, 30 °C, 180 rpm, and 50 mL, respectively. The residual fluoride concentration was measured following the method as stated above. Study reveals that irrespective of coal mines, shale, activated at 550 °C, shows the best fluoride removal efficacy. Therefore, further studies on fluoride removal were performed with heat-activated shale obtained at 550 °C. The effect of initial pH of solution was examined by varying it from 3 to 13. After 24 h, samples were collected and analyzed for residual fluoride concentration.
Comparative study on fluoride removal using natural and heat-activated shale
To compare the fluoride removal efficacy of natural shale and its heat activated form, simulated fluoride solution having initial concentration of 10 mg/L was contacted with 3.5 g of each type of shale individually. Samples were collected after each 8-h interval and analyzed for residual fluoride concentration. Results reveal that irrespective of types of shale, heat-activated forms have better fluoride removal efficacy. Therefore, further studies were made with heat-activated shale.
Kinetic and equilibrium studies on adsorption of fluoride and selection of best adsorbent
During kinetic study, initial concentration of fluoride was varied in the range of 5 to 15 mg/L in a batch contactor. Size and dose of adsorbent particle, volume of the solution, pH, temperature, and shaking speed were maintained at 90 μ, 3.5 g, 50 mL, 3.2, 30 °C, and 180 rpm, respectively, during removal of fluoride using heat-activated shale. The samples were collected after 2-h interval and analyzed for residual fluoride concentration. During the experiments on optimization of operating parameters for removal of fluoride using natural shale, it is seen that after 24 h of contact time, there is a negligible increase in removal of fluoride and the solution attains equilibrium. Therefore, kinetic studies on removal of fluoride were conducted for 24 h with each of the heat-activated shale samples. Variation of pH of the solution was also investigated during kinetic study of fluoride removal using heat-activated shale samples. Equilibrium study was performed at three different temperatures viz., 25, 30, and 35 °C, respectively. Simulated solution of fluoride having the initial concentration in the range of 5 to 15 mg/L was contacted with 3.5 g of heat-activated shale in batch contactor separately. Volume of the solution, particle size, pH, and stirring speed were maintained at 50 mL, 90 μ, 3.2 and 180 rpm, respectively. Samples were collected after 24 h and analyzed for residual concentration of fluoride. To investigate desorption of fluoride from spent adsorbent, the later was treated at different pH conditions, such as pH 3.2, 7.2, and 12.2, respectively. The resulting solution was then tested for fluoride concentration using ion-selective fluoride electrode (Ion Meter 4Star, B36531, Thermo Scientific, India).
Effect of different co-ions
The fluoride contaminated wastewater is usually associated with other co-ions like nitrate, chloride, carbonate or bicarbonate, and phosphate, which can compete with fluoride ions for active binding site of adsorbent during adsorption process. In the present study, the effects of chloride, nitrate, carbonate, and phosphate were investigated. The solutions were prepared from their sodium and potassium salts, and concentration of each co-ion was varied in the range of 10–100 mg/L where the initial fluoride concentration was maintained at 10 mg/L with shaking speed 180 rpm, volume 50 mL, temperature 30 °C, pH 3.2, particle size 90 μ, and adsorbent dose 3.5 g.
