Abstract
This study successfully applied an improved valvometry technique to measure waterborne copper (Cu), based on valve activity dynamics of the freshwater clam Corbicula fluminea. The improved valvometry technique allows the use of free-range bivalves and avoids causing stresses from experimental artifacts. The proposed daily valve rhythm models and a toxicodynamics-based Hill model were linked to predict valve dynamic responses under different Cu exposures with a circadian valve rhythm endpoint. Cu-specific detection threshold was 5.6 (95 % CI 2.1–9.3) and 19.5 (14.6–24.3) μg L−1 for C. fluminea, based on response times of 300 and 30 min, respectively. Upon exposure to Cu concentrations in excess of 50 μg L−1, the alteration of valve rhythm behavior was correlated with Cu concentration within 30 min, indicating notable sensing ability. This study outlines the feasibility of an in situ early warning dynamic biomonitoring system for detection of waterborne Cu based on circadian valve activities of C. fluminea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to direct contact with terrestrial biomes, the contamination of freshwater ecosystems as a result of human activities has become a growing public concern worldwide in the last few decades. Among the more common pollutants, heavy metals are of particular concern due to their persistence, bioaccumulatory potential, and toxicity. In order to accurately assess potential ecological and human health risks posed by heavy metals, it is crucial to be able to rapidly, continuously, and accurately monitor their waterborne concentrations. Various chemical analysis methods, such as atomic absorption (AAS), atomic fluorescence spectroscopy (AFS), atomic emission spectroscopy (AES), and inductively coupled plasma-mass spectrometry (ICP-MS), have conventionally been used for this purpose and are essential tools to detect waterborne metals. However, all these technologies are labor-intensive, costly, and not suitable for application to the continuous and real-time in situ monitoring of water quality (Liao et al. 2005; Jou and Liao 2006). Biomonitoring, a process that uses the behavioral and/or physiological changes of aquatic organisms under various stresses, has been proposed as a less expensive and more effective alternative for the monitoring of metals in aquatic ecosystems (Borcherding and Wolf 2001). Consequently, many researchers have been dedicated to the development and in situ application of biological early warning systems (BEWS) in recent years for the continuous monitoring of water quality to conserve aquatic ecosystems and protect species health.
Because of their wide distribution, ease of collection, environmental sensitivity, and ability to accumulate high concentrations of heavy metals, freshwater bivalves are recommended by a number of studies as ideal species for biomonitoring purposes (Legeay et al. 2005; Liao et al. 2005). The Asian clam, Corbicula fluminea, a filter-feeding bivalve living in the upper layers of sediments, is an economically important species with a wide distribution throughout the world (Ortmann and Grieshaber 2003). In addition to natural populations, aquaculture of C. fluminea is also a growing industry within Taiwan, because an extract of this species contains markedly high concentrations of ornithine, an amino acid that has been reported to improve human liver function (Jou and Liao 2006). C. fluminea aquaculture sites are now widely distributed throughout the coastal areas of Taiwan. Recently, C. fluminea has been suggested as an ideal sentinel organism for use in the biomonitoring of heavy metals in freshwater ecosystems. Several studies have employed this species for the monitoring of waterborne metals, including cadmium (Tran et al. 2003; Legeay et al. 2005; Jou and Liao 2006), arsenic (Santos et al. 2007; Liao et al. 2009; Chen et al. 2012), and copper (Cu) (Tran et al. 2004; Jou and Liao 2006; Liao et al. 2007). Consequently, in order to better assess the feasibility of potential biomonitoring techniques utilizing C. fluminea in the field, delineating the physiological or behavioral responses produced by the effects of specific heavy metals on this species is necessary.
There are various biological endpoints, including both lethal and sublethal effects that are used for biomonitoring purposes. Behavioral endpoints, due to their relative sensitivity, ecological relevance, and ease to assess, are particularly useful in both laboratory and field settings. Most of the studies dealing with the behavioral endpoints of bivalves exposed to waterborne metals have been based on observations of burrowing activities, valve closing behavior, valve movement response, or siphon extension. For example, Bonnard et al. (2009) proposed that the burrowing behavior of the bivalve Scrobicularia plana provides an ecologically relevant tool for environmental monitoring. Sobrino-Figueroa and Caceres-Martinez (2009) indicated that alterations in valve movement and closing speed of the bivalve Argopecten ventricosus caused by toxic metals can be used as a reliable biomarker in a biomonitoring setting. El-Shenawy (2004) studied the effects of several heavy metals on the behavioral response of the bivalve Ruditapes decussatus and recommended siphon extension as a suitable indicator for online, in situ monitoring of various waterborne metals. To enhance the feasibility of biomonitoring techniques in practice, it is therefore important to delineate the specific stress-response profiles of bivalves exposed to specific contaminants.
Copper is ubiquitous in the aquatic environment as a consequence of various mining, agricultural, and industrial activities, as well as arising naturally from geochemical processes (Bonnard et al. 2009). Cu is an essential micronutrient for plants, animals, and humans as a constituent of several enzymes and as a redox catalyst in a variety of metabolic pathways (Devez et al. 2005). Cu deficiency may result in damage to biological functions and even mortality. On the other hand, it is well recognized that elevated concentrations of Cu are toxic to organisms as well. In Taiwan, Cu contamination in aquatic ecosystems has received particular attention due to a mass mortality of oysters along coastal areas caused by high levels of Cu pollution in 1986. Due to its environmental and toxicological significance in aquatic ecosystems, therefore, online, long-term biomonitoring of Cu by bivalves has been widely studied and evaluated by a number of research studies in recent years (Liao et al. 2005; Jou and Liao 2006; Bonnard et al. 2009; Jou et al. 2009).
