Abstract
The levels of 16 polycyclic aromatic hydrocarbons (PAHs) were determined in 8 passive air samples (PAS) and 6 lichen transplants (Ramalina fastigiata) deployed for a period of 2 months in different zones of Tarragona County (Catalonia, Spain), an area with an important number of chemical and petrochemical industries. The accumulated amount of the sum of the 16 PAHs ranged between 1363 to 7866 ng/sample in air samples. The highest concentration was found in the neighborhood of Puigdelfí (village of Perafort), in the vicinity of a big oil refinery and well under the potential influence of the petrochemical emissions. In lichen samples, the sum of the 16 PAHs ranged between 247 and 841 ng/g (dry weight), being the greatest value also observed in Puigdelfí. Data on the levels and profiles of PAHs in both passive monitoring methods were compared. A significant positive linear correlation was found between the concentrations of low molecular weight PAHs in lichens and the amounts accumulated in passive air samples (R = 0.827, P < 0.05), being especially significant the correlation of 4-ring PAHs (R = 0.941, P < 0.05). These results strongly suggest that lichens can be used to monitor gas-phase PAHs, providing data that can be quantitatively translated into equivalents for air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large group of organic compounds with two or more fused aromatic rings (Jaward et al. 2004; Nadal et al., 2004a; Wang et al. 2010; Xia et al. 2013). PAHs are products of incomplete combustion processes of natural (e.g., volcanoes, forests fires, and grassland combustion) or anthropogenic origin (e.g., incineration, power generation, domestic heating, vehicles emissions, and industrial activities) (Jaward et al. 2004; Wang et al. 2010; Estellano et al. 2012). Therefore, the environmental PAH burdens are expected to be different depending on the proximity of emission sources. In recent years, the toxic character of PAHs has been recognized (Estellano et al. 2012). Hence, the United States Environmental Protection Agency (USEPA) developed a list of 16 priority PAHs (Bohlin et al. 2010; Wang et al. 2010; Choi et al. 2012; Harner et al. 2013), 7 of them being classified as probable human carcinogens under the B2 group (Leung et al. 2015). Other agencies, such as the International Agency for Research on Cancer (IARC), have also classified some PAHs as carcinogens or possible carcinogens to humans. Among them, benzo[a]pyrene (BaP) has been identified as one of the most toxic (Bohlin et al. 2010; Xia et al. 2013).
Petrochemical industries have been pointed out as one of the most important anthropogenic sources of PAHs (Nadal et al. 2011). One of the largest chemical and petrochemical industrial complexes in Southern Europe is located in Tarragona County (Catalonia, Spain) (Nadal et al. 2004a, 2009). This area is composed of a total of 33 companies, including a big oil refinery, as well as a number of chemical and petrochemical industries. In addition, the presence of a highway and several roads with heavy traffic has a notable impact on the local environment (Nadal et al. 2004a, b). Since 2002, a large multicompartmental environmental monitoring program has been carried out by analyzing PAHs as well as other chemical pollutants in soil and vegetation samples from different areas of Tarragona County (Nadal et al. 2004a, b, 2007, 2009, 2011; Schuhmacher et al. 2004).
The need to monitor the occurrence of PAHs in air has led to the development of a large range of methods and devices for sampling (Zabiegała et al. 2010). Passive air samplers (PAS) with polyurethane foam (PUF) have become a viable alternative to commonly used active air sampling equipment since those devices are cheaper, smaller, simpler to handle, and they do not require electricity and maintenance (Mari et al. 2008). These characteristics assist the deployment in remote areas and the simultaneous collection of samples at different locations and/or scales (Pozo et al. 2004; Bohlin et al. 2008; Zabiegała et al. 2010; Estellano et al. 2012; Vilavert et al. 2013, 2014).
At the same time, the use of lichens (symbioses of fungi and algae) as bioindicators has proved to be very useful for the evaluation of environmental levels of PAHs (Augusto et al. 2013a; Loppi et al. 2015). Lichens are one of the most used organisms to monitor atmospheric deposition of several pollutants (Augusto et al. 2013a). They are easy to sample, while they allow monitoring of a large number of sampling sites and several pollutants within the same matrix (Augusto et al. 2010). In the past, PAHs accumulated in lichens have been compared with those measured in the particulate-phase of air by using filters from active air samplers (Augusto et al. 2013b). However, a valid comparison between PAHs in lichens and those in the air gas-phase is still missing. Very recently, Loppi et al. (2015) tried to overcome this gap, establishing a correlation between the levels of six PAHs measured in passive air samplers and those analyzed in lichen transplants of Evernia prunastri. The correlation between PAHs in lichens and in the gas-phase of air is crucial to optimize the use of lichens to monitor these compounds. Therefore, it is fundamental to understand whether other species of lichens are also related to airborne PAHs and lichens can be broadly applied for environmental monitoring.
