Abstract
We conducted an evaluation of alterations produced in the valve closing speed of juvenile Argopecten ventricosus (Catarina scallop) exposed to the metals cadmium, chromium and lead, because of the connection of this response to the state of health of the mollusk. Bioassays were conducted with 50 juveniles (length 3 ± 0.5 cm) exposed to 0.02, 0.1, 0.2 mg Cd l−1; 0.1, 0.5, 1.0 mg Cr l−1; 0.04, 0.2, 0.4 mg Pb l−1 and 0.8 and 1.6 mg Cd + Cr + Pb l−1 for 480 h. The average valve closing speed at the end of the experiment was under 1 s in the control group, from 2 to 3.6 s in the bioassays with cadmium, from 1.4 to 3.4 s with chromium, from 3 to 12 s with lead, and from 12 to 15 s with the metal mixtures. It was found that there are significant differences between the values recorded in assays with metals and the control (P < 0.05). The retardation of valve closing in the organisms exposed to toxic substances is probably caused by damage to the sensory cilia located on the edge of the mantle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Catarina scallop (Argopecten ventricosus) (Pacific calico scallop) is an important fishing resource in the state of Baja California Sur, Mexico, as the state is responsible for 75–90% of production nationwide (Sagarpa 2005). Fifty years ago, consumption of the scallop was local, but in the 1970s, exports of the product to the U.S. began. Since then, production of this mollusk has declined between 16 and 86% (Sagarpa 2005). This decline is attributed solely to overfishing, as there are cases where scallop populations have disappeared entirely, as has happened in Ensenada de la Paz (Baqueiro 1982). Here, to date, no studies have been conducted to identify other factors that may cause the disappearance of bivalve beds, despite the fact that concentrations of the metals cadmium, chromium, copper, nickel and lead recorded in their sediments (4.2 ± 0.9 μg Cd g−1, 93.2 ± 28.3 μg Cr g−1, 43.2 ± 12.3 μg Ni g−1 y 52.7 ± 15.7 μg Pb g−1. Méndez et al. 1998; Green-Ruiz 2000; Shumilin et al. 2001) are higher than the levels established by Long et al. (1995) (ERL, effects range-low: 1.2 μg Cd g−1, 81 μg Cr g−1, 20.9 μg Ni g−1, 46.7 μg Pb g−1), which cause noxious effects in benthic organisms. In the Ensenada de la Paz, Catarina scallops are raised in a nursery managed by UABCS to obtain organisms which are used in restocking programs, making it important to carry out biomonitoring studies for the continuous rapid detection of harmful effects caused by contaminants present in there to protect the health of the species.
Changes in behavior of bivalves caused by xenobiotics have been used as biomarkers in monitoring programs. Scallops are the only bivalves that have the ability to swim long distances. This occurs in response to a variety of abiotic and biotic factors, including to escape from predators (Jenkins and Brand 2001; Powers and Kittinger 2002), to select habitat and changes in seasonal temperature (Winter and Hamiliton 1985; Maguire et al. 1999).
Behaviors similar to narcosis (excessive opening of valves), torpor, has been observed in bivalves exposed to low concentrations of toxic substances (El-Shenawy 2004) and in organisms near to spawning (Cáceres-Martínez, personal communication); such behaviors have also been reported in mollusks in conditions of stress (Tyurin 1991; García-March et al. 2006; Cáceres-Martínez, personal communication).
Valve closing speed is connected to the health of the organism, since adductor muscle lesions caused by parasites or infections, or alterations in sensory cells located on the tentacles at the edge of the mantle, cause changes in valve closing (Beninger and Le Pennec 1991).
Since no research has been done on the behavior of A. ventricosus exposed to toxic metals, in this study we conducted an assessment of behavioral alterations and changes in valve closing speed of juvenile Pacific calico scallop exposed to the metals Cd, Cr and Pb, which are found in high concentrations in Ensenada de la Paz, Baja California Sur, Mexico. (Méndez et al. 1998; Green-Ruiz 2000; Shumilin et al. 2001), to evaluate the use of these responses as reliable biomarkers in biomonitoring studies.