Results and discussions
Characterization of shale samples
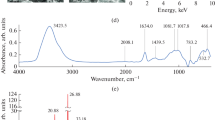
Specific gravity, bulk density, moisture content, and total organic carbon of natural shale (MBS and SBS) and heat-activated shale (HAMBS550 and HASBS550) have been determined and presented in Table 1. Specific gravity is more for heat-activated shale than its natural form. This is obvious because adsorbents become more compact as occluded gases, moisture, and water of hydration are lost during heat activation process. As expected, moisture content decreases with heat activation. Again, total organic carbon (TOC) values of native and heat-activated shales have been found to be 1.07 % for MBS, 2.4 % for HAMBS550, 2.03 % for SBS, and 3.69 % for HASBS550, which indicates that TOC of heat-treated shale is more than that of its native form. It can be stated that even if some volatile organic carbon may be released during heat treatment process, due to compaction of mass of shale, higher value of TOC of heat-activated shale is observed. A considerable amount of weight loss has been observed when native shale samples are heated in muffle furnace at 550 °C to prepare the heat-activated shale. From Table 1, it is seen that while BET surface area remains the same for both MBS (11.02 m2/g) and its heat-activated form (HAMBS550 11.02 m2/g), the surface area of SBS (9.19 m2/g) is more than that of its heat-activated form (HASBS550 8.39 m2/g). The decrease in BET surface area may be attributed to the compaction or elimination of pores during heat activation stage. Similar observation has been made by Wang et al. (2005) during heat activation of fly ash and red mud. SEM micrographs of shale samples (MBS, SBS) and their fluoride-loaded forms are shown in Fig. 1a–d. From the SEM micrographs of MBS and its fluoride-loaded form, no distinct difference in effect of fluoride adsorption on the surface of the shale is observed. On the other hand, a smoothening effect is observed for fluoride-loaded SBS in comparison to its natural form. Elemental analysis obtained from EDAX study of shale samples are given in Table 2. Basically, EDAX allows localization of elements and quantitative detection in the shale samples. From the table, it is clear that SBS is richer in carbon content than MBS. EDAX spectra of fluoride-loaded MBS and SBS are shown in Fig. 2a, b. Fluoride peak in the EDAX spectra confirms the adsorption of fluoride on shale. FTIR studies of heat-activated shale samples (HAMBS550, HASBS550) with and without fluoride loading are shown in Figs. 3 and 4, respectively. FTIR graph of HAMBS550 shows three peaks at wave numbers of 626, 1034, and 3621 cm−1 representing C–Cl (strong) and –OH (strong and sharp) bonds respectively. After being loaded with fluoride, −OH (strong and sharp) bond disappears and the wave numbers of C–Cl (strong) change from 626 cm−1 to 619 cm−1 as evident from Fig. 3a and b. Shale, being a mineral, is very likely to contain chloride in its natural form. Presence of –OH bonds signifies the occurrence of hydroxyl group containing compounds. However, the disappearance of –OH group after fluoride loading may be due to the replacement of these groups by fluoride from the inorganic materials. FTIR graph of HASBS550 (Fig. 4a) gives four peaks at wave numbers of 718, 1032, 3410 and 3696 cm−1 representing C–Cl (strong) and –OH (strong and sharp) bonds respectively. After being loaded with fluoride, HASBS550 shows three peaks at wave numbers of 631, 1044 and 3436 cm−1 shows three representing C–Cl (strong), C–F (strong), and –OH, −H (strong and sharp) bonds, respectively as shown in Fig 4b. XRD of MBS shows its mineralogical compositions like montmorillonite (NaMgAlSiO2 (OH) H2O; and Si3.74 Al2.03 Fe0.03 Mg0.02 · O11), kaolinite (Al2Si2O5 (OH)4), siderite magnesiancalcian (Ca0.1Mg0.33Fe0.57 (CO3)), quartz (SiO2; hexagonal), gismondine (CaAl2Si2O8 · 4H2O), sodium salicylate (C7H5NaO3), tridymite (SiO2), in the form of characteristic peaks as already shown by Sadhu et al. (2013). The pattern obtained from XRD study (Fig. 5a, b) of SBS shows sharp reflection in low 2θ values. It indicates the presence of different minerals like kaolinite (Al2Si2O5 (OH)4), huanghoite (BaCe(CO3)2 F), melilite ((Ca,Na)2(Al,Mg,Fe2)(Si,Al)2.O7), cryptophyllite (K2Ca[Si4O10].5H2O), gismondine (CaAl2Si2O8 · 4H2O), and lemoynite ((Na,K)2CaZr2Si10O26, 6H2O). After treatment with fluoride, fluorite (CaF2) and villiaumite (NaF) are found from XRD pattern analysis.
The pHzpc of MBS and SBS obtained by salt addition method have been found to be 7.5 ± 0.1 and 8 ± 0.2, respectively. This indicates the presence of negatively charged surface groups on adsorbent. Bhaumik et al. (2012) stated that adsorption of anion is favored at pH < pHzpc. Therefore, the removal of fluoride with shale sample should be favored at low initial pH condition.
