Abstract
The excess of fluoride ions in potable water is an important public health problem. This study has evaluated the thermal treatment process of a sludge from a water treatment plant at five different temperatures (200, 300, 400, 500, and 600 °C) to find a low-cost and eco-friendly adsorbent for fluoride removal. The sludge characterization was evaluated by thermogravimetric analysis, point of zero charge, scanning electron microscopy coupled with energy dispersive spectroscopy, X-ray diffraction, Fourier transform infrared spectroscopy, and N2 physisorption analysis, aiming to identify changes caused by the thermal treatment and its impact on F− adsorption. All the thermally treated samples were submitted to the same operational conditions in an adsorption experiment and compared with the raw sample. The thermally treated sludge showed the best adsorbent performance at 200 °C. By the use of this adsorbent material, the fluoride removal percentage was 98.13%, and the adsorption capacity was 1.05 mg g−1, resulting in a final fluoride concentration close to zero and meeting the recommendation of the World Health Organization (WHO). These results indicate that the thermal treatment of a water treatment plant sludge could be viable. On one side, thermal treatment is an option to treat problematic waste (sludge) generated in large amounts. On the other hand, this thermally treated sludge can be used as a largely available, accessible, eco-friendly, and low-cost adsorbent for fluoride removal from aqueous media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water treatment plant sludge is a waste resulting from the conventional water treatment process, specifically from the coagulation–flocculation step. This happens because colloidal particles and impurities in raw water are destabilized and then agglomerated into larger aggregates by chemical agents (Maraschin et al. 2020). Lastly, the sediment is decanted or filtered. As the demand for drinking water increases, the treatment process results in higher volumes of sludge that lack alternatives for correct disposal. The disposal of this waste often ends up being carried out directly in water bodies, which is not adequate due to contamination caused by chemicals used as coagulants in the treatment process (Ahmad et al. 2016a). Some studies suggest reusing it as a coagulant (Jangkorn et al. 2011) or as a substrate in constructed wetlands and in agricultural practice and other land-based uses (Ahmad et al. 2016b). Sludge contains a significant amount of organic and inorganic compounds as oxides of aluminum with small amounts of non-decomposed silicates (Teh et al. 2016; Jeon et al. 2018). This property gives a large surface area and adsorption capacity to the material (Siswoyo et al. 2019). So, it can be used as a potential, effective, and low-cost adsorbent.
In parallel, a serious public health problem is the excess of fluoride ions in drinking water. Fluoride is an inorganic ion present naturally in groundwater and has an important role at low concentrations in human health, especially for young children (Loganathan et al. 2013). Its maximum recommended concentration is 1.5 mg L−1, which is supposed to be enough to avoid dental caries (WHO 1998). However, people can develop fluorosis when continuously exposed to higher concentrations. Fluorosis is caused by fluoride deposition and can be dental in the first stage and skeletal in an advanced stage and lead to adverse effects, including cancer, digestive disorders, and neurological damage (Kazi et al. 2018). Groundwater is the major source of domestic water for people living in rural and semi-urban areas in most parts of the world (Ahamad et al. 2018). It is estimated that around 200 million people are affected by excess fluoride ions in drinking water in at least 25 countries (Singh et al. 2020). A simple and effective technology that can remove this excess and make the water potable with a low-cost is necessary.
Possible water defluorination methods are coagulation/precipitation, ion exchange, membrane separation, electrochemical treatment, and adsorption (Barathi et al. 2019). Among these options, adsorption generally stands out because of its effectiveness, convenience, ease of operation, simplicity of design, economic and environmental considerations. This unit operation is preferred when low-cost adsorbents with an effective ability to remove fluoride ions are used, as close as possible neutral pH of drinking water (Loganathan et al. 2013). Several materials are related as possible adsorbents for F− removal from aqueous solutions, such as activated alumina, activated carbon, chitosan, bone char, and others (Kazi et al. 2018). However, these materials have limitations such as efficiency and reduced adsorption capacity, restriction on the pH range of the medium, treatment difficulties, and elevated cost (Xu et al. 2017). In this way, there is continuous research for low-cost adsorbents capable of uptake F− from waters. Here, an adsorbent derived from a problematic waste (water treatment plant sludge) is proposed for water defluorination, solving two environmental issues.