The aim of this study was to determine the air levels of 16 PAHs from different areas of Tarragona County, Spain by means of passive sampling devices. Furthermore, the PAH levels and profiles in lichen transplants (Ramalina fastigiata) were correlated with those measured in air. Finally, the temporal trends of PAHs in air samples were determined by comparing current data with previously reported results.
Materials and methods
Sampling
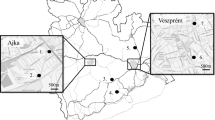
A total of eight passive air samplers and eight lichen transplants were deployed for a period of 2 months (November 26, 2014–January 26, 2015) in different areas of Tarragona County, Spain. The distribution of the sampling sites was as follows: two samples were deployed under the potential influence of the petrochemical complex, which includes a big oil refinery (Puigdelfí = P1 and Constantí = P2); two samples close to a zone of chemical industries (La Laboral = C3 and La Canonja = C4); two samples in urban areas (Tarragona = U5 and Vila-Seca = U6); and two samples in background sites, 30 km away from the area of influence of all the suspected sources of contamination (Cambrils = B7 and Torredembarra = B8) (Fig. 1). Sampling sites were located at less than 2 km from the considered pollution sources.
Passive air samplers
PAS containing polyurethane foam (PUF) discs were purchased from Newterra (Beamsville, ON, Canada) (diameter 14 cm; thickness 1.2 cm; surface area 360 cm2; density 0.035 g cm−3). Prior to use, PUFs were precleaned by Soxhlet extraction for 24 h by using dichloromethane (DCM) (purity 99.5 %, Scharlab, S.L., Sentmenat, Spain). Subsequently, they were dried in a vacuum desiccator, stored in precleaned brown glass jars, and sealed with high density polyethylene caps (Bohlin et al. 2008; Pozo et al. 2009). Once in the field, PUF discs were inserted in the PAS.
Lichen transplants
Lichens of the fruticose species Ramalina fastigiata (Pers. Ach.) were collected in November 2014 in mountains of L’Albiol (ca. 30 km to the north of the sampling area). Healthy R. fastigiata thalli of about 2–4 cm long were collected from the available phorophytes, notably from pine and oak trees, and transplanted within 48 h to the same eight sampling sites as PAS. Transplants consisted of nylon bags of 15 × 15 cm filled with approximately 5 g of lichens, which were hung in handrails of monitoring stations or home balconies. After 2 months of exposure, transplants were collected, stored in brown glass bottles, protected from sunlight, and immediately stored at 4 °C. All samples (transplants after exposure and background samples) were analyzed for PAHs.
Chemical analysis
PAHs in PUFs were extracted using an accelerated solvent extraction (ASE) 200 (DIONEX, Sunnyvale, USA) through loading the sampled filters into 33 mL extraction cells. The solvent used for foams was DCM (99.9 % pure, supplied by Prolabo, VWR Spain). ASE was conducted with a 6-min preheat time, a static time of 6 min, an extraction temperature of 120 °C, an extraction pressure of 1500 psi, two subsequent cycles, a purge time of 120 s, and a flushing volume of 100 %. The extracts obtained were evaporated to about 1 mL using an R-114 rotary evaporator (Büchi, Flawil, Swiss). Before the evaporation step, 400 μL of dimethylformamide was added to prevent loss of analytes. Prior to the chromatographic analysis, internal standards (IS) (d8-naphtalene, d10-acenaphthene, d10-phenanthrene, d12-chrysene, and d12-perylene) were added, and analytes were again dissolved in 1 mL of DCM.
The 16 USEPA priority PAHs, namely naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-c,d)pyrene, dibenzo(a,h)anthracene, and benzo(g,h,i)perylene, were determined and quantified in the extracts by gas chromatography/mass spectrometry (GC/MS) by means of a QP2010 Ultra System (Shimadzu Corporation, Tokyo, Japan). The chromatographic separation was carried out on a ZB-50 analytical column (50 % phenyl-dimethylpolysiloxane, 30 m × 0.25 mm id; 0.25 μm film thickness) from Micron Phenomenex (Torrance, California, USA). For the analysis, a volume of 1 μL of the sample extract was injected in splitless mode, with a column rate of 1 mL/min. The compounds were quantified by a target ion using internal standard calibration and identified by retention times and qualifier ions. To calculate extraction recoveries, two PUFs were spiked with 20 μL of a 0.1 mg/L standard solution, one before and one after the extraction, being afterward analyzed. In addition, a blank PUF was also analyzed, and the peak signals were subtracted from the above. The extraction recoveries were calculated by comparing the peak area ration (analyte versus IS) of the two analysis, being ranged between 66 and 116 %, for benzo(k)fluoranthene and acenaphthylene, respectively (Table S1). The instrumental linear range was between 1, 5, 10, or 20 μg L−1 and 1000 or 5000 μg/L, depending on the analyte. In all cases, determination coefficients (r 2) were higher than 0.99 except for indeno(1,2,3-c,d) pyrene that was 0.98. The limits of detection (LODs) and the limits of quantitation (LOQs) were estimated from the instrumental signal and taking into account extraction recoveries. For LODs, a signal/noise ratio of three was considered, while LOQs were fixed to the lowest instrumental calibration point. Thus, LODs ranged from 0.3 to 5 ng/PUF, while LOQs ranged from 1 to 20 ng/PUF (Table S1).