Removal of fluoride using shale samples
Optimization of operating parameters for removal of fluoride using natural shale
Effect of pH on adsorption of fluoride onto shale samples has been seen by varying it from 3 to 13. Maximum removal of 46.4 and 50.1 % has been recorded at an initial pH 3.2 for MBS and SBS, respectively, with 10 mg/L initial concentration and 70 g/L adsorbent dose and contact time of 24 h. From Fig. 6, it is seen that when pH increases from 3 to 9, removal of fluoride decreases from 46.4 to 13 % for MBS and from 50.1 to 3.5 % for SBS. pHzpc of MBS and SBS have been found to be 7.5 and 8.0, respectively. It is well known that surface charge of adsorbent will become negative when pH of the solution exceeds the value of pHzpc (Thakre et al. 2010). Therefore, the decrease in fluoride removal at a higher pH (pH > pHzpc) may be attributed to the accumulation of excess negative charges over the surface which repels fluoride ion strongly and inhibits its adsorption on the surface. Similar observations were made by Goswami and Purkait (2012). On the other hand, removal was seen to increase monotonously as pH decreases below pHzpc. This may be due to the accumulation of positive ions (mainly H+) on adsorbent at lower pH values for which fluoride readily gets adsorbed on the surface. This is in conformity with the observations made by Srimurli et al. (1998) and Karthikeyan et al. (2005). Fluoride removal efficiencies of MBS and SBS have been seen to increase with increase in adsorbent dose. For MBS, removal increases from 48 to 57.8 % with increase in adsorbent dose from 30 to 100 g/L as shown in Fig. 7. When adsorbent dose is higher than 70 g/L, the removal becomes independent of it. For, SBS, the highest removal is observed at adsorbent dose of 70 g/L as shown in Fig. 7. Thus, 70 g/L has been chosen to be the optimum adsorbent dose. The increase in percentage removal with increase in adsorbent dose is attributed to the increase in adsorbent sites available for the same amount of fluoride anions. Effect of variation of size of particle on fluoride removal has been seen by varying it from 53 to 212 μ (Fig. 8). Removal has been found to be inversely proportional to the size of particle (Fig. 8). Maximum removal is obtained with 90 μ particle. Thus, 90-μ particle is chosen for further removal studies.
Optimization of operating parameters for removal of fluoride using heat activated shale
HAMBS350, HAMBS450, HAMBS550, HASBS350, HASBS450, and HASBS550 have been used in the removal of fluoride to assess their adsorption capacity. Simulated fluoride solution of 10 mg/L has been contacted with 70 g/L shale samples having 90-μ particle size at pH 3.2. After 24 h, samples have been collected and analyzed for residual fluoride concentration and the results are shown in Fig. 9. From the figure, it is clear that, HAX550 (“X” stands for MBS and SBS) shows the maximum fluoride removal in comparison with HAX350 and HAX450 under identical condition. Since HAX550, i.e., HAMBS550 and HASBS550, has been found to be the most efficient, shale samples, heat activated at 550 °C, have been used for defluoridation process; 88.3 and 88.5 % removals of fluoride have been obtained with HAMBS550 and HASBS550, respectively, in comparison to their natural forms (MBS 50 % and SBS 48 %) under identical condition. Therefore, it can be stated that the removal efficiency of natural shale samples is lower than that of their heat-activated forms. Figure 10 embodies the effect of initial pH on the percentage removal of fluoride from simulated solution using HAMBS550 and HASBS550. Figure reveals that maximum and minimum removals have been achieved at an initial pH 3 and pH 13, respectively, for both HAMBS550 and HASBS550.
Comparative study on fluoride removal using natural and heat-activated shale
To compare the removal efficiency, natural and heat-activated shale samples are placed in BOD shaker keeping other parameters constant such as size of adsorbent 90 μ, wt of the adsorbent 70 g/L, temperature 30 °C, shaking speed 180 rpm, and pH 3.2. Figure 11 reveals that heat-activated forms have better fluoride removal efficacy in comparison to their natural forms. Therefore, further studies have been made with heat-activated shale.