In this study, sludge from a local water treatment plant was thermally treated at five different temperatures, and its fluoride adsorption performance was compared with the raw sludge. All the sludges (raw and treated) were characterized using thermogravimetric analysis (TGA), point of zero charge (PZC), zeta potential (ZP), scanning electron microscopy (SEM) coupled with energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and N2 physisorption analysis, aiming to identify changes caused by the thermal treatment and its impact on adsorption. All sludges were applied for water defluorination by adsorption at the same experimental conditions.
Materials and methods
Thermal treatment of sludge
The raw sludge was obtained from the decanter in a local water treatment plant (Santa Maria, RS, Brazil). The sample was oven-dried at 80 °C for 12 h. Then, samples of 50 g were thermally treated at five different temperatures (100, 200, 300, 400, 500, and 600 °C) in oxidizing atmosphere furnace for 2 h with a heating rate of 10 °C min−1. All materials were sieved using a 400 mesh sieve (particle size lower than 38 µm) to get homogeneous samples for characterization and use. The samples were named as In_natura, 100C, 200C, 300C, 400C, 500C and 600C.
Sludge characterization
Several characterization techniques were used to verify the impact of the thermal treatment on the sludge characteristics and its adsorptive potential, as follows: (I) weight loss was measured by thermogravimetric analysis (TGA) with 50 mL min−1 flow air, temperature from 25 to 1000 °C, and a heating rate of 10 °C min−1 (Shimadzu, TGA-50/50H model); (II) samples weight was measured in analytical balance (Bel, M214Ai model) before and after thermal treatment; (III) point of zero charge (PZC) was determined. 0.2 g of each material was added into a 250 mL Erlenmeyer flask containing 80 mL 0.1 mol L−1 NaCl solution. The initial pH (from 2 to 12) was adjusted with HCl 1.0 mol L−1 and NaOH 1.0 mol L−1, and the final pH was measured after stirring of 24 h at 120 rpm and room temperature; (IV) surface morphology and chemical composition of the samples were investigated by scanning electron microscopy (SEM) (Tescan, Vega3 SB model) coupled with energy dispersive spectroscopy (EDS); (V) the sludge’s crystalline structure was analyzed by X-ray diffraction (XRD) (Rigaku, Miniflex® 300) using Cu Ka radiation (λ = 1.54051 Å) in the range of 2θ = 5–100° at 30 kV and 10 mA; (VI) the adsorbents functional groups were characterized by Fourier transform infrared spectroscopy (FT-IR) (Shimadzu, IR Prestige model) using KBr pellets in a wavelength range of 400–4500 cm−1; (VII) N2 physisorption analysis with Brunauer–Emmett–Teller (BET) surface area, pore volume, and average pore size were measured by BET surface analyzer (Micromeritics, ASAP 2020 model).
Defluorination assays
Defluorination assays were carried out in batch mode using a dubnoff shaker (Marconi, MA095 model) at 25 °C. A synthetic fluoride solution was prepared with 0.221 g of NaF in 1 L of distilled water and then diluted to attain an initial F− concentration of 5.34 mg L−1. The pH of these solutions was adjusted to 6.0. The experiments were then carried out using 0.5 g of the sludge sample with 100 mL of fluoride solution in a 250 mL polypropylene Erlenmeyer flask, which was stirred at 150 rpm for 2 h. This procedure was applied to all sludge samples considered in this study. The solution’s pH was measured using a pH meter (Thermo Scientific, Orion Star A211 model), and the fluoride concentration was measured by ion chromatography (Metrohm, 930 Compact IC Flex model) after filtering the 10 mL of the liquid through a syringe filter (diameter 25 mm, pore size 0.22 μm). All the experiments were carried out in triplicate, and all chemicals used were of analytical grade. The statistical analysis of the data obtained for each material’s adsorption potential was carried out through the application of the Analysis of Variance (ANOVA) test and the comparison of means by the Tukey test at the significance level of 5%. The results were expressed in terms of fluoride removal percentage R (%) (Eq. 1) and adsorption capacity q (mg g−1) (Eq. 2):
where C0 and C are the initial and final concentrations of fluoride ions (mg L−1), respectively, m is the mass of adsorbent (g), and V is the volume of solution (L).
Results and discussion
Evaluation of the thermal treatment
Raw sludge obtained in a local water treatment plant (Santa Maria, RS, Brazil) was thermally treated at five different temperatures (100, 200, 300, 400, 500, and 600 °C) for 2 h with a heating rate of 10 °C min−1. This thermal treatment’s impact on the sludge characteristics was evaluated by TGA, weight loss, point of zero charge (PZC), SEM, EDS, XRD, FT-IR, and BET.
