Abstract
The difficulties in dewatering waste-activated sludge (WAS) using mechanical devices have caused great problems in sludge transportation and disposal. Herein, coagulation and flocculation are combined with the use of a magnetic field as a clean and low-energy physical treatment method to enhance the dewaterability of municipal and citric acid–processing WAS. It is shown that the use of the magnetic field had a significant effect on the capillary suction time (CST) of municipal WAS but not on the specific resistance filtration (SRF) and CST of the citric acid WAS. The differences in the magnetic field effects were due to differences in the sludge properties. For municipal WAS, the particle size decreased, the zeta potential remained unchanged, and the viscosity decreased, whereas in the citric acid WAS, the particle size increased, the absolute value of the zeta potential decreased, and the viscosity increased. In addition, these effects were also confirmed with studies of the water state and micro-morphology analyses. It is shown that the acidification of the municipal WAS and coagulation of citric acid WAS were likely the reasons for the enhancement of their dewaterability, respectively. This study confirmed that the use of a magnetic field combined with coagulation/flocculation may serve as an effective sludge conditioning method; however, the treatment conditions may vary with the sludge type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waste-activated sludge (WAS) is the by-product of activated sludge processes in wastewater treatment. Its moisture content is usually more than 98% by mass (Mowla et al. 2013). Along with the increasing generation of wastewater, large amounts of WAS are produced in wastewater treatment plants daily and are causing issues in the wastewater and solid waste disposal industries. Reducing the moisture content (MC) in WAS to less than 60% is key for cost-effectively decreasing the WAS volume, and thus further reducing the handling, storage, and transportation cost (Mowla et al. 2013). Mechanical dewatering is the most widely used method for reducing MC in WAS in wastewater treatment plants; however, the mechanical dewatering method without any pretreatment of the WAS can only achieve MC of 80–98% (Wang et al. 2010). Considering the economic and efficiency benefits, coagulation/flocculation is one of the most commonly used conditioning approaches prior to mechanical dewatering of WAS (Wei et al. 2018). During coagulation/flocculation, small colloidal particles in the sludge form larger flocs or compact cakes that facilitate sedimentation and are easily centrifuged or compressed during the mechanical dewatering. However, even when the WAS is pretreated using coagulation/flocculation, the MC reduction may not be enough. WAS conditioned using traditional polyacrylamide still retains a MC higher than 80% after mechanical dewatering in many wastewater treatment plants in China (Xia et al. 2016).
Physical conditioning has been used in combination with coagulation/flocculation to improve the pretreatment performance. In recent years, the use of magnetic fields in sludge conditioning has gained interest because of its ability to change the metabolisms of microorganisms in sludge and thereby affect the characteristics of the extracellular polymeric substances (EPS) (Cai et al. 2017) and modify the structure and polarization of sludge molecules (Krzemieniewski et al. 2004). It was suggested that the factors that reflect the dewaterability of sludge, such as the specific resistance of filtration (SRF), the water content of the sludge cake (Wsc), and the capillary suction time (CST), all decreased after placing the WAS in magnetic fields; however, these changes were limited (Krzemieniewski et al. 2003; Xue and Chen 2014; Zielinski et al. 2018). In combination with coagulation, the application of magnetic fields may affect the colloidal stability of the particles by altering the structure of water molecules and ions adsorbed on their particle surface or in the medium, and consequently enhance the coagulation and sedimentation (Okada et al. 1991; Higashitani 1996). Despite these potential enhancements in coagulation, the application of magnetic fields to sludge conditioning combined with coagulation/flocculation has rarely been studied (Zielinski et al. 2018). In addition, the optimization of the magnetic field strength, together with types and doses of coagulants and flocculants, has hardly been reported. Consequently, the use of magnetic fields in combination with coagulation/flocculation on the conditioning of different types of sludge has never been discussed and compared. This leaves a significant gap in research on sludge conditioning.
Municipal wastewater sludge is the exclusive majority of sludge produced in the world (Bennamoun 2012); hence, research on improving its dewaterability has frequently been reported (Pambou et al. 2016; Wang et al. 2018; Liang et al. 2019). Citric acid is a widely used organic acid in a variety of industries (Angumeenal and Venkappayya 2013). It is produced through fermentation processes of crops such as corns and yams, which results in a high volume of WAS during treatment of the processing wastewater (Ding et al. 2018). As it has a high organic content, citric acid WAS is considered difficult to dewater (Skinner et al. 2015). In this study, both municipal and citric acid–processing WAS were conditioned for dewaterability improvement, and the effect and mechanisms were compared and discussed in terms of the sludge types.