PAHs in lichen samples were extracted using the Soxhlet method, as described by Augusto et al. (2010). Approximately 2 g of lichen sample was extracted in a Soxhlet for 24 h with 300 mL of acetonitrile (99.8 % pure, Optigrade supplied by Promochem, Germany). After extraction, the extracts were concentrated by rotary vacuum evaporation and cleaned up in a florisil column (aolid phase extraction cartridges, Extrabond Florisil 2 g, 12 mL, supplied by Scharlab S.L.) with 30 mL of acetonitrile as eluting solvent. Then, the extracts were evaporated and concentrated with a gentle stream of purified N2 to 1 mL. The 16 USEPA priority PAHs in the lichen extracts were also quantified by GC/MS using a gas chromatograph (Hewlett-Packard G1099A) coupled with a mass spectrometer (Agilent MSD5973). An HP-5MS 5 % phenyl methyl siloxane capillary column (30 m, 0.25 mm id, 0.25 μm film thickness) (Agilent 19091S-433) was used for separating the PAHs. For the analysis, a volume of 1 μL of the sample extract was injected in splitless mode, with a column rate of 1.4 mL/min. The masses monitored by the detector were set as follows: from 40 to 600 m/z.
PAHs in lichen samples were quantified by a target ion using internal standard calibration and identified by retention times and qualifier ions. Recoveries were achieved by spiking a portion of lichen with a solution of the 16 USEPA PAHs and with d10-fluorene prior to extraction. Recovery percentages differed according to individual PAHs, ranging between 56 and 151 %. The instrumental linear range was between 0.6, 1 or 5 and 500 μg/L, depending on the analyte. Determination coefficients (r 2) were higher than 0.98, except for naphthalene (r 2 = 0.82) and acenaphthylene (r 2 = 0.96). LODs ranged from 0.5 to 5 ng/g, while LOQs ranged from 1 to 20 ng/g (Table S1).
Data analysis
For the calculations, those PAHs presenting levels below the LOD or LOQ were assumed to have a concentration equal to one half of the LOD or LOQ. Pearson linear correlation coefficients between lichens and air passive samplers were calculated for each PAH ring group, low molecular weight (LMW), and high molecular weight (HMW) PAHs, as well as for the total of the 16 PAHs. Significant correlations were displayed in scatterplots.
Results and discussion
Levels of polycyclic aromatic hydrocarbons in air samples
The total amount of the 16 USEPA priority PAHs (Σ16PAHs), as well as that of each individual congener, in different areas of Tarragona County (Catalonia, Spain) measured between November 2014 and January 2015 are summarized in Table 1. Most international studies using passive air samplers translate concentrations measured in PUF into equivalents for air. For that purpose, published calibrations between PAH concentrations measured by active samplers and PAS are generally used (Shoeib and Harner 2002; Harner et al. 2013). Because calibrations tend to vary to some extent depending on the number of factors, such as wind intensity, rainfall, or temperature, this procedure may envisage results. For this reason, as well as because this study was aimed at establishing the validity of lichen transplants, amounts of PAHs accumulated in the PUFs instead of air equivalent concentrations were used.
Air PAH levels ranged from 1363 to 7866 ng/sample, with a mean value of 3414 ng/sample. These values are consistent with a preliminary survey conducted in this area in winter of 2013 (Domínguez-Morueco et al. 2014), in which PAH levels found in PAS deployed for 2 months ranged from 670 to 7530 ng/sample (Table 2). In addition, these levels are in agreement with other international studies. Data on PAHs in air measured by means of PAS published in recent years in the scientific literature are summarized in Table 2. Estellano et al. (2014) found an accumulation of PAHs of 2724–6222 ng/sample at four locations in urban and suburban sites of Puglia region (Italy), while Harner et al. (2013) found a range of 525–6300 ng/sample in the atmosphere of the Alberta oil sands (Canada). Similar levels were also reported by Kennedy et al. (2010) in South Australia (2169–7033 ng/sample). Contrastingly, other authors have found relatively higher amounts of PAHs in a number of countries (e.g., India, Korea, Mexico, and Sweden) (Bohlin et al. 2008; Choi et al. 2012; Cheng et al. 2013). In turn, the studies conducted by Estellano et al. (2012) in the Tuscany region, Italy, or the ones by Bohlin et al. (2008) in Lancaster, UK, reported lower PAH values in PAS than those currently found in Tarragona County (Table 2).