The aim of this study was to determine the dose-response relationship of C. fluminea exposed to waterborne Cu, which would enhance one’s ability to design biomonitoring systems for continuous, in situ monitoring of Cu levels in water. Both valve closing and siphon extension were chosen as the behavioral endpoints in the present study. The ecotoxicological parameters of these two sublethal effects were estimated and compared to determine the sensitivity of both behavioral responses under Cu stress, a crucial metric when assessing the feasibility of such systems for biomonitoring within aquatic ecosystems.
Materials and methods
The protocol for implementing behavioral ecotoxicology modeling of freshwater clam valve rhythm in response to waterborne Cu is shown in Fig. 1 and is described in the subsequent sections. The acclimation information related to tested specimens as sentries and environmental conditions are described in the Supplementary materials.
Valve movement observation
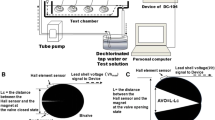
In this study, valvometry was performed based on the valve movement observation protocol described in our recent study (Jou et al. 2013), allowing a well-established sequence framework for quantifying the daily valve behavioral rhythms in a free-range setting. The configuration of clam acclimation and observation aquariums with a follow-through circulation system is illustrated in Fig. 2a. An inductance-based valvometry device by attaching a pair of lightweight electrical coils to the valves was constructed to be used as the sensor for determining the degree of bivalve openness (Fig. 2b, c). One of the two electrical coils (Fig. 2b) was connected to a function generator (Fig. 2d) supplying an oscillating sinusoidal potential difference at a frequency of 4 kHz with a voltage amplitude of 10 V. The inductance generated in the other coil attached to the other clam valve was used to output signal voltages according to the degree of valve openness (Fig. 2b).
The magnitude of the induced signal voltage, which was inversely proportioned to the distance between the two electrical coils glued on the valves, was transformed to indicate the status of shell position of each clam. The inductance-based valvometry apparatus can detect variations in the distance between two mutual inductances, one glued onto each valve. Through the use of a previously developed valvometry system (see Fig. 2b–h), which included 16 inductance-based valvometry devices (Fig. 2b–d) associated with corresponding isolated signal processing circuits (Fig. 2e), a data acquisition interface card (NI DAQPad-6259, Fig. 2f), and a monitor-specific compiled LabVIEW program (Fig. 2g), the dynamic valve activities of 16 free-range clams were recorded dynamically and in real-time (Fig. 2h) using a personal computer (PC).
A clam extends its siphon for filtering plankton, and the extension of the siphon is in accordance with the magnitude (%) of bivalve shell gape. Valve activities can be identified by using three primary indicators: (1) siphon extension (SE), (2) siphon withdrawal (SW) (but valve opening [VO] is regarded as an unsteady state [US]), and (3) valve closing (VC). The previous study results demonstrated that the magnitudes of shell gape of 20 and 50 % can be adopted as the thresholds of VC and SE states, respectively, to digitalize valve movements in C. fluminea (Jou et al. 2013).
Three key behavioral activities of the bivalves were monitored in both unpolluted and Cu-polluted environments: (1) the time-varying proportion of clams showing VC and SE states in a given group of clams, (2) the single time point average magnitude of shell gape in a given group of clams, and (3) the average daily duration of VC/VO (DVC/DVO) in a given group of clams. Hence, this newly developed clam-based behavioral monitoring system can measure and record the relative shell positions of unconstrained clams. Valve movements in C. fluminea before each Cu exposure experiment were continuously recorded and quantified as the reference status (0 μg L−1) to establish the background valve activities in the 16 tested animals.
Behavioral toxicity assays
In this study, the behavioral endpoints were VC and SW responses. The time for the probability of spontaneous valve closure at its lowest was observed in order to minimize any confounding SW/VC effects due to exposure to unspecific waterborne metals. The bivalve exposure experiments were performed in the laboratory. Based on the valve behavioral assay proposed by Jou et al. (2013), the bivalve behavioral movements in response to Cu were observed as SW and VC states. More precise conversions of SE and VO behavioral responses (to SW and VC states, respectively) in a group of clams after the acute addition of Cu to the water can be quantified using the following equations:
where R
SW(t
R, C
w) and R
VC(t
R, C
w) are the observed response proportion (%) under SW and VC states at any given response time t
R, N
SEoriginal state
(t
0) and N
VOoriginal state
are the total number of observed specimens showing either the SE and VC states before Cu exposure at the initial time t
0, N
SE → SW(t
R)and N
VO → VC(t
R) are the corresponding change number from SE/VO to SW/VC at any given response time t
R−
, \( {R}^{\overline{\mathrm{DVO}}}\left({C}_{\mathrm{w}}\right) \) is the normalized response value (%) based on the 24-h-based mean DVO (%) in a group of clams exposed to Cu concentration C
w, and \( {\overline{\mathrm{DVO}}}_{\mathrm{unpolluted}} \) and \( {\overline{\mathrm{DVO}}}_{C_{\mathrm{w}}} \) are the corresponding mean DVO in a group of clams exposed to unpolluted water and waterborne Cu.
Tested clams were randomly settled into groups of eight in each of the two test tanks to obtain the dose-response profiles with various Cu exposure concentrations under different response times (Fig. 3a). Cu chloride (CuCl2) stock solution was diluted to the required concentration using deionized water. C. fluminea were exposed under various Cu concentrations of 20, 50, 100, 200, and 500 μg L−1 with a mean pH of 8.26 and a mean temperature of 22.2 °C. Three replicates were observed for each Cu exposure concentration with 16 clams per replicate. No mortality was observed during the behavioral assays. A Perkin-Elmer Model 5100PC atomic absorption spectrometer (Perkin-Elmer, Shelton, CT, USA) equipped with an HGA-300 graphite furnace atomizer was used to measure the total Cu concentration in the water. The limit of detection was 0.1 μg Cu L−1. Valve behavioral toxicity assays were carried out from April 10 to 24, 2011.