Kinetic and equilibrium studies and selection of best adsorbent
Kinetic studies have been done at different initial fluoride concentrations in the range of 5–15 mg/L. The variations of percentage removal with time are shown in Fig. 12a, b. It has been observed that, with increase in initial fluoride concentrations, the fluoride removal decreases. For HAMBS550, maximum removal of 95.46 % has been seen at initial fluoride concentration of 5 mg/L. This value decreases to 82 % at an initial fluoride concentration of 15 mg/L. For HASBS550, maximum removal of 90.57 % has been seen at initial fluoride concentration of 5 mg/L. This value decreases to 49.2 % at an initial fluoride concentration of 15 mg/L. Decrease in removal efficiency with increase of initial fluoride concentration is mainly due to competition of more number of fluoride ions for the same number of available adsorption sites. It has also been seen that after 24 h of contact time, there is a negligible increase in the removal of fluoride and the solution attains equilibrium. Kinetic data have been analyzed using Lagergren first-order model and Ho pseudo-second-order model (Svilovic et al. 2010; Thakre et al. 2010), separately, and the values of the parameters are shown in Table 3. Ho pseudo-second-order kinetic model has been found to be the best as evident from the values of correlation coefficients. The nature of adsorption of the adsorbate on the adsorbent can be predicted using adsorption isotherm models. Adsorption isotherm is generally described by the Langmuir and Freundlich equations (Hamdi and Sarsa 2007). Equilibrium studies have been done to determine the adsorption capacity of the adsorbent. Equilibrium data have been analyzed using both Langmuir and Freundlich adsorption isotherm models individually, and the values are shown in Table 4. Equilibrium data have been found to fit satisfactorily to Freundlich adsorption isotherm model. Heat of adsorption is a characteristic property of the adsorption process and more specifically to type of adsorption and the extent of adsorption (Bhaumik et al. 2012). In the present study, the adsorption process has been found to be exothermic in nature having heat of adsorption (∆H) 5.104 × 10−3 J/mol as obtained from van’t Hoff equation (Table 5). Similar observations have been made by Bhaumik et al. (2012).
It is seen that the highest removal takes place when initial pH is 3.2. This is too low for drinking water. However, during adsorption process, the pH increases to 6.8 for HAMBS550 and 7.2 for HASBS550 bringing the pH of treated water within the desirable range (Fig. 13). Mechanism for removal of Fluoride-ion with clay or similar adsorbents has been proposed by several scientists (Srimurli et al. 1998; Ma and Eggleton 1999; Karthikeyan et al. 2005; Hamdi and Sarsa 2007, Goswami and Purkait 2012). These involve reaction of proton in solution with hydroxyl group on surface of solid adsorbent producing a positively charged surface species, and aqueous fluoride ion reacts with this to produce a surface-adsorbed fluoride and releases water, overall effect being replacement of hydroxyl group in shale by fluoride. Other possible paths for the same overall reaction have also been suggested (Puka 2004, Thakre et al. 2010). In this work, FTIR studies were made which definitely shows that hydroxyl groups existed on fresh HAMBS550 and HASBS550 and after adsorption, fluoride ions have been detected but no hydroxyl ion. This observation definitely agrees with the overall scheme of the replacement of hydroxyl group by fluoride ion.
During desorption study, it was expected that since low initial pH favors the adsorption of fluoride onto shale, desorption would occur at higher pH. However, the results do not show the same. Adsorbed fluoride has not been recovered at any pH condition. Therefore, it can be concluded that fluoride once adsorbed onto shale cannot easily be leached. Again, fitting of Ho-pseudo second-order model indicates the Chemi-sorptive binding of fluoride ion on shale. Thus, fluoride-loaded shale sample can safely be used in road construction and any other civil works.
Competitive effect of different co-ions
Since in ground water, major four anions such us chloride, carbonate, nitrate and phosphate are found, during fluoride removal study, the effect of these co-ions have been investigated (Thakre et al. 2010). The removal of fluoride has been varied from 60.2 to 91 %, from 56 to 48 %, and from 60.2 to 48 % when initial concentration of phosphate, nitrate, and chloride have been varied individually from 10 to 100 mg/L, respectively (Fig. 14). Initial concentration of fluoride has been kept 10 mg/L in each of the solution. However, negligible amount of fluoride removal has been seen in the presence of carbonate ions when initial concentration of carbonate ion has been varied from 10 to 100 mg/L. Similar observations have been made by Viswanathan et al. (2009). Thus, the order of inhibitive effect of these co-ions is as follows: carbonate > nitrate > chloride > phosphate. Interference on removal of fluoride of these co-ions depends on the sorbent surfaces, concentration, charge, and size of the anions (Viswanathan et al. 2009).