A seasonal pattern of PAHs in air was found. Airborne concentrations found in this study, performed in winter (mean temperature 9 °C; range 3–15 °C), were notably higher than PAH amounts observed in summer of 2014 (mean temperature 24 °C) in the same area under study (unpublished data) when levels ranged 2396–4743 ng/sample (Table 2). Seasonal differences have been largely reported in the scientific literature, with higher concentrations of PAHs typically found in cold seasons (Delhomme and Millet 2012; Jyethi et al. 2014). Furthermore, this pattern also agree with data on PAH concentrations in air obtained by means of active sampling devices in Tarragona County (Ramirez et al. 2011), as well as with other international studies (Kennedy et al. 2010; Wang et al. 2010; Chen et al. 2011; Estellano et al. 2014).
The highest Σ16PAH amount (7866 ng/sample) was observed in the sampling point P1, corresponding to the neighborhood of Puigdelfí (village of Perafort). This value is very similar to that found in the village of Vilallonga del Camp, a very close town, in the 2013 campaign, when a PAH amount of 7530 ng/sample was found (Domínguez-Morueco et al. 2014). Both sampling points are situated in the vicinity of a big oil refinery and well under the potential influence of petrochemical emissions. This fact agrees with the results from other international monitoring studies, in which a certain increase of PAHs have been associated to the presence of chemical industries (Pozo et al. 2012).
Phenanthrene was the predominant hydrocarbon in air, with a mean contribution of 49 % of the total (range 43–54 %). Moreover, relatively high contributions were also noted for acenaphthylene and fluoranthene (mean contribution of 16 and 10 %, respectively). The profile observed in this study was consistent with the results reported by other authors who showed a predominance of 3- and 4-ring PAHs (Bohlin et al. 2008; Choi et al. 2012; Pozo et al. 2012; Estellano et al. 2012, 2014). Since they are predominantly present in the gas-phase, LMW PAHs may be more easily captured by the PAS. In contrast, PAHs with 5–6 benzene rings have a higher molecular weight and lower volatility, tending to remain in the particulate-phase (Choi et al. 2012; Estellano et al. 2012; Pozo et al. 2012).
Polycyclic aromatic hydrocarbon levels in lichen transplants
Lichens were transplanted from a background site to eight different locations of Tarragona County, grouped into four areas: chemical, petrochemical, urban, and background. Unfortunately, from the eight transplants deployed in the field, two (P2 and U6) were missing at the end of the sampling period. The concentrations of individual PAHs and Σ16PAHs measured in lichen transplants after 2 months of exposure are displayed in Table 1. The analysis allowed the quantitation of 14 out of the 16 USEPA PAHs.
Σ16PAH concentrations in lichens after exposure varied between 247 ng/g (for the urban site U5) and 841 ng/g (for the petrochemical site P1), with an average of 480 ng/g. Preexposed lichens revealed a concentration of 307 ng/g. After being transplanted from a presumably background area to an exposed area, the lichens are expected to become enriched or depleted according to pollutant loads existent in the exposed area. In our case, four transplants became enriched for Σ16PAHs, while the remaining two were depleted.
Although the first studies using lichens to measure PAHs were published some time ago (Carlberg et al. 1983), only until recently lichens have started to be used as routine monitors of PAHs. In the past, most studies were based on the use of native lichens to estimate the environmental concentrations of PAHs (Studabaker et al. 2012). In contrast, only few studies report the use of lichen transplants in areas where native lichens could not be found (Table 3).
PAH concentration ranges obtained in lichens transplanted to the area of Tarragona County are in accordance with other studies (Table 3). In order to establish a valid comparison, the obtained values were divided by the exposure period (number of months). Therefore, the cumulative effect over time is neglected, and comparisons between studies can be conducted with a high degree of reliability. The range of PAH levels in lichens sampled in Tarragona is in the high part of the range in comparison to data from Italy, the country where lichens have been more extensively used for the monitoring of PAHs. In Tarragona County, the maximum PAH concentration was 420 ng/g per month, while the greatest level in Italy was found in an Italian industrial area, reaching 344 ng/g per month (Table 3). Notwithstanding, it must be highlighted that different lichen species were used in each study, the number of quantified PAHs was different, and sampling was performed in different years and seasons. For example, concentrations of Σ16PAHs measured in native lichens (Parmotrema hypoleucinum) collected in the southwest of Portugal in a petrochemical industrial area similar to Tarragona were found to decrease by a factor of ten during the summer season (from a maximum of 556 ng/g in winter to a minimum of 58 ng/g during summer) (Augusto et al. 2013b). This strong variation was significantly correlated with climate factors, such as temperature, relative humidity, and wind speed. These parameters, together with the use of different lichen species and the existence of different pollution sources in each study, may contribute to differences in PAH concentrations observed in lichens.