Flowchart showing the modeling methodology for: a I the R VC/R SW-based response time profiles based on an empirical three-parameter Hill model under different Cu exposure concentrations; II the R VC/R SW-based time-varying dose-response profiles based on the TD-based Hill model; III the R VC/R SW-based EC50 profiles as the effective Cu concentration causing 50 % of total valve behavioral response of bivalves at different response times; and b a primary approach to an early warning system for modeling changes in valve behavioral rhythm due to exposure to waterborne Cu (see text for a detailed description of the symbols)
Modeling approach
The overall observed data of bivalve behavioral movement in response to Cu were analyzed to construct the respective R SW/VC-based response time profiles (Fig. 3a–i) based on an empirical three-parameter Hill model under different Cu exposure concentrations. The empirical three-parameter Hill model can be expressed as follows:
where R SW/VC is the observed response proportion (%) of a given number of clams showing the SW/VC state at the same time to the total population, ETSW/VC50 is the effective time to yield the SW/VC response under a specific Cu concentration equivalent to half of the maximal response R SW/VCmax , t R is the exposure time (min), and the exponent n SW/VC is a fitted Hill coefficient that is a measure of cooperativity according to SW/VC state. The ETSW/VC50 values were estimated under different Cu exposure concentrations. By fitting the toxicodynamics (TD)-based Hill model, the R SW/VC-based time-varying dose-response profiles to the overall observed data were constructed (Fig. 3a, II). The TD-based Hill model can be calculated based on Eq. (4) as
where ECSW/VC50 is the effective Cu concentration (μg L−1) that yields a SW/VC response reaching half of the maximal response R SW/VCmax , C w is the waterborne Cu concentration (μg L−1), and the exponent n SW/VC is a fitted Hill coefficient that is a measure of cooperativity according to SW/VC state. The ECSW/VC50 values were estimated at different response times from 0 to 300 min (Fig. 3a, III).
Figure 3b shows the primary approach used in this study for modeling early warning changes in the valve behavioral rhythm of clams subjected to Cu stress. By incorporating the derived ECSW/VC50 time and fitted response time-dependent n SW/VC value profiles into the constructed dose-response models, a response surface function of the response time t R and waterborne Cu concentration C w can be acquired to determine the time-varying effective profiles. Thus, a Hill model-based dose-response function was further deduced to describe the overall valve movement behaviors (VMBs) of bivalves in response to waterborne Cu at any given response time.
where R SW/VC(t R, Cw) is the observed SW/VC-based response (%) at response time t R, ECSW/VC50(t R) is the time-dependent effective Cu concentration that yields half of the SW/VC-based maximal response R SW/VCmax (μg L−1), and the exponent n SW/VC(t R) is a time-dependent SW/VC-based Hill coefficient.
For the unpolluted environment condition, a time-varying three-parameter lognormal function was applied to describe the proportions of each valve behavioral response of free-range C. fluminea:
where A log is the amplitude, B log is the maximum value of t, C log can be derived from the area under curve (\( \mathrm{A}\mathrm{U}\mathrm{C}={A}_{\log}\cdot {B}_{\log}\cdot {C}_{\log}\cdot \sqrt{2\pi \exp \left({C}_{\log}^2\right)} \)), and D is the basic offset in the y-axis. f(t, 0) can be defined at the SE and VC states under the uncontaminated environmental condition to build the respective time-varying valve rhythm proportion functions of φ VC(t, 0) and ψ SE(t, 0) for databases of local clam-based biomonitoring systems.
The variation of valve response rhythm after acute Cu addition can be approached by combining Eq. (6) and the fitted time-varying valve rhythm proportion functions of φ VC(t, 0) and ψ SE(t, 0):
where φ VC(t, C w) and ψ SE(t, C w) are the time-varying valve rhythm proportion functions for describing clams under VC and SE states, respectively, when exposed to concentration C w. The above conceptual schematic flowchart (Fig. 3b) depicts a primary predictive mechanism to model early warning changes in valve behavioral rhythm subjected to waterborne Cu.
Data analysis
The mathematical expressions of nonlinear regression of the TableCurve 2D (Version 5, AISN Software Inc., Mapleton, OR, USA) package were employed to carry out all model fittings (Eqs. (3)–(6)) to the observed data in laboratory experiments. The Crystal Ball software (Version 2000.2, Decisioneering, Inc., Denver, CO, USA) was used to obtain the optimum distribution of sampling data. When the number of sampling data points exceeded 40, the chi-square test was used to test the sampling data; when it was lower than 40, the Kolmogorov-Smirnov test was used.
Statistical significance was judged as p < 0.05. Maximum-likelihood estimators were used to estimate the distribution of all measured data, including the corresponding magnitudes (%) of bivalve shell gape at VC and SE states in clams. Monte Carlo simulation was performed to generate the 2.5 and 97.5 percentiles as the 95 % confidence interval (CI) for fitted model parameters. The Crystal Ball software was used to implement the Monte Carlo simulation.
Results
Daily valve rhythm observation
Figure 4 shows the daily valve movement monitored from 16 C. fluminea clams under the condition of spontaneous behavior. Using the pattern of shell gaping magnitude (%), a 14-day continuous recording of individual daily valve activity of the 16 individuals is shown in Fig. 4a. Figure 4b shows the corresponding relationships between valve activities and the magnitude (%) of shell gape in C. fluminea, with the valve opening magnitudes of 20 and 50 % used to differentiate VC/VO and SW/SE states. The corresponding digitized results shown in Fig. 4c, d exhibit the VC/VO and SW/SE states of the 16 clams acquired over 14 days, respectively. The time-varying profiles of the proportion of a given number of clams showing VC, US, and SE states at the same sampling time in a day to the total number of clams are respectively shown in Fig. 4e–g. Based on statistical analysis of the observed data in each day (n = 14) shown in Fig. 4e. g, the β distribution was determined to be optimal.