Method
Juvenile Catarina scallops were obtained from the “culture park” or nursery located in a cove near the Port of Pichilingue (24°15.364′N, 110°19.137′W), 500 m south of the facilities of the Universidad Autónoma de Baja California Sur (UABCS). The organisms were transported to the CICIMAR-IPN (Centro Interdisciplinario de Ciencias Marinas) Experimental Biology Laboratory, where they remained in acclimation for 6 days (APHA et al. 1994), under the following conditions: salinity 36 ppm, temperature 20 ± 2°C and constant bubbling. They were fed daily with a mixture of the microalgae Tetraselmis sp. and Chaetoceros sp. at a density of 300,000–800,000 cell/ml, cultured with Guillard F/2 medium (Stein 1973).
In bioassays, which were conducted with water changes every 48 h, 10 scallop juveniles (length 3 ± 0.5 cm) were exposed to three concentrations of the metals Cd, Cr and Pb, in quintuplicate, for 20 days (480 h). Two metal concentrations correspond to those established in NOM 001-SERMANAT (Norma Oficial Mexicana) for discharge into coastal waters (0.1, 0.2 mg Cd l−1; 0.5, 1.0 mg Cr l−1 and 0.2, 0.4 mg Pb l−1); the third corresponds to a 1:10 dilution (0.02 mg Cd l−1, 0.1 mg Cr l−1 and 0.04 mg Pb l−1). In addition, 2 concentrations of the mixture of the three metals were assayed (1.6 mg l−1 of metals: 0.2 mg Cd + 1.0 mg Cr + 0.4 mg of Pb l−1; 0.8 mg metals l−1: 0.1 mg Cd + 0.5 mg Cr + 0.2 mg Pb l−1).
Metals were added from standard solutions: (FAO 1987; APHA et al. 1994) CdCl2, Baker 99% purity, K2Cr2O7 Merk 99.5% purity and Pb(NO3)2 Baker 99% purity in 1% acidified distilled water and kept at 4°C. Nominal concentrations were confirmed by flame atomic absorption spectrophotometry (Perkin Elmer model 3100) (ASTM 1994).
Ten-liter aquariums with 10 organisms per aquarium were used (50 organisms per concentration of metal and mixtures). The conditions prevailing during the bioassay were: salinity: 36 ppm, temperature: 22 ± 1°C, dissolved oxygen: 7.2 ± 0.2, feeding: daily with a mixture of the microalgae Tetraselmis sp. and Chaetoceros sp. (300,000–800,000 cell/ml).
Observations were made to detect behavioral changes in each assay with metals and mixtures as of the start of the bioassays. In addition, the valve closing speed of each of the bivalves was determined every 24 h by stimulating the organisms with a glass rod and measuring shell closing time with a stopwatch.
The data obtained in the bioassays were subjected to an exploratory analysis through the Kolmogorov-Smirnov test to determine normality. Data were then analyzed using the ANOVA test (variance analysis) and a multiple comparison with the Tukey test was also conducted to determine the statistical significance of the differences found between the control and the various treatments with metals and their mixtures. A significance level of 0.05 was used for the analysis. (Zar 1996).
Results
At the end of the experiments, no mortality was observed in the control group or in the assays with concentrations of 0.02 and 0.1 mg Cd l−1, 0.1 and 0.5 mg Cr l−1, 0.04 and 0.2 mg Pb l−1, 0.8 mg l−1 Cd +Cr +Pb. At higher concentrations, the organisms began to die at 8 days (192 h); survival at 20 days (480 h) of exposure to the toxic substances was 78, 84, 80 and 76% in the assays with 0.2 mg Cd l−1, 1 mg Cr l−1, 0.4 mg Pb l−1 and 1.6 mg l−1 of the mixture of metals, respectively.