Comparison between passive air sampling and lichen transplants
In order to compare the accumulation of PAHs between PAS and lichens, data for the six sites, where both monitors were existent at the end of the sampling, were used. This means that P2 and U6 samples were excluded from the statistical treatment. Σ16PAH values ranged between 247 and 840 ng/g in lichen transplants and between 1947 and 7866 ng/sample in PAS, both deployed at the same sampling sites during the same period (2 months). In both monitors, PAS and lichens, the highest concentration was found at site P1, located near the petrochemical complex, whereas the lowest level was found at U5 (urban area), where the traffic intensity was rather low.
The PAH ring profile found in PAS and lichens at each sampling site is displayed in Fig. 2. Air samples revealed a PAH profile dominated by LMW PAHs, especially 3-ring (accounting for more than 70 % of the total concentration) and 4-ring PAHs (contributing for approximately 20 %). In lichen samples, PAH profiles seem to be also dominated by 3- and 4-ring PAHs, together accounting for 60–70 % of the profile. High molecular weight PAHs exist mainly in the particulate-phase of air. Thus, they are not likely to be adsorbed by PAS. The high percentage of 3-ring PAHs (>70 %) measured in air samples from Tarragona County is in agreement with other studies performed across Europe (Jaward et al. 2004; Estellano et al. 2012, 2014; Loppi et al. 2015).
Besides intercepting PAHs existent in the gas-phase of air (mainly LMW PAHs), lichens also accumulate PAHs sorbed to the particulate matter (Fig. 2). In previous studies, PAH profiles in lichens (P. hypoleucinum and Xanthoria parietina) were compared with those found in the particulate phase of air by active air samplers (Augusto et al. 2010, 2013b). Lichens showed higher contributions of LMW PAHs when compared to air, whereas particulate phase of air presented higher contributions of 5- and 6-ring PAHs in comparison to lichens (Augusto et al. 2013b). Regardless of the difference of profiles, a significant correlation was found for PAH concentrations in lichens and in particulate-phase of air (Augusto et al. 2013b).
The correlations of PAH concentrations in lichens and the amount accumulated in PAS exposed at the same sampling sites for the same time span are summarized in Table 4. A significant positive linear correlation for LMW PAHs was found between PAS and lichens (R = 0.827, P < 0.05). This correlation was mainly supported by the significant correlation of the 4-ring PAHs (R = 0.941, P < 0.05). Because correlations were performed with a limited amount of data (six pairs), the respective scatterplots were also graphically represented (Fig. 3). A linear relationship of LMW PAHs, and very especially of 4-ring PAHs, between lichens and PAS, was observed.
These results are in agreement with those recently found by Loppi et al. (2015) when comparing PAHs in PAS and lichen transplants exposed for 3 months at the same sampling sites in Italy, despite the differences of the lichen species (Evernia prunastri vs. R. fastigiata) and the sampling season (spring vs. winter). The accumulation of PAHs by lichens can be influenced by the lichen species, the surface/volume ratio and algae content being important aspects (Augusto et al. 2013a). The increase of surface/volume ratio leads to a higher accumulation of PAHs as more lichen area is exposed to atmospheric pollution. The lichen R. fastigiata is a fruticose lichen with a branched bushy-type structure with a high surface/volume ratio. In turn, the lichen E. prunastri used by Loppi et al. (2015) is a foliose lichen but attached at one point and appearing fruticose. E. prunastri has a lower surface/volume ratio than R. fastigiata. Very recently, and after tracking gas-phase PAHs inside lichens, Augusto et al. (2015) observed that these compounds easily enter the lichen thallus and accumulate in the algal. Therefore, the amount of algae is likely to influence the amount of gas-phase PAHs accumulated by lichens.
Even though different lichen species were used, our results are in accordance with those reported by Loppi et al. (2015). In both investigations, a linear relationship between PAHs in lichens and PAS was established. However, the linearity of this relationship was smaller than that found by Loppi et al. (2015). It could be due to the fact that the sampling in Spain was performed during winter, where the contribution of particle-associated PAHs is bigger than in summer and spring. As lichens accumulate PAHs as both gas- and particulate-phases, during winter, lichens more easily reflect the particulate-phase of PAHs. As the temperature increases, the contribution of gas-phase PAHs in air, as well as in lichens, also increases. Thus, the correlation of PAHs between PAS and lichens is expected to become stronger in summer.