The thermal profile of raw sludge, which allows one to identify different occurrences due to temperature increase under the oxidizer atmosphere, is depicted in Fig. 1. Concerning the weight loss curve (black), two main regions can be identified, i.e., a strong weight loss from 25 to 500 °C followed by a less intense weight loss from 500 to 1000 °C. About the heat flow curve (blue), the main peaks were found at 155, 550, and 900 °C. The peak at 155 °C is due to removing free or physically linked water from the sludge. The weight loss from 25 to 500 °C can be attributed to the water removal and organic material oxidation. The peak at 550 °C is associated with water expulsion from aluminum hydroxide and its phase change to γ-Al2O3, so-called dehydroxylation. The peak close to 900 °C is due to the formation of characteristically crystalline oxides as Al2O3. The weight loss from 500 to 1000 °C can be attributed to removing water linked with inorganics and decomposition of inorganics like carbonates. These results were corroborated by the weight loss that occurred during the thermal treatment. Table 1 shows that the weight loss increased with the temperature and ranged from 20 to 36%.
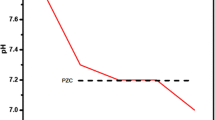
The point of zero charge (PZC) of all sludges (In_natura, 100C, 200C, 300C, 400C, 500C and 600C) is depicted in Fig. 2. Experimentally, the point of zero charge for the In_natura sample (dried raw sludge) was 5.5 (Fig. 2). As long as the calcination temperature increased, there was also the increase of point of zero charge in the interval between 5.0 and 6.5, with values of 5.4, 5.5, 5.7, 6.0, 6, 0, and 6.2 respectively for each material. It was perceived that the biggest increase happened between two samples, the calcinated at 300 °C and 400 °C one, being the probable annulation of superficial charges due to the increase in the calcination temperature and the consequent complete removal of organic matter, which was corroborated by the analysis of TGA.
In an adsorption operation, PZC is directly linked with the material surface charge, and consequently, with the sludges’ potential to uptake fluoride. It is known that at solution pH values lower than the adsorbent PZC, such material has its surface positively charged. On the other hand, in a medium with a pH higher than the PZC, the surface is negatively charged (Gao et al. 2020). The fluoride ions (F−), in turn, have a negative charge, so it is important to work under conditions in which the adsorbent material is positively charged. This happens in mediums whose pH is below PZC. However, very low pH values are related to the formation of hydrofluoric acid (HF), besides the complexes AlFe2+ and AlFe+, which result in a lesser adsorption (Kumari and Khan 2018). In this work, the pH of the solutions was adjusted to 6.0, and the PZC of the materials ranged from 5.5 to 6.2, depending on the thermal treatment temperature. This effect on fluoride adsorption will be better evaluated in “Potential of thermally treated sludge for water defluorination”.
The SEM images of all samples are presented in Fig. 3. Table 2 presents the atomic percentages of the main elements. The mapping of Al, Fe, C, and Si for In_natura and 600C sludge is presented in Fig. 4. An agglomeration of irregular particles of different sizes for both In_natura and thermally treated samples can be seen in Fig. 3. A well-distributed surface is perceived, with characteristic roughness according to all materials over the different calcination temperatures with no significant change in this aspect. In Table 2, In_natura sludge presented 26.1% of carbon, but after the thermal treatment at 600 °C, this content was eliminated. For aluminum in different materials, the average values of 4.89 for In_natura and 9.68% for 600C do not represent a significant change, given the losses that happened. It was noted that the elements are well distributed on the material surface, although they are in different quantities (Fig. 4). It was perceived small presence of carbon, especially at the highest temperature in which there is complete elimination of organic material. The predominant forms involving Al, Fe, and Si are hydroxides and oxides, depending on the calcination temperature to which the samples were submitted.
The microstructure is affected by chemical reactions during the thermal treatment as dehydration of Al(OH)3, Al2O3 formation, and amorphous silica crystallization (Tantawy 2015). The same was concluded by other studies that did not find crystalline traces on the sludge surface and reported its amorphous structure (Babatunde et al. 2008). The amorphous and porous nature of Al and Fe hydroxides makes the sludge a place of adsorption for most anions.