Behavioral responses observed in the bioassays with juvenile A. ventricosus exposed to metals were the following: escape movements, maximum valve opening (gaping), and variations in shell closing speed.
Escape movements
This type of behavior consisted of a kind of “swimming” through expulsion of water from the mantle, quickly opening and closing valves, thereby causing forward movement. These movements were observed in the individuals exposed to the mixture Cd + Cr + Pb (1.6 mg l−1) at the beginning of exposure to the metals. These movements ceased after the first 10 h of exposure to the toxic substances (Table 1).
Maximum valve opening (gaping)
Scallops open their valves, remaining in this position for 3–10 s (Fig. 1). These movements are repetitive. This type of behavior was observed in the organisms exposed to cadmium (0.2 mg l−1) after 120 h, in those treated with chromium (1 mg l−1) after 240 h, and those exposed to the mixture Cd + Cr + Pb (0.8 and 1.6 mg l−1) after 48 h of exposure. This type of movement was observed until completion of the bioassays (480 h) (Table 1).
Valve closing speed
In the experiments, it was observed that average valve closing time in the organisms exposed to toxic substances was higher than that for the controls.
It was also found that there are significant differences between the values recorded in assays with metals and the control (P < 0.05).
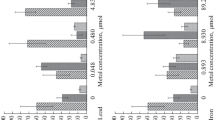
The average speed recorded in the control group was under 1 s. During the first 5 days of exposure, the organisms exposed to cadmium at concentrations of 0.02 and 0.1 mg l−1 closed their valves in a time period similar to that observed for the controls (Fig. 2); subsequently, at 20 days, the valve closing response was slower, observed to be from 2 to 2.8 s (Fig. 2).
In the assays with chromium, after 10 days of exposure (240 h) an increase in the time required to close valves was observed of between 0.68 and 2 s; and, at 20 days, the average time required to close valves was between 1.4 and 3.4 s (Fig. 2).
Juveniles exposed to lead took 3–12 s to close their valves at 20 days (480 h) of exposure.
Scallops exposed to the mixtures of metals (0.8 and 1.6 mg l−1) showed alterations in valve closing after 96 h of exposure; at the end of the bioassays, valve closing speed was observed at 15 and 12 s, respectively.
Upon completion of the bioassays, an inspection of the surviving organisms was conducted using a dissecting microscope (Olympus mod 20). Lesions were detected in sensory cilia at the start of the mantle in organisms exposed to lead (0.4 mg l−1) and the mixture of metals (0.8 and 1.6 mg l−1) (Fig. 3).
Discussion
The use of novel techniques for determining the presence of contaminants in water and sediments is required to prevent deterioration of aquatic ecosystems. The continuing discharge of wastewater into the coastal environment of Ensenada de la Paz is the main challenge facing sustainability of the ecosystem, due to growth in demand for residential area services, fishing and aquaculture, and tourism and recreational activities in the area.
It is estimated that 300,000 m3 of wastewater comes daily from the city of La Paz, in addition to the pollutants generated by the operations of PEMEX (Pétroleos Mexicanos) fuel deposits and the Punta Prieta thermoelectric plant, located in eastern Ensenada (INEGI 2005).
Behavioral evaluation has been proposed as a monitoring technique to detect environmental changes caused by contaminants. Shin et al. 2002 and Cooper and Bidwell 2006, have evaluated the burial conduct (burial rate) of mollusks as a response to exposure to metals and pesticides present in sediment. Variations in valve opening and closing time are also considered to be responses to metal exposure (Hughes et al. 1987; Inda and Cuturrufo 1999), but this response is not specific to toxic compounds since it is also provoked by factors such as temperature and low oxygen tension (Roger et al. 1990; Borcherding 2006).