The performance of daily valve movement monitoring. a Patterns of magnitude (%) of shell gape over a 14-day continuous recording of individual daily valve rhythm activities in 16 clams. b The relationship between valve activities (VC, VC, SW, and SE) and the valve opening magnitudes of 20 and 50 % in C. fluminea, which are used as the threshold of the VC/VO and SW/SE states. Digitalized results for c VC/VO and d SW/SE states of 16 clams. Profiles of time-varying proportions (with mean and standard deviation) of a given number of clams showing e VC, f US, and g SE states at the same sampling time (time interval 2 min) to the total number of clams. Daily valve rhythm profiles with 95 % CI obtained using a statistics-based approach and the fitted three-parameter lognormal model, describing bivalve behavioral movements of clams exposed to unpolluted environment under h VC and i SE states (see text for detailed descriptions)
For representing daily valve activity rhythms, the maximum-likelihood method was adopted to perform a Monte Carlo simulation (n = 10,000) to obtain the probability distribution of the time-varying proportion of VC/SE behavior of the total clams. The profiles describing daily valve behavioral movements of clams in VC and SE states under the unpolluted condition are shown in panels h and i of Fig. 4, respectively, with 95 % CI and the corresponding fitted three-parameter lognormal model. The results revealed that the time-varying proportion of VC/SE behavior of C. fluminea follows an impressive daily trace. The fitted three-parameter lognormal models for valve daily rhythm of VC and SE can be represented, respectively, as
where φ VC(t, 0) and ψ SE(t, 0) are the daily valve rhythm functions of time-varying proportions of VC and SE states, respectively. The results also indicated that the VC and SE responses of clams occur predominantly in the morning (0600–0800) and in the evening (1900–2100).
During the 14-day observed duration, the circadian shell gaping magnitude (represented by mean ± SD) in 16 C. fluminea at the same time is shown in Fig. 5a. The time-varying average magnitude of shell gape (Fig. 5a: \( \overline{M}(t) \)%) was calculated by the mean value of shell gaping magnitude in clams at the same time. The circadian valve rhythmic period as shown in Fig. 5a was compared with the periodic variation of valve daily rhythmic proportion under SE status shown in Fig. 4g, revealing that both valve daily rhythmic periods are near identical. The difference between Figs. 4g and 5a is that the circadian valve rhythmic differential degree of peak/trough of the former (ψ SE(t)%, 55.4/18.3 and 41.1/14.3) was more predominant than that of the latter (\( \overline{M}(t) \)%, 44.6/22.3 and 36.9/16.9) at the same time. The magnitudes of shell gape of 50 % as the determining thresholds of SE statuses were adopted to digitalize the valve movement for quantifying time-varying proportion showing SE statuses (Fig. 4g: ψ SE(t)%) in a group of clams.
The time-varying average magnitudes (\( \overline{M}(t) \)%) of shell gape under SE and VO states are shown in panels b and c of Fig. 5, respectively. The total average magnitude of shell gape in a group of clams under SE and VO states were 75.0 ± 2.6 % (mean ± SD) and 73.0 ± 2.9 % that exhibits a near level, respectively. The above results demonstrated that the quantification of time-varying proportion showing SE statuses (Fig. 4g: ψ SE(t)%) in a group of clams has better identifiable capability to assess valve behavioral rhythm than that of the time-varying average magnitude of shell gape (Fig. 5a: \( \overline{M}(t) \)%).
Valve behavioral reactions to waterborne Cu
A circadian continuous recording of individual valve activities (i.e., SE/SW and VO/VC) of the 16 C. fluminea is shown in Fig. S1 (see Supplementary materials), displaying their changes from SE/VO to SW/VC states at response time t R from 0700 to 1200 after the addition of Cu to the system. Figure 6a, b shows the fitted empirical three-parameter Hill model-based exposure time (min) SW and VC response profiles and the corresponding fitted parametric values under specific Cu concentrations of 20, 50, 100, 200 and 500 μg L−1. The Hill model and a 10,000 trial Monte Carlo simulation provided an adequate parametric fit for the data analysis (χ 2 goodness of fit, p > 0.5) with high r 2 values (Fig. 6a: 0.81–0.99, p < 0.05; Fig. 6b: 0.93–0.99, p < 0.05). The fitted Hill coefficients (n SW/n VC) ranged from 2.56/4.38 to 3.98/6.12, indicating positive cooperativity. The estimated ETSW/VC50 values varied with the increase of Cu exposure concentration and decreased notably from 27.1/56.6 (95 % CI 21.2–32.9/53.4–59.9) min at 20 μg L−1 Cu to 11.2/22.7 (10.6–11.7/21.2–24.2) min at 500 μg L−1 Cu. When Cu exposure concentration was >200 μg L−1, the evaluated R SW/VCmax response values measured up to 100 %. The ETSW50 values were notably lower than the corresponding ETVC50 values of each specific Cu exposure concentration.