The X-ray diffraction patterns of all sludges (In_natura, 100C, 200C, 300C, 400C, 500C and 600C) are presented in Fig. 5. The In_natura sample (Fig. 5a) and the other calcinated materials have a predominantly amorphous character (Fig. 5b). Peaks that indicate poorly organized, shapeless, or without pattern particles are perceived. Kaolinite and quartz can be identified in the samples, which permits us to state that the material is composed of SiO2, Al2O3, and Fe2O3. The characteristic peaks of SiO2 can be identified in about 2θ = 20° and 50° (*); and of Al2O3 in 2θ = 27°, 36°, 39°, 55° and 78° (*) (Fig. 5a). When compared to the calcinated sample, it is possible to observe the reduction of the intensity of peaks related to the kaolinite, especially in 20° indicating that such crystalline phase amortization happened (Duarte et al. 2019; León-Mejía et al. 2018). The same did not happen to the other peaks.
The FT-IR spectra of In_natura and thermally treated sludges are presented in Fig. 6a and b, respectively. It was verified that most bands are kept through calcination in the region of 1600–3500 cm−1. The unmodified material (Fig. 6a) has a band of 3400–3700 cm−1 associated with the OH bonds stretching. Even with calcination, the band was still broad and strong. The In_natura material presents characteristic bands of other materials of the same nature, highlighting silica in 469, 797, and 1036 cm−1 and aluminum hydroxide in 550, 1700, and 3400 cm−1. The band in 1400 cm−1 is associated with carbonate, besides COO− symmetrical stretch, OH deformation, and C–O stretchings of phenolic groups in 1400–1465 cm−1. In the present study, the disappearance of that band in around 1384 cm−1, while Jeon et al. (2018) verified the band’s disappearance 1404 cm−1 for calcinated sludge at 500 °C. Thus, from 400 °C, the complete degradation of organic material was perceived since the carbon atomic percentage decreased. The organic material presents several characteristic bonds between 1050 and 1725 cm−1, which may be C=C aromatic type and conjugated carbonyl C=O in 1635–1643 cm−1 (Geng et al. 2009).
The band’s strong absorbance between 1035 and 1090 cm−1 was associated with stretching of the Si–O bond in the tetrahedral group [SiO4]. The band between 750 and 800 cm−1 was associated with the vibration of the bond Al–O in the tetrahedral group [AlO4]. The band close to 475 cm−1 is attributed to the bridged oxygen vibration, based on the deformational vibration of Si–O–Si and Si–O–Al bonds, in this case, in 470 cm−1 and kept in all materials (Sebdani et al. 2015). Bands of 912 and 539 cm−1 are associated with Al–O bonds and their alterations, which suggests the presence of amorphous forms of aluminum oxide and hydroxide (alumina structure) and favors the fluoride ions adsorption. Except for eliminating organic material, it was verified that the material maintained its characteristics with no contamination.
The values of surface area, pore volume, and pore size are depicted in Table 3. The surface area increased with calcination temperature until 400 °C, reaching 87.67 m2 g−1; however, it started decreasing at higher temperatures. The 600 °C calcinated material area of 55.14 m2 g−1 is even smaller than the 200 °C calcinated material area of 70.07 m2 g−1. In a certain way, the increase of surface area would increase adsorption capacity. Yet, it was perceived that the material had more significant changes in morphological and structural aspects besides the surface area. Considering the temperatures 500 °C and 600 °C, the decrease of surface area and the increase of average pore size may be attributed to the aluminum oxide and hydroxide crystallization (Jeon et al. 2018). The average pore size increased to higher temperatures, 13.27 mn for 500 °C calcinated sludge and 19.22 nm for 600 °C. In this case, the mesopores so far blocked by organic material are revealed as such material’s decomposition happens with calcination (Jeon et al. 2018).
The N2 adsorption–desorption plot from BET analysis of sludge calcinated at 200 °C and 600 °C are depicted in Fig. 7. It was concluded that for calcinated materials at 200 °C and 600 °C, the same structure was maintained. According to the International Union of Pure and Applied Chemistry, the isotherms were of type IV, typical of mesoporous materials between 2 and 50 nm (Thommes et al. 2015). This behavior agrees with the values of average pore size, which were presented in Table 3 (from 10 to 20 nm).