The responses observed in juvenile A. ventricosus exposed to the metals Cd, Cr and Pb and their mixture were escape behavior through swimming, maximum valve opening (gaping), and alterations in valve closing speed.
Swimming behavior (motile escape) of the scallops is a mechanism to escape predators. In the assays conducted with A. ventricosus, this type of behavior was detected after exposing the organisms to the mixture of metals (1.6 mg l−1 Cr + Pb + Cd). Although this kind of response in bivalves caused by exposure to toxic metals had not been previously reported, changes in swimming pattern and speed have been observed in crustaceans (Gerhardt 1999; Gerhardt et al. 2002; Untersteiner et al. 2003).
Maximum valve opening (gaping) has been observed in specimens that are about to begin spawning, and has also been observed in organisms that are in a state of stress (Cáceres, personal communication). As mentioned above, adult A. ventricosus exposed to cadmium and chromium showed this type of behavior, but did not spawn. This response probably indicates a kind of “irritation” caused by exposure to the metals, causing valve opening.
Valve closing speed is connected to the health of the organism, since adductor muscle lesions caused by parasites or infections, or alterations in sensory cells located on the tentacles at the edge of the mantle cause valve closing disturbances (Beninger and Le Pennec 1991; Zhadan and Semenkov 1984). The retardation in valve closing in organisms treated with the toxic substances is possibly a result of the damage caused by the metals to the sensory cilia located on the edge of the mantle; these lesions cause the altered valve closing response, since the receptors that receive mechanical stimuli are located on these structures.
Based on our results, although behavioral responses such as escape swimming and maximum valve opening (gaping) are not specific to exposure to metals, alterations in valve closing speed do reflect possible damage due to these toxic substances. However, additional studies are required to be able to propose this behavioral response as a tool for biomonitoring of polluted sites.
References
APHA, AWWA, WPFC (1994) Métodos estandar para el exámen de aguas y aguas de desecho, 64th edn. Interamericana, Mexico
ASTM (American Society for Testing and Materials) (1994) Standard test methods for elements in water by metals atomic absorption spectroscopy. USA ASTM Committee on Standards 11, Philadelphia, Pennsylvania, USA
Baqueiro C (1982) Distribución y abundancia de moluscos de importancia comercial en Baja California Sur. Delegación Federal de Pesca, BCS
Beninger P, Le Pennec M (1991) Functional anatomy of scallops. In: Shumway SE (ed) Scallops: biology, ecology and aquaculture. Elsevier, Amsterdam, pp 133–223
Borcherding J (2006) Ten years of practical experience with the Dreissena-Monitor, a biological early warning system for continuous water quality monitoring. Hydrobiol 556:417–426
Cooper N, Bidwell J (2006) Cholinesterase inhibition and impacts on behavior of the Asian clam Corbicula fluminea after exposure to an organophosphate insecticide. Aquatic Toxicol 76:258–267
El-Shenawy NS (2004) Heavy-metal and microbial depuration of the clam Ruditapes decussatus and its effect on bivalve behavior and physiology. Environ Toxicol 19:143–153
FAO (1987) Manual of methods in aquatic environment research. Part 10. Short-term static bioassays. FAO Fisheries Technical Paper 247, Roma
García-March J, Sanchís Solsona MA, García-Carrascosa AM (2006) Shell gaping behaviour of Pinna nobilis L., 1758: circadian and circalunar rhythms revealed by in situ monitoring. Mar Biol 150:861–871
Gerhardt A (1999) Recent trends in online biomonitoring for water quality control. In: Gerhardt A (ed) Biomonitoring of polluted water. Trans Tech Publications, Environmental Science Forum, Uetikon-Zuerich, Switzerland, pp 95–118