Conclusion
Shale, a mine waste-generated as removed overburden in coal mines, is used in the present study to remove fluoride from simulated solution. Two coal mines viz., Mahabir colliery and Sonepur Bazari colliery, have been chosen for collection of shale samples. To increase the efficacy of shale, heat activation of natural shale samples have been done at a higher temperature. It is seen that the removal efficiency of natural shale samples is lower than their heat-activated forms. FTIR study reveals the elimination of hydroxyl ion [OH−] on fluoride-loaded shale samples. Two reaction schemes have been proposed which accounts for the elimination of hydroxyl ion [OH−] from shale after adsorption of fluoride on it. HAMBS550 and HASBS550 show maximum removal of 88.3 and 88.5 %, respectively, at initial fluoride concentration of 10 mg/L, size of adsorbent 90 μ, pH 3.2, adsorbent dose 70 g/L, temperature 30 °C, and shaking speed 180 rpm. pH of the simulated fluoride solution increases from 3.2 to 6.8 and 7.2 during treatment with HAMBS550 and HASBS550, respectively, making it fit for drinking purposes. Therefore, it can be stated that heat-activated forms can efficiently be used for fluoride removal. No desorption of fluoride from the spent shale has taken place. Thus, fluoride-loaded spent adsorbent can safely be used in road construction or any other civil works. Therefore, the utilization of shale in the removal of fluoride does not only assure the supply of safe drinking water but also mitigate the problem of solid waste disposal.
Nomenclature
C o Initial fluoride concentration (mg/L)
C Residual liquid phase concentration of fluoride at any time (mg/L)
C e Residual liquid phase concentration of fluoride at equilibrium time (mg/L)
q e Solid phase concentration of fluoride at equilibrium (mg/g)
q t Solid phase concentration of fluoride at any time t (mg/g)
t Contact time (h)
k 1 Adsorption rate constant of Lagergren first-order adsorption rate equation (h−1)
k 2 Adsorption rate constant of pseudo-second-order adsorption rate equation (g mg−1 h−1)
q max Maximum adsorption capacity of the adsorbent (mg/g)
K F Freundlich isotherm constant [(mg/g) (L/mg)1/n]
K Langmuir isotherm constant (L/g)
∆ H Heat of adsorption (J/mol)
References
Adhikari SK, Tipnis UK, Harkare WP, Govindan KP (1989) Defluoridation during desalination of brackish water by electrodialysis. Désalin 71:301–312
Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Moller KN, Sarr M, Johnson CA (2008) Statistical modelling of modelling of global geogenic fluoride contamination in groundwater. Environ Sci Technol 42:3662–3668
Bower CA, Hatcher JT (1967) Adsorption of fluoride by soils and minerals. J Soil Sci 3:151–154
Bhaumik R, Mondal NK, Das B, Roy P, K. Pal KC, Das C, Banerjee A, Datta JK (2012) Eggshell Powder as an Adsorbent for Removal of Fluoride from Aqueous Solution: Equilibrium, Kinetic and Thermodynamic Studies, E-J Chem. 9:1457–1480.
British Fluoridation Society (2012) One in a million: the facts about water fluoridation., http://www.bfsweb.org/onemillion/onemillion2012.html. Accessed on 3 March 2015
Bureau of Indian Standard (BIS) (2012) Indian standard specification for drinking water. IS10500:1–24. Drinking water [FAD 25: Drinking Water]. https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf.Accessed 28 Nov 2015
Daifullah AAM, Yakout SM, Elreefy SA (2007) Adsorption of fluoride in aqueous solutions using KMnO4 modified activated carbon derived from steam pyrolysis of rice straw. J Hazard Material 147:633–643
Das N, Pattanaik P, Das R (2005) Defluoridation of drinking water using activated titanium rich bauxite. J Colloid Interface Sci 292:1–10
Dhagat A, Goyal B, Sailo L (2013) Removal of Cr (VI) in aqueous solution using iron oxide coated sand (IOCS). Int J Scientific Eng Res 4:1–4
Goswami A, Purkait MK (2012) The defluoridation of water by acidic alumina. Che Engg Res Design 90:2316–2324
Guo Q, Reardon EJ (2012) Fluoride removal from water by meixnerite and its calcinations product. Appl Clay Sci 56:7–15
Hamdi N, Sarsa E (2007) Removal of fluoride from acidic wastewater by clay mineral: effect of solid–liquid ratios. Desalin 206:238–244
Hamdi N, Sarsa E (2009) Retention of fluoride from industrial acidic wastewater and NaF solution by three Tunisian clayey soils. Fluoride 42:39–45
Hanumantharao Y, Kishore M, Ravindhranath K (2011) Preparation and development of adsorbent carbon from Acacia farnesiana for defluoridation. Int J Plant Anim Environ Sci 1(3):209–223
Islam M, Patel RK (2007) Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. J Hazard Material 143:303–310
Karthikeyan G, Pius A, Alugumuthu G (2005) Fluoride adsorption studies of montmorillonite clay. Indian J Chem Tech 12:263–272
Kennedy JH (1990) Analytical chemistry principles, 2nd ed. W.B. Saunders, New York
Lahnid S, Tahaikt M, Elaroui K, Idrissi I, Hafsi M, Laaziz I, Amor Z, Tiyal F, Elmidaoui A (2008) Economic evaluation of fluoride removal by electrodialysis. Desalin 230:213–219
Latha SS, Ambika SR and Prasad SJ (1998) Fluoride contamination status of groundwater in Karnataka. http://www.iisc.ernet.in/currsci/mar25/articles13.htm. Accessed on 27 February 2015.