Because levels of each PAH in air and lichens can be strongly influenced by climate factors, similar experimental calibrations of PAHs in PAS and in lichens, covering different seasons and lichen species, must be repeated. During summer, with high temperatures, LMW PAHs tend to exist mainly in the gas-phase of air, whereas in winter, concentrations of HMW PAHs tend to increase due to an intensification of emissions from seasonal sources. As PAS preferentially adsorb compounds in the gas-phase and lichens capture compounds existent in gas- and particulate-phase, differences should be expected when comparing both monitors over different seasons.
Conclusions
The levels of 16 PAHs were determined in PAS and lichen transplants (Ramalina fastigiata) deployed for a period of 2 months in different zones of Tarragona County (Catalonia, Spain), an area with an important number of chemical and petrochemical industries.
Σ16PAH concentrations in air samples ranged from 1363 to 7866 ng/sample, phenanthrene being the predominant hydrocarbon. The highest concentration was found in the neighborhood of Puigdelfí (village of Perafort) in the vicinity of a big oil refinery and well under the potential influence of petrochemical emissions. The PAH concentrations in lichen transplants varied between 247 and 841 ng/g, with the highest value also reported in the same sampling point as for the PAS. When both monitoring methods were compared, a significant positive linear correlation was found for LMW PAHs. These results strongly suggest that lichens can be used to monitor gas phase PAHs, providing data that can be quantitatively translated into equivalents for air. However, since PAHs in air may be strongly influenced by climate/meteorological factors, it is essential to perform similar experiment covering different seasons and lichen species.
References
Augusto S, Máguas C, Matos J, Pereira MJ, Branquinho C (2010) Lichens as an integrating tool for monitoring PAH atmospheric deposition: a comparison with soil, air and pine needles. Environ Pollut 158(2):483–489. doi:10.1016/j.envpol.2009.08.016
Augusto S, Máguas C, Branquinho C (2013a) Guidelines for biomonitoring persistent organic pollutants (POPs), using lichens and aquatic mosses—a review. Environ Pollut 180:330–338. doi:10.1016/j.envpol.2013.05.019
Augusto S, Pereira MJ, Máguas C, Branquinho C (2013b) A step towards the use of biomonitors as estimators of atmospheric PAHs for regulatory purposes. Chemosphere 92(5):626–632. doi:10.1016/j.chemosphere.2013.03.068
Augusto S, Sierra J, Nadal M, Schuhmacher M (2015) Tracking polycyclic aromatic hydrocarbons in lichens: it’s all about the algae. Environ Pollut. doi:10.1016/j.envpol.2015.08.013
Bohlin P, Jones KC, Tovalin H, Strandberg B (2008) Observations on persistent organic pollutants in indoor and outdoor air using passive polyurethane foam samplers. Atmos Environ 42(31):7234–7241. doi:10.1016/j.atmosenv.2008.07.012
Bohlin P, Jones KC, Strandberg B (2010) Field evaluation of polyurethane foam passive air samplers to assess airborne PAHs in occupational environments. Environ Sci Technol 44(2):749–754. doi:10.1021/es902318g
Carlberg GE, Ofsad EB, Drangsholt H, Steinnes E (1983) Atmospheric deposition of organic micropollutants in Norway studied by means of moss and lichen analysis. Chemosphere 12:341–356. doi:10.1016/0045-6535(83)90109-1
Chen Y, Feng Y, Xiong S, Liu D, Wang G, Sheng G, Fu J (2011) Polycyclic aromatic hydrocarbons in the atmosphere of Shanghai, China. Environ Monit Assess 172(1–4):235–247. doi:10.1007/s10661-010-1330-x
Cheng H, Deng Z, Chakraborty P, Liu D, Zhang R, Xu Y, Luo C, Zhang G, Li J (2013) A comparison study of atmospheric polycyclic aromatic hydrocarbons in three Indian cities using PUF disk passive air samplers. Atmos Environ 73:16–21. doi:10.1016/j.atmosenv.2013.03.001
Choi SD, Kwon HO, Lee YS, Park EJ, Oh JY (2012) Improving the spatial resolution of atmospheric polycyclic aromatic hydrocarbons using passive air samplers in a multi-industrial city. J Hazard Mater 241–242:252–258. doi:10.1016/j.jhazmat.2012.09.039