Potential of thermally treated sludge for water defluorination
The potential of In_natura and thermally treated sludges for water defluorination was tested by batch adsorption. The fluoride removal percentage and adsorption capacity of all sludges are depicted in Fig. 8a and, the final fluoride concentration in the liquid phase is depicted in Fig. 8b. The sludges calcinated at 200 °C and 300 °C showed the highest adsorption capacity of 1.05 mg g−1, resulting in 98.13% removal compared to In_natura, with 0.946 mg g−1 capacity (88.58% removal), and 600 °C with 0.634 mg g−1 (59.36% removal). Sludge calcinated at 600 °C had the worst performance as fluoride adsorbent. It is probably because aluminum oxides/hydroxides crystallization occurs during thermal treatment, its reduced surface area, and the complete removal of organic matter. It was the only material that did not reach the potability standard of 1.5 mg L−1 (Fig. 8b). On the other hand, at temperatures below 500 °C, some organic material remained in the sludge. From the removal results obtained, it can be said that these functional groups’ presence is favorable to fluorine adsorption.
Also, concerning removals close to 100%, it can be considered that acid solution initial pH favors fluoride adsorption according to the material point of zero charge. It was possible not only to decrease the concentration of fluoride ions to the potability standard but also to eliminate it. In a large-scale treatment, an alternative may be the mixture of two streams, one with excess and one free from fluoride ions for dilution until the appropriate concentration value is reached. Based on these results, it can be stated that the sludge treated at lower temperatures is an advantage for lower energy consumption.
A comparison of the fluoride adsorption in other adsorbent materials is shown in Table 4. These results show that materials considered as waste or by-products need high dosages and pH adjustment to obtain great adsorption capacities. It would probably be necessary to adjust the final pH value to 6.0 to obtain the real situation’s potability standard in a drinking water treatment. The material prepared in this work, in turn, is efficient with lower dosages and in the real pH for water defluorination. In this context, therefore, in the present study, the 200C sludge presented an interesting value and can be considered reasonable given the process conditions. The calcined materials at higher temperatures did not present satisfactory results, considering the greater energy expenditure to obtain them and the low adsorptive potential presented.
By the Tukey test of comparison of means, the removal results for all calcination temperatures except 200 and 300 °C were considered different; only those of these two materials did not differ at the 5% significance level. Thus, the thermal treatment was effective in changing the adsorption capacity of the materials.
Conclusion
Sludge from a water treatment station in Santa Maria, RS (Brazil) was calcinated at five different temperatures. The thermally treated materials were compared with the unmodified material concerning its defluorination potential. The characterization techniques verified that the process of calcination completely removed the organic material and reduced the material surface area. However, groups OH and Al–O had not been modified. The amorphous structure kept the same characteristic peaks of silica and alumina, which are oxides usually found in materials of that nature. The best water defluorination performance was verified for calcinated materials at 200 and 300 °C (98.13% removal and 1.05 mg g−1 of adsorption capacity). The unmodified sludge obtained 88.58% and 0.95 mg g−1 and the calcinated at 600 °C, 59.36%, and 0.63 mg g−1, respectively. At higher calcination temperatures, at 500 °C and 600 °C, there was a reduction in the adsorption. These results indicated that the low-temperature calcination is viable to manage the sludge generated in the water treatment station (Santa Maria, RS, Brazil). Also, the sludge can be transformed into a locally available and low-cost adsorbent for fluoride removal from waters. This way, it can be applied to large-scale treatment processes that provide safe potable water to the population.
References
Ahamad KU, Singh R, Baruah I, Choudhury H, Sharma MR (2018) Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder. Groundw Sustain Dev 7:452–458
Ahmad T, Ahmad K, Ahad A, Alam M (2016a) Characterization of water treatment sludge and its reuse as coagulant. J Environ Manag 182:606–611
Ahmad T, Ahmad K, Alam M (2016b) Sustainable management of water treatment sludge through 3‘R’ concept. J Clean Prod 124:1–13
Babatunde AO, Zhao YQ, Yang Y, Kearney P (2008) Reuse of dewatered aluminium-coagulated water treatment residual to immobilize phosphorus: batch and column trials using a condensed phosphate. Chem Eng J 136(2–3):108–115