Liu J, Xu Z, Li X, Zhang Y, Zhou Y, Wang Z, Wang X (2007) An improved process to prepare high separation performance PA/PVDF hollow fiber composite nanofiltration membranes. Sep Purif Technol 58:53–60
Ma C, Eggleton RA (1999) Cation exchange capacity of kaolinite. Clay Clay Miner 47:174–180
Meenakshi S, Viswanathan N (2007) Identification of selective ion-exchange resin for fluoride sorption. J Colloid Interface Sci 308:438–450
Piddennavar R, Krishnappa P (2013) Review on defluoridation techniques of water. Int J of Eng Sci 2:86–94
Pollick HF (2004) Water fluoridation and the environment: current perspective in the United States. Int J Occup Environ Health 10:343–350
Puka LR (2004) Fluoride Adsorption Modelling and the Characterization of Clays for Defluoridation of Natural Waters, Magister Scientia in Chemistry, Faculty of Science, Rand Afrikaans University, Johannesburg, South Africa.
Sadhu K, Mukherjee A, Shukla SK, Adhikari K, Dutta S (2013) Adsorptive removal of phenol from coke-oven wastewater using Gondwana shale, India: experiment, modeling and optimization. Desalin Water Treat 52:6492–6504
Srivastav AL, Singh PK, Weng CH, and Sharma YC (2014) Novel Adsorbent Hydrous Bismuth Oxide for the Removal of Nitrate from Aqueous Solutions, J Hazard Toxic Radioact Waste. doi: 10.1061/(ASCE)HZ.2153-5515.0000235
Srimurli M, Pragathi A, Karthikeyan J (1998) A study on removal of fluorides from drinking water by adsorption onto low-cost materials. Env Pollution 99:285–289
Svilovic S, Rusic D, Basic A (2010) Investigations of different kinetic models of copper ions sorption on zeolite 13X. Desalin 259:71–75
Tang Y, Wang J, Gao N (2010) Characteristics and model studies for fluoride and arsenic adsorption on goethite. J Env Sci 22:1689–1694
Tentli-Sequeira A, Solache-Ríos M, Martínez-Miranda V, Linares-Hernández I (2014) Comparison of aluminum modified natural materials in the removal of fluoride ions. J Colloid Interface Sc 418:254–260
Thakre D, Rayalu S, Kawade R, Meshram S, Subrt J, Labhsetwar N (2010) Magnesium incorporated bentonite clay for defluoridation of drinking water. J Hazard Material 180:122–130
Viswanathan N, Sundaram CS, Meenakshi S (2009) Removal of fluoride from aqueous solution using protonated chitosan beads. J Hazard Material 161:423–430
Wang S, Boyjoo Y, Choueib A, Zhu ZH (2005) Removal of dyes from aqueous solution using fly ash and red mud. Water Res 39:129–138
World Health Organization (2004) Guidelines for drinking water quality, 3rd edn. WHO, Geneva, ISBN 9241546387
Zhao M, Xiang Y (2004) Natural rubber vulcanizate reinforced by modified coal-shale-based fillers. Inc J Appl Polym Sci 93:1397–1400
Acknowledgement
This work is supported by a grant from Department of Science and Technology, Govt. of West Bengal, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
On behalf of all co-authors, the corresponding author would like to declare that the work described has not been published before: that it is not under consideration for publication anywhere else; that its publication has been approved by all co-authors, as well as National Institute of Technology Durgapur, the Institute where the work has been carried out. The authors declare that they have no conflict of interest. On behalf of all authors, the corresponding author is taking all of the responsibilities for submission of the following research paper.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Biswas, G., Dutta, M., Dutta, S. et al. A comparative study of removal of fluoride from contaminated water using shale collected from different coal mines in India. Environ Sci Pollut Res 23, 9418–9431 (2016). https://doi.org/10.1007/s11356-015-5815-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5815-6