Diagram showing the modeled experimental data representing valve behavioral reactions to waterborne Cu. The fitted Hill model-based exposure time a SW and b VC response profiles and the corresponding fitted parametric values under specific Cu concentrations of 20, 50, 100, 200, and 500 μg L−1, respectively. The fitted Hill model-based Cu concentration c SW and d VC response relationships varied with different response times of 10, 15, 30, 60, 120, and 300 min, respectively. The time-dependent e ECSW50(t R)/ECVC50(t R) and f n SW/n VC profiles with the experimental data and curve fittings, based on the behavioral endpoints of SW and VC responses. g The fitted Hill model-based concentration-response profile (C w) with 95 % CI
By fitting the TD-based Hill model, the R SW/VC-based time-varying dose-response profiles to the overall observed data were constructed and are shown in Fig. 6c, d. The figures show that the fitted Hill model-based Cu concentration SW/VC response relationships varied with different response times of 10, 15, 30, 60, 120, and 300 min. The results revealed that the Hill model and a 10,000 trial Monte Carlo simulation provided an adequate parametric fit for the data (χ 2 goodness of fit, p > 0.5) with high r 2 values (Fig. 6c: 0.97–0.99, p < 0.05; Fig. 6d: 0.88–0.99, p < 0.05). The fitted Hill coefficients (n SW/n VC) varied with the increase of exposure time, decreasing notably from 1.55/4.23 to 1.18/1.04. Based on the fitted concentration-response model (Fig. 6c, d), the ECSW/VC50 values were estimated to be 73.2/70.6, 43.7/48.8, 19.5/35.9, 8.8/19.8, 6.6/6.5, and 5.6/5.0 μg L−1 for valve response times of 10, 15, 30, 60, 120, and 300 min, respectively. Figure 6c, d also reveals that the addition of low Cu exposure concentrations causes a significant change from SE/VO to SW/VC states between 30 and 60 min, suggesting that SW/VC-based valve activities can be used as sensitive indicators of low concentrations of waterborne Cu.
A nonlinear exponential model of a + b · exp(−t R/c), where a, b, and c are fitted parameters, was used to best fit the estimated ECSW/VC50 and n SW/n VC values (as shown in Fig. 6c, d) with exposure times from 10 to 300 min (time interval 5 min). The modeled equations of ECSW50(t R) plus ECVC50(t R) and n SW(t R) plus n VC(t R) are shown in panels e and f of Fig. 6, respectively. Furthermore, from the comparison of the time-dependent ECSW50(t R) and ECVC50(t R) profiles shown in Fig. 6e, it was revealed that the SW-based valve response is the most suitable behavioral endpoint for the detection of low Cu exposure concentrations within 60 min of Cu addition.
Based on the continuous recordings (Supplementary Fig. S1a, c) exhibiting individual daily VO rhythmic state of the observed 16 C. fluminea exposed to unpolluted environment and waterborne Cu, the above observed data under specific Cu exposure concentrations of 0, 20, 50, 100, 200, and 500 μg L−1 was analyzed and quantified to acquire the corresponding mean DVO values (%) of 33.4, 30.9, 23.5, 20.5, 13.5, and 12.5 %, respectively. The above calculated data of a 24-h-based mean DVO percentage (%) and Eq. (3) were used to normalize the valve daily DVO response (\( {R}^{\overline{\mathrm{DVO}}}\left(\%\right) \)) under unpolluted environment and Cu exposure. The fitted Hill model-based concentration-response profile (\( {R}^{\overline{\mathrm{DVO}}} \)(C w )) with 95 % CI is shown in Fig. 6g. In light of the results shown in Fig. 6g, the relationships between the valve DVO response (\( {R}^{\overline{\mathrm{DVO}}}\left(\%\right) \)) and Cu concentration fitted by the Hill model (r 2 = 0.97), indicating that \( {\mathrm{EC}}^{\overline{\mathrm{DVO}}}50 \), is estimated to be 66.79 μg L−1 with a \( {R}_{\max}^{\overline{\mathrm{DVO}}}=64.31\;\% \) and n = 1.89.
From the circadian continuous recording (Supplementary Fig. S1b) exhibiting the VO rhythmic state of the 16 C. fluminea subjected to Cu stress, it can be found that tested specimens have a sufficient acclimation capability during the 1-day observed duration after Cu addition. On account of the above results, the mean DVO-based maximum response is notably more or less 64.31 % and it is not to gain 100 % under these exposure conditions. In general, a clam-based BEWS can rapidly send out an alarm within a few hours after acute Cu addition. In terms of a biologically sensitive endpoint, the valve DVO response (\( {R}^{\overline{\mathrm{DVO}}}\left(\%\right) \)) is not appropriately used as a quick detecting index for constructing a clam-based biological early warning system.
Valve rhythm activities in response to Cu
To investigate the dynamic response of valve rhythm activities after Cu addition, the clams were exposed to Cu concentrations of 20, 50, 100, 200, and 500 μg L−1 at 0700 in order to continuously observe the alteration in SE/VO states. Valve response data were collected between the morning hours of 0500 and 1200. The above experimental results are shown in Fig. 7. It can also be found that the valve rhythm response exhibited was noticeable within 30 min of exposure to concentrations of Cu in excess of 50 μg L−1, indicating the potential effectiveness of the system’s application to the rapid detection of even low-level increases in Cu concentration.
Model prediction and validation
Here, the proposed daily valve rhythm models of SE/VC in clams in an unpolluted situation (Eqs. (10) and (11), Fig. 4h, i) and the Hill model-based time- and concentration-specific SW/VC response function (Eq. (6), Fig. 6c–f) were integrated into the time-varying proportion functions of valve rhythm in response to Cu (Eqs. (8) and (9)) in order to predict changes in the valve behavioral rhythm of clams subjected to Cu stress at any given response time. The simulation results based on experimental data in the morning hours from 0600 to 1200 (as shown in Fig. 8a, b) indicate that the predicted valve rhythm activity changes in SE and VC states of clams exposed to different Cu concentrations (20 to 500 μg L−1 h) are in agreement with observed data (Fig. 7a–e and k–o).