Delhomme O, Millet M (2012) Characterization of particulate polycyclic aromatic hydrocarbons in the east of France urban areas. Environ Sci Pollut Res 19(5):1791–1799. doi:10.1007/s11356-011-0701-3
Domínguez-Morueco N, Vilavert L, Schuhmacher M, Domingo JL, Nadal M (2014) Passive air sampling of PAHs at local scale: preliminary results in Tarragona County, Spain. Organohalogen Compd 76:384–387
Estellano VH, Pozo K, Harner T, Corsolini S, Focardi S (2012) Using PUF disk passive samplers to simultaneously measure air concentrations of persistent organic pollutants (POPs) across the Tuscany region, Italy. Atmos Pollut Res 3(1):88–94. doi:10.5094/APR.2012.008
Estellano VH, Pozo K, Silibello C, Mulder MD, Efstathiou C, Tomasino MP, Furano F, Donadio I, Focardi F (2014) Characterization of urban pollution in two cities of the Puglia region in southern Italy using field measurements and air quality (AQ) model approach. Atmos Pollut Res 5:34–41. doi:10.5094/APR.2014.005
Guidotti M, Stella D, Owczarek M, de Marco A, de Simona C (2003) Lichens as polycyclic aromatic hydrocarbons bioaccumulators used in atmospheric pollution studies. J Chromatogr A 985:185–190. doi:10.1016/S0021-9673(02)01452-8
Guidotti M, Stella D, Dominici C, Blasi G, Owczarek M, Vitali M, Protano C (2009) Monitoring of traffic-related pollution in a province of central Italy with transplanted lichen Pseudevernia furfuracea. Bull Environ Contam Toxicol 83:852–858. doi:10.1007/s00128-009-9792-7
Harner T, Su K, Genualdi S, Karpowicz J, Ahrens L, Mihele C, Schuster J, Charland JP, Narayan J (2013) Calibration and application of PUF disk passive air samplers for tracking polycyclic aromatic compounds (PACs). Atmos Environ 75:123–128. doi:10.1016/j.atmosenv.2013.04.012
Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC (2004) Passive air sampling of polycyclic aromatic hydrocarbons and polychlorinated naphthalenes across Europe. Environ Toxicol Chem 23:1355–1364. doi:10.1897/03-420
Jyethi DS, Khillare PS, Sarkar S (2014) Risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons in school children. Environ Sci Pollut Res 21(1):366–378. doi:10.1007/s11356-013-1912-6
Kennedy K, Macova M, Bartkow ME, Hawker DW, Zhao B, Denison MS, Mueller JF (2010) Effect based monitoring of seasonal ambient air exposures in Australia sampled by PUF passive air samplers. Atmos Pollut Res 1:50–58. doi:10.5094/APR.2010.008
Leung AO, Cheung KC, Wong MH (2015) Spatial distribution of polycyclic aromatic hydrocarbons in soil, sediment, and combusted residue at an e-waste processing site in southeast China. Environ Sci Pollut Res 22:8786–8801. doi:10.1007/s11356-013-1465-8
Loppi S, Pozo K, Estellano VH, Corsolini S, Sardella G, Paoli L (2015) Accumulation of polycyclic aromatic hydrocarbons by lichen transplants: comparison with gas-phase passive air samplers. Chemosphere 134:39–43. doi:10.1016/j.chemosphere.2015.03.066
Mari M, Schuhmacher M, Feliubadaló J, Domingo JL (2008) Air concentrations of PCDD/Fs, PCBs and PCNs using active and passive air samplers. Chemosphere 70(9):1637–1643. doi:10.1016/j.chemosphere.2007.07.076
Nadal M, Schuhmacher M, Domingo JL (2004a) Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environ Pollut 132(1):1–11. doi:10.1016/j.envpol.2004.04.003
Nadal M, Schuhmacher M, Domingo JL (2004b) Metal pollution of soils and vegetation in an area with petrochemical industry. Sci Total Environ 321(1–3):59–69. doi:10.1016/j.scitotenv.2003.08.029
Nadal M, Schuhmacher M, Domingo JL (2007) Levels of metals, PCBs, PCNs and PAHs in soils of a highly industrialized chemical/petrochemical area: temporal trend. Chemosphere 66:267–276. doi:10.1016/j.chemosphere.2006.05.020
Nadal M, Mari M, Schuhmacher M, Domingo JL (2009) Multi-compartmental environmental surveillance of a petrochemical area: levels of micropollutants. Environ Int 35(2):227–235. doi:10.1016/j.envint.2008.06.001
Nadal M, Schuhmacher M, Domingo JL (2011) Long-term environmental monitoring of persistent organic pollutants and metals in a chemical/petrochemical area: human health risks. Environ Pollut 159(7):1769–1777. doi:10.1016/j.envpol.2011.04.007