Gerhardt AL, Janssens B, Mo Z, Wang C, Yang M, Wang Z (2002) Short-term responses of Oryzias latipes (Pisces: Adrianichthyidae) and Macrobrachium nipponense (Crustacea: Palaemonidae) to municipal and pharmaceutical wastewater in Beijing, China: survival, behaviour, biochemical biomarkers. Chemosphere 47:35–47
Green-Ruiz C (2000) Geoquímica de metales pesados y mineralogía de la fracción arcillosa de los sedimentos de cuatro puertos del Golfo de California. Tesis Doctoral ICMyL. Mazatlan, Sinaloa. 329 p
Hughes JM, Chapman HF, Kitching RL (1987) Effects of sublethal concentrations of copper and freshwater on behaviour in an estuarine gastropod Polinices sordidus. Mar Pollut Bull 18:127–131
Inda J, Cuturrufo M (1999) Valve closure by juvenile scallops as a protective mechanism in response to elevated levels of environmental copper. Bull Environ Contam Toxicol 63:520–523
INEGI (Instituto Nacional de Estadística, Geografía e Informática) (2005) Anuario Estadístico del Estado de Baja California Sur, México
Jenkins S, Brand A (2001) The effect of dredge capture on the escape response of the great scallop, Pecten maximus (L.): implications for the survival of undersized discards. J Exp Mar Biol Ecol 266:33–50
Long E, Mac Donald D, Smith S, Calder F (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97
Maguire J, O’Connor D, Burnell G (1999) An investigation into behavioural indicators of stress in juvenile scallops. Aquaculture 7:169–177
Méndez L, Acosta B, Alvarez T, Lechuga C (1998) Trace metal distribution along the Southern coast of Bahia de LaPaz, Gulf California, Mexico. Bull Environ Contam Toxicol 61:616–622
Powers SP, Kittinger JN (2002) Hydrodynamic mediation of predator–prey interactions: differential patterns of prey susceptibility and predator success explained by variation in water flow. J Exp Mar Biol Ecol 273:171–187
Roger B, Gnaiger E, McMahon R, Dietz T (1990) Behavioral and metabolic responses to emersion and subsequent reimmersion in the freshwater bivalve, Corbicula fluminea. Biol Bull 178:251–259
SAGARPA (2005) Anuario Estadístico de Pesca. Conapesca, México
Shin P, Ng A, Cheung R (2002) Burrowing responses of the short-neck clam Ruditapes philippinarum to sediment contaminants. Mar Pollut Bull 45:133–139
Shumilin E, Paez-Osuna F, Green-Ruiz C, Sapozhnikov D, Rodriguez-Meza G, Godinez-Orta L (2001) Arsenic, antimony, selenium and other trace elements in sediments of the La Paz lagoon, Peninsula of Baja California, Mexico. Mar Pollut Bull 42:174–178
Stein JR (1973) Handbook of phycological methods. University Press, Cambridge
Tyurin AN (1991) Behavioural reactions of the scallop, Mizuhopecten yessoensis, and the mussel, Crenomytilus grayanus, to reduced salinity and oxygen and exposure to synthetic detergents. J Hydrobiol 24:13–19
Untersteiner H, Kahapka J, Kaiser H (2003) Behavioural response of the cladoceran Daphnia magna Straus to sublethal copper stress—validation by image analysis. Aquat Toxicol 65:435–442
Winter M, Hamiliton P (1985) Factors influencing swimming in bay scallops Argopecten irradians (Lamarck, 1819). J Exp Mar Biol Ecol 88:227–242
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey
Zhadan PM, Semenkov PG (1984) An electrophysiological study of the mechanoreceptory function of abdominal sense organ of the scallop Patinopecten yessoensis (Jay). Comp Biochem Physiol 78A:865–870
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobrino-Figueroa, A., Cáceres-Martínez, C. Alterations of valve closing behavior in juvenile Catarina scallops (Argopecten ventricosus Sowerby, 1842) exposed to toxic metals. Ecotoxicology 18, 983–987 (2009). https://doi.org/10.1007/s10646-009-0358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0358-6