The differentiation between the predicted and observed SE/VC-based valve rhythm responses under Cu exposure concentrations of 20, 50, 100, 200, and 500 μg L−1 were validated to be 5.38/6.45, 4.05/4.16, 4.56/4.32, 1.51/1.62, and 1.45/2.33 % by calculation of the root-mean-squared errors (RMSE). The results also demonstrate that the modeling approach employing Eqs. (8) and (9) based on the R SW and R VC indices calculated by Eqs. (1) and (2) has a satisfactory prediction capability for describing valve rhythm response variations.
In order to determine the effects of various valvometry settings and associated acclimation conditions on daily valve rhythm observations, the average magnitude of shell gape in bivalves was used as an index for the comparison of daily valve rhythms in the present work with those in previously published studies (Sluyts et al. 1996; Ortmann and Grieshaber 2003; Jou et al. 2009), as shown in Fig. 9.
In this study, the relative voltage/distance-based valve rhythmic traces were based on the average valve opening magnitude (%), exhibiting a twin peak/trough pattern over a 12-h period. Figure 9 shows that the degree of voltage-based valve alteration was greater than that of the distance-based alteration. The distance-based measuring index has been favored for the expression of valve rhythm traces in previous publications (Nagai et al. 2006; Chambon et al. 2007; Basti et al. 2009; Tran et al. 2010; Gnyubkin 2010; Sow et al. 2011). Tables 1 and 2 present the comparison of daily valve mean DVOs and rhythm variations under various conditions.
Table 3 shows a comparison between the estimated EC50 values and experimental data published by Tran et al. (2004), indicating that the test specimens exposed to Cu under SW response-based observations exhibited a range of EC50 values similar to those previously published.
Discussion
Daily valve rhythm observations
Higgins (1980) reported that when plankton and algae concentrations are favorable for bivalve feeding, they have a longer daily valve opening time. Through this modulation of feeding time, bivalves exhibit natural rhythmic valve alterations (Borcherding 1994; Williams and Pilditch 1997). Garcia-March et al. (2008) indicated that bivalves have an apparent valve opening/closing response to sunrise/sunset. Gnyubkin (2010) also described that the circadian rhythm of Mediterranean mussels was influenced by variations in light conditions (i.e., sun altitudes in different seasons or variations in artificial illumination) resulting in a distinct daily pattern of repeated valve opening. Hence, rhythmic behavioral patterns of valve opening and closing appear to be greatly influenced by specific environmental factors (e.g., temperature, pH, photoperiod, feeding fashion, etc.). In addition, the experimental setup employed (i.e., fixed or unconstrained conditions) influences valve behavioral pattern observations.
The valvometry measuring method used in this study is inspired by the ideas described by Tran et al. (2003). The improved valvometry technique resulted in minimal artificial stress being placed on the clams, thus reducing the probability of eliciting abnormal valve closing reactions. In this work, the efforts were to minimize the influences of the abovementioned environmental factors in order to readily characterize the rhythmic pattern of daily valve movements using the fitted three-parameter lognormal model developed through statistical analysis.
The improved inductance-based valvometry technique was used to observe and measure valve movements, revealing the daily SE/VC-based valve behavioral activities over two 12-h periods. In this study, a computational mechanism based on relative quantification (Jou et al. 2013) was used to normalize the valve opening magnitude (%) of each clam without respect to the difference in body size among individuals. These observed data are associated with confidence intervals in order to characterize the intrinsic valve rhythms of the SE/VC state, while still allowing for the inclusion of variables related to regional variation in C. fluminea behavior.
Estimations of the response time-specific ECSW/VC50 values
The Hill model (Eqs. (4) and (5)) was used to construct time-specific concentration-response relationships (Eq. (6)) in order to determine R SW/VC-based EC50 values. The R VC-based EC50 values determined in this study were relatively higher than other SW- and previously published VC-based responses over a 60-min integrated time period. The results also revealed that Cu detection thresholds of 5.6 (95 % CI 2.1–9.3) μg L−1 for 300 min and 19.5 (95 % CI 14.6–24.3) μg L−1 for 30 min are allowable when using EC50(t R) profiles. One of the objectives of this study was to determine the valve responses of 50 % in clams as a time-varying function of Cu exposure in order to rapidly detect acute changes in aquatic Cu concentrations. Higher Cu concentrations can elicit valve responses in a relatively short exposure period, whereas the detection of lower concentrations requires a much longer integration time.
The modeled time-specific dose-response profiles could potentially be compiled as a database for reference in the use of such rapid detection systems. This study did not consider the natural variation in background Cu concentrations but, instead, focused on the nominal value. According to published standards of environmental water quality in Taiwan, the Cu concentration in public aquatic areas should be less than 30 μg L−1. The minimum detectable Cu concentrations can be estimated based on the length of the exposure period and an expected sensitivity threshold. The R SW/VC-based valve rhythm response data, as generated in an uncontaminated environment, is suitable for future reference within a database that might be used for implementing a quantitative and comprehensive assessment of a clam-based BEWS in aquatic environments.
Implications
Traditional techniques of assessing the degree of contamination in aquatic environments suffer from issues of slowness, nonspecificity, and cost. Yet another concern is that such assessments involve measuring potentially toxic agents and risk of exposure via water samples or internal target organ specimens of study organisms; ideally, best laboratory practices would minimize risk to scientists though. The dynamic nature of exposure responses is virtually impossible to accurately model using existing chemical assessment techniques, especially when the pollution event itself is dynamic. Recent advances in sensing technologies allow for the provision of real-time data collection and the synchronic measurement of multiple agents using a single apparatus. This improved valvometric instrumental conversion technique can evaluate the valve behavioral reaction when exposed to other metals (e.g., Cd, Hg, and As) or a mixture of metals. Furthermore, this valvometry technique can be employed to study the sensitivity of the valve closure response following the addition of acute contaminants in aquatic environments.