Pozo K, Harner T, Shoeib M, Urrutia R, Barra R, Parra O, Focardi S (2004) Passive-sampler derived air concentrations of persistent organic pollutants on a north–south transect in Chile. Environ Sci Technol 38(24):6529–6537. doi:10.1021/es049065i
Pozo K, Harner T, Lee SC, Wania F, Muir DCG, Jones KC (2009) Seasonally resolved concentrations of persistent organic pollutants in the global atmosphere from the first year of the GAPS study. Environ Sci Technol 43(3):796–803. doi:10.1021/es802106a
Pozo K, Harner T, Rudolph A, Oyola G, Estellano VH, Ahumada-Rudolph R, Focardi S (2012) Survey of persistent organic pollutants (POPs) and polycyclic aromatic hydrocarbons (PAHs) in the atmosphere of rural, urban and industrial areas of Concepción, Chile, using passive air samplers. Atmos Pollut Res 3(4):426–434. doi:10.5094/APR.2012.049
Protano C, Guidotti M, Owczarek M, Fantozzi L, Blasi G, Vitali M (2014) Polycyclic aromatic hydrocarbons and metals in transplanted lichen (Pseudoevernia furfuracea) at sites adjacent to a solid-waste landfill in central Italy. Arch Environ Contam Toxicol 471–481. doi:10.1007/s00244-013-9965-6
Ramirez N, Cuadras A, Rovira E, Marcé RM, Borrull F (2011) Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites. Environ Health Perspect 119:1110–1116. doi:10.1289/ehp.1002855
Schuhmacher M, Nadal M, Domingo JL (2004) Levels of PCDD/Fs, PCBs, and PCNs in soils and vegetation in an area with chemical and petrochemical industries. Environ Sci Technol 38(7):1960–1969. doi:10.1021/es034787f
Shoeib M, Harner T (2002) Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ Sci Technol 36:4142–4151. doi:10.1021/es020635t
Studabaker WB, Krupa S, Jayanty RKM, Raymer JH (2012) Measurement of polynuclear aromatic hydrocarbons (PAHs) in epiphytic lichens for receptor modelling in Athabasca Oil Sands Region (AOSR): a pilot study. In: Percy KE (ed) Dev Environm Sci vol. 11, Amsterdam, The Netherlands pp. 391–425
Vilavert L, Nadal M, Rovira J, Schuhmacher M, Domingo JL (2013) Air passive sampling for the screening of inhalation risks of POPs near an incineration plant. Hum Ecol Risk Assess 19(3):620–634. doi:10.1080/10807039.2012.701990
Vilavert L, Nadal M, Schuhmacher M, Domingo JL (2014) Seasonal surveillance of airborne PCDD/Fs, PCBs and PCNs using passive samplers to assess human health risks. Sci Total Environ 466–467:733–740. doi:10.1016/j.scitotenv.2013.07.124
Wang XY, Li QB, Luo YM, Ding Q, Xi LM, Ma JM, Li I, Liu P, Cheng CL (2010) Characteristics and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in Shanghai, China. Environ Monit Assess 165(1–4):295–305. doi:10.1007/s10661-009-0946-1
Xia Z, Duan X, Tao S, Qiu W, Liu D, Wang Y, Wei S, Wang B, Jiang Q, Lu B, Song Y, Hu X (2013) Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ Pollut 173:150–156. doi:10.1016/j.envpol.2012.10.009
Zabiegała B, Kot-Wasik A, Urbanowicz M, Namieśnik J (2010) Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal Bioanal Chem 396(1):273–296. doi:10.1007/s00216-009-3244-4
Acknowledgments
This work has been supported by the Spanish Ministry of Economy and Competitiveness (Mineco), through the project CTM2012-33079 and by Mancomunitat d’Incineració dels Residus Urbans, Tarragona, Spain. Noelia Domínguez-Morueco also received a PhD fellowship (BES-2013-066313) from the Mineco (FPI Predoctoral Contracts for Doctors Training 2013), while Sofia Augusto acknowledges the European Union Seventh Framework Programme for the Marie Curie Intra-European Fellowship for Career Development (FP7-PEOPLE-2013-IEF-624022). Antoni Sans and Ferran Clua, from the Departament de Territori i Sostenibilitat (Generalitat de Catalunya, Spain), are acknowledged for their assistance in the deployment of PAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Domínguez-Morueco, N., Augusto, S., Trabalón, L. et al. Monitoring PAHs in the petrochemical area of Tarragona County, Spain: comparing passive air samplers with lichen transplants. Environ Sci Pollut Res 24, 11890–11900 (2017). https://doi.org/10.1007/s11356-015-5612-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5612-2