Barathi M, Kumar ASK, Rajesh N (2019) Impact of fluoride in potable water - an outlook on the existing defluoridation strategies and the road ahead. Coord Chem Rev 387:121–128
Bhaumik R, Mondal NK, Das B, Roy P, Pal KC, Das C, Baneerjee A, Datta JK (2012) Eggshell powder as an adsorbent for removal of fluoride from aqueous solution: equilibrium, kinetic and thermodynamic studies. EJ Chem 9:1457–1480
Duarte AL, Da Boit K, Oliveira MLS, Teixeira EC, Schneider IL, Silva LFO (2019) Hazardous elements and amorphous nanoparticles in historical estuary coal mining area. Geosci Front 10:927–939
Dwivedi AD, Dubey SP, Gopal K, Tandon VK (2010) A comparative investigation for strengthening the adsorptive phenomenon by activated natural minerals and plant waste-carbon for defluoridation in water milieu. Desalination 263:189–199
Gao M, Wang W, Yang H, Ye BC (2020) Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc-magnesium-aluminum ternary oxide microspheres. Chem Eng J 380:122459
Geng W, Nakajima T, Takanashi H, Ohki A (2009) Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 88:139–144
Jangkorn S, Kuhakaew S, Theantanoo S, Klinla-or H, Sriwiriyarat T (2011) Evaluation of reusing alum sludge for the coagulation of industrial wastewater containing mixed anionic surfactants. J Environ Sci 23:587–594
Jeon EK, Ryu S, Park SW, Wang L, Tsang DCW, Baek K (2018) Enhanced adsorption of arsenic onto alum sludge modified by calcination. J Clean Prod 176:54–62
Kazi TG, Brahman KD, Baig JA, Afrid HI (2018) A new efficient indigenous material for simultaneous removal of fluoride and inorganic arsenic species from groundwater. J Hazard Mater 357:159–167
Kumari S, Khan S (2018) Effect of Fe3O4 NPs application on fluoride (F) accumulation efficiency of Prosopis juliflora. Ecotoxicol Environ Saf 166:419–426
León-Mejía G, Machado MN, Okuro RT, Silva LF, Telles C, Dias J, Niekraszewicz L, Da Silva J, Henriques JAP, Zin WA (2018) Intratracheal instillation of coal and coal fly ash particles in mice induces dna damage and translocation of metals to extrapulmonary tissues. Sci Total Environ 625:589–599
Leyva-Ramos R, Rivera-Utrilla J, Medellin-Castillo NA, Sanchez-Polo M (2010) Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chem Eng J 158:458–467
Loganathan P, Vigneswarana S, Kandasamy J, Naidu R (2013) Defluoridation of drinking water using adsorption processes. J Hazard Mater 248–249:1–19
Maraschin M, Ferrari KFSH, Silva APH, Carissimi E (2020) Aluminum sludge thickening: Novel helical pipes for aggregation by dual flocculation and thickening by filtration applied to water treatment plants. Sep Purif Technol 24:116560
Mohammad A, Majumder CB (2014) Removal of fluoride from synthetic waste water by using “bio-adsorbents.” Int J Res Eng Technol 3:776–785
Mohan D, Sharma R, Singh VK, Steele P, Pittman CU Jr (2012) Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: equilibrium uptake and sorption dynamics modeling. Ind Eng Chem Res 51:900–914
Sebdani MM, Mauro JC, Jensen LR, Smedskjaer MM (2015) Structure-property relations in calcium aluminate glasses containing different divalent cations and SiO2. J Noncryst Solid 427:160–165
Singh S, German M, Chaudhari S, Sengupta AK (2020) Fluoride removal from groundwater using zirconium impregnated anion exchange resin. J Environ Manag 263:110415
Siswoyo E, Qoniah I, Lestari P, Fajri JA, Sani RA, Sari DG, Boving T (2019) Development of a floating adsorbent for cadmium derived from modified drinking water treatment plant sludge. Environ Technol Innov 14:100312
Tantawy MA (2015) Characterization and pozzolanic properties of calcined alum sludge. Mater Res Bull 61:415–421
Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55:4363–4389
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Tor A, Danaoglu N, Arslan G, Cengeloglu Y (2009) Removal of fluoride from water by using granular red mud: batch and column studies. J Hazard Mater 164:271–278
WHO (1998) Guidelines for drinking water quality, 2nd edn. Addendum to health criteria and other supporting information. World health organization, Geneva, vol 2, pp 123–152
Xingbin S, Chengju X, Zhaochao H (2010) The fluoride-adsorption capacity and influencing factors study of Zeolite. Int Conf Chall Environ Sci Comput Eng 1:358–361
Xu L, Chen G, Peng C, Qiao H, Ke F, Hou R, Li D, Cai H, Wan X (2017) Adsorptive removal of fluoride from drinking water using porous starch loaded with common metal ions. Carbohydr Polym 160:82–89
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pigatto, R.S., Somavilla, E.A., Carissimi, É. et al. Thermally treated sludge obtained from a coagulation–flocculation water treatment process as a low-cost and eco-friendly adsorbent for water defluorination. Braz. J. Chem. Eng. 38, 451–460 (2021). https://doi.org/10.1007/s43153-021-00117-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00117-2