Clams protect themselves from waterborne contaminants by closing their shells and reducing feeding activities; this permits them to retain biotic function and minimize toxicant damage (Kadar et al. 2001). Two different valve positions can be selected as monitoring responses in signal analyses with respect to behavioral activities of the clam. In the present study, the previously developed inductance-based valvometry technique based on the quantification of SE/VC states in C. fluminea (Jou et al. 2013) was integrated as a dynamic response, Cu-specific detection mechanism. Experiments utilizing various Cu concentrations revealed that lower concentrations can induce apparent and detectable reactions as changes of SE/SW and VO/VC states. The implication of this result is that an optimal sensitivity threshold can be determined according to the expected response time, and thus, at what time an early warning should be sent out in a clam-based biomonitoring system can be assessed in terms of SE/SW and VO/VC reactions.
For any toxicant detection mechanism, the false positive is one important issue that must be especially addressed. In the present methodology, ECSW/VC50, the effective concentration causing 50 % of C. fluminea to exhibit SW/VC reactions, is high enough that any potential risk of false positives should be avoidable. Additionally, the field application of such systems in natural environments is necessarily influenced by many environmental factors, and so these must be determined and quantified in the laboratory prior to in situ use. Upon field application, such a system will be subject to equipment malfunctions as well as changes in water quality. For example, precipitation may cause changes in water temperature, dissolved oxygen, pH, and suspended solids. Hence, careful evaluation of response patterns to a variety of environmental stimuli is quite critical to the development of an accurate monitoring system, as is monitoring common water quality parameters such as temperature, dissolved oxygen, pH, and conductivity (van der Schalie et al. 2001).
In addition, other factors such as gender, age, and general physiology of the organisms can also influence in the responsiveness of the individuals when exposed to contaminants such as Cu. In the natural environment, aquatic organisms are frequently exposed to mixtures of contaminations, where the toxicity of the combinations of chemicals will be approximately additive, synergistic, potentiated, or antagonistic. The combined valve rhythm toxicity effects of metal mixtures on freshwater clam should be considered in future research that could monitor in real-time the multimetal toxicity under environmental relevant situations. In future research, a statistic model and auxiliary water quality sensors will be employed to carefully evaluate the time-varying clam daily rhythm in the field for providing an accurate estimate of waterborne metals (see Supplementary Fig. S2). When the accurate estimated metal concentration exceeds the threshold concentration (e.g., EC50(t R = 60 min)) and sustains a given number of minutes, the alarm threshold is triggered to send out a warning.
In future research, more environmental parameters could be added to improve the performance and guard against false positives even more strongly. Future research could include the determination of the influence of additional real-life scenario environmental factors, with the aim of optimizing a working model for field application. While the mechanical aspects of the working system are ready for field trial, it is likely that various environmental factors might distort clam behavior to such a degree that false positives and false negatives would be encountered frequently. The inclusion and consideration of additional factors will likely lead to a substantial increase in the limit of detection associated with the system. However, a working BEWS that dramatically reduces the lag period in one’s detection of pollutant fluctuations could be developed based on this technology.
Conclusions
Through the use of reliable statistical modeling approaches, the proposed daily valve rhythm models and the TD-based Hill model can be utilized to predict valve dynamic responses under different Cu exposure concentrations, taking into account circadian valve rhythm endpoints, while using a precise valvometry system to validate the simulation. The ultimate aim was to design a dynamic BEWS based on an accurate quantification of C. fluminea valve activities and observations of the dose-response relationship. The integration of the TD-based Hill model and a region-dependent endogenous daily valve rhythm model provides an important theoretical advantage that can potentially delineate both physiological and environmental factors affecting the valve behavior of C. fluminea in response to Cu.
References
Basti L, Nagai K, Shimasaki Y, Oshima Y, Honjo T, Segawa S (2009) Effects of the toxic dinoflagellate Heterocapsa circularisquama on the valve movement behaviour of the Manila clam Ruditapes philippinarum. Aquaculture 291:41–47
Bonnard M, Romeo M, Amiard-Triquet C (2009) Effects of copper on the burrowing behavior of estuarine and coastal invertebrates, the Polychaete Nereis diversicolor and the bivalve Scrobicularia plana. Hum Ecol Risk Assess 15:11–26
Borcherding J (1994) The ‘Dreissena-Monitor’-improved evaluation of dynamic limits for the establishment of alarm thresholds during toxicity tests and for continuous water control. In: Hill IR, Heimbach F (eds) Freshwater field tests for hazard assessment of chemicals. Lewis, Boca Raton, pp 477–484
Borcherding J, Wolf J (2001) The influence of suspended particles on the acute toxicity of 2-chloro-4-nitro-aniline, cadmium, and pentachlorophenol on the valve movement response of the zebra mussel (Dreissena polymorpha). Arch Environ Contam Toxicol 40:497–504
Chambon C, Legeay A, Durrieu G, Gonzalez P, Ciret P, Massabuau JC (2007) Influence of the parasite worm Polydora sp. on the behavior of the oyster Crassostrea gigas: a study of the respiratory impact and associated oxidative stress. Mar Biol 152:329–338
Chen WY, Jou LJ, Chen SH, Liao CM (2012) A real-time biomonitoring system to detect arsenic toxicity by valve movement in freshwater clam Corbicula fluminea. Ecotoxicology 21:1177–1187
Devez A, Gomez E, Gilbin R, Elbaz-Poulichet F, Persin F, Andrieux P, Casellas C (2005) Assessment of copper bioavailability and toxicity in vineyard runoff waters by DPASV and algal bioassay. Sci Total Environ 348:82–92
El-Shenawy NS (2004) Heavy-metal and microbial depuration of the clam Ruditapes decussatus and its effect on bivalve behavior and physiology. Environ Toxicol 19:143–153
Garcia-March JR, Solsona MAS, Garcia-Carrascosa AM (2008) Shell gaping 104 behaviour of Pinna nobilis L., 1758; circadian and circalunar rhythms revealed by in situ monitoring. Mar Biol 153:689–698
Gnyubkin VF (2010) The circadian rhythms of valve movements in the mussel Mytilus galloprovincialis. Russ J Mar Biol 36(6):419–428
Higgins PJJ (1980) Effects of food availability on the valve movement and feeding behaviour of juvenile Crassostrea virginica (Gmelin). I. Valve movement and periodic activity. Exp Mar Biol Ecol 45:229–244
Jou LJ, Chen WY, Liao CM (2009) Online detection of waterborne bioavailable copper by vale daily rhythms in freshwater clam Corbicula fluminea. Environ Monit Assess 155:257–272
Jou LJ, Lin SC, Chen BC, Chen WY, Liao CM (2013) Synthesis and measurement of valve activities by an improved online clam-based behavioral monitoring system. Comput Electron Agric 90:106–118
Jou LJ, Liao CM (2006) A dynamic artificial clam (Corbicula fluminea) allows parsimony on-line measurement of waterborne metals. Environ Pollut 144:172–183
Kadar E, Salanki J, Jugdaohsingh R, Powell JJ, McCrohan CR, White KN (2001) Avoidance responses to aluminium in the freshwater bivalve Anodonta cygnea. Aquat Toxicol 55:137–148
Legeay A, Achard-Joris M, Baudrimont M, Massabuau JC, Bourdineaud JP (2005) Impact of cadmium contamination and oxygenation levels on biochemical responses in the Asiatic clam Corbicula fluminea. Aquat Toxicol 74:242–253
Liao CM, Jau SF, Lin CM, Jou LJ, Liu CW, Liao VHC, Chang FJ (2009) Valve movement response of the freshwater clam Corbicula fluminea following exposure to waterborne arsenic. Ecotoxicology 18:567–576
Liao CM, Jou LJ, Chen BC (2005) Risk-based approach to appraise valve closure in the clam Corbicula fluminea in response to waterborne metals. Environ Pollut 135:41–52
Liao CM, Jou LJ, Lin CM, Chiang KC, Yeh CH, Chou BYH (2007) Predicting acute copper toxicity to valve closure behavior in the freshwater clam Corbicula fluminea supports the biotic ligand model. Environ Toxicol 22:295–307
Nagai K, Honjo T, Go J, Yamashita H, Oh SJ (2006) Detecting the shellfish killer Heterocapsa circularisquama (Dinophyceae) by measuring bivalve valve activity with a Hall element sensor. Aquaculture 255:395–401
Ortmann C, Grieshaber MK (2003) Energy metabolism and valve closure behaviour in the Asian clam Corbicula fluminea. J Exp Biol 206:4167–4178
Santos HM, Diniz MS, Costa PM, Peres I, Costa MH, Alves S, Capelo JL (2007) Toxicological effects and bioaccumulation in the freshwater clam (Corbicula fluminea) following exposure to trivalent arsenic. Environ Toxicol 22:502–509
Sluyts H, Van Hoof F, Cornet A, Paulussen J (1996) A dynamic new alarm system for use in biological early warning systems. Environ Toxicol Chem 15:1317–1323
Sobrino-Figueroa A, Caceres-Martinez C (2009) Alterations of valve closing behavior in juvenile Catarina scallops (Argopecten ventricosus Sowerby, 1842) exposed to toxic metals. Ecotoxicology 18:983–987
Sow M, Durrieu G, Briollais L, Ciret P, Massabuau JC (2011) Water quality assessment by means of HFNI valvometry and high-frequency data modeling. Environ Monit Assess 182:155–170
Tran D, Ciret P, Ciutat A, Durrieu G, Massabuau JC (2003) Estimation of potential and limits of bivalve closure response to detect contaminants: application to cadmium. Environ Toxicol Chem 22:914–920
Tran D, Fournier E, Durrieu G, Massabuau JC (2004) Copper detection in the Asiatic clam Corbicula fluminea: optimum valve closure response. Aquat Toxicol 66:333–343
Tran D, Haberkorn H, Soudant P, Ciret P, Massabuau JC (2010) Behavioral responses of Crassostrea gigas exposed to the harmful algae Alexandriumminutum. Aquaculture 298:338–345
van der Schalie WH, Shedd TR, Knechtges PL, Widder MW (2001) Using higher organisms in biological early warning systems for real-time toxicity detection. Biosens Bioelectron 16:457–465
Williams BG, Pilditch CA (1997) The entrainment of persistent tidal rhythmicity in a filter-feeding bivalve using cycles of food availability. J Biol Rhythm 12:173–181
Acknowledgments
This work was supported by the Ministry of Science and Technology of the Republic of China under Grant MOST 100-2313-B197-005. The authors acknowledge two anonymous reviewers for making significant contributions to improve the quality of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Henner Hollert
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 7510 kb)
Rights and permissions
About this article
Cite this article
Jou, LJ., Chen, BC., Chen, WY. et al. Sensory determinants of valve rhythm dynamics provide in situ biodetection of copper in aquatic environments. Environ Sci Pollut Res 23, 5374–5389 (2016). https://doi.org/10.1007/s11356-015-5735-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5735-5