The objectives of this study were to (a) investigate the optimal conditions to enhance the dewaterability of two different types of sludge using a magnetic field combined with coagulation/flocculation; (b) examine the changes on the sludge properties at their respective optimal condition; and (c) analyze the conditioning mechanisms of two different sludge types.
Material and methods
Sludge samples and chemicals
Municipal WAS samples were collected from the secondary sedimentation tank at a municipal wastewater treatment plant in Beijing, China. Citric acid–processing wastewater WAS were collected from the secondary sedimentation tank at a citric acid production company in Shandong province, China. Samples were stored at 4 °C before use. Raw WAS were completely mixed before taking samples for experiments. The properties of the WAS samples are listed in Table 1. The pH was measured using a digital pH meter (HQ440d, Hach, USA). CST, Wsc, and SV30 were analyzed as described in section “Sludge dewaterability analysis.” The measurements of the MC, volatile suspended solids (VSS), and total suspended solids (TSS) were conducted in accordance with the standard methods for the examination of water and wastewater (American Public Health Association 2005). Ferric chloride (FeCl3, analytical grade) was purchased from Fuchen (Tianjin) Chemical Reagent Company (Tianjin, China), and cationic polyacrylamide (PAM) with a molecular weight (MW) 4,000,000 was purchased from Haoyu Chemical Ltd. of city of Renqiu (Hebei province, China).
Experimental procedures
Single-factor experiment
The effects of the respective doses of FeCl3 and PAM on the SRF and CST of the municipal and citric acid WAS were evaluated. FeCl3 is a traditional coagulant that causes the sludge to compact and settle, while PAM is a polymeric flocculant that enhances coagulation at minimal added concentrations. For economic considerations, FeCl3 doses of 1.0–6.0 g/L and PAM doses of 1–20 mg/L were applied to both types of WAS. FeCl3 and PAM were dissolved in Milli-Q water before use. The predetermined volume of the FeCl3 solution was added to 150 mL of sludge sample, followed by rapid mixing at 200 r/min for 1 min, or the predetermined volume of the PAM solution was added to 150 mL sludge, followed by slow mixing at 30 r/min for 10 min. The CST and SRF were analyzed subsequently. The detailed procedures were described in section “Sludge dewaterability analysis.”
Response surface methodology (RSM) design
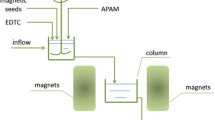
The Box-Behnken design (BBD) (Montgomery 1991) was selected to evaluate the combined effects of the strength of static magnetic field, the dose of FeCl3, and the dose of PAM during conditioning. The magnetic field was provided by two static magnets. The SRF and CST were examined as the response values. Fifteen runs were conducted for each experiment. The optimal conditions were determined by minimizing SRF and CST, giving SRF a weight of 5 and CST a weight of 3.
Sludge dewaterability analysis
The sludge dewaterability was evaluated based on the measured SRF and CST. The SRF was measured using the vacuum filtration method. One hundred milliliters of sludge sample was poured into a standard Buchner funnel with a filter. The sample was filtered under gravity for 1 min before vacuum filtration, and the pressure was controlled at 0.07 MPa. The volume and time of the sample passing through the funnel were recorded. Filtration was terminated at 20 min or until the pressure uncontrollably dropped, whichever came first. The SRF was determined according to Eqs. 1 and 2.
where r is the SRF (m/kg), P is pressure during filtration (Pa), A is the surface area of filter (m2), b is slope of filtration equation –dt/dV = bV+a, μ is kinetic viscosity (kg s m−2), C is the dry solid weight per unit volume sludge on the filtrate media (kg m−3), Cb is the solid particle concentration of the cake (dried weight of filtered cake/volume of cake before drying) (g/mL), and C0 is the solid particle concentration of the sludge (dried weight of sludge/volume of sludge) (g/mL).
The CST was measured using a standard CST apparatus (TR04-304M, Triton Electronics Ltd., UK). Detailed procedures were described in Ding et al. (2018).
Sludge properties’ characterization
The composition of the EPS was evaluated. A modified heat extraction method was performed to extract slime EPS (S-EPS), loosely bound EPS (LB-EPS), and tightly bound EPS (TB-EPS) from the sludge samples. The concentrations of the extracted proteins and the polysaccharides were determined as described in Ding et al. (2018).
The excitation emission matrix (EEM) fluorescence spectra of S-EPS, LB-EPS, and TB-EPS were analyzed. The extracted S-EPS, LB-EPS, and TB-EPS samples were filtered through 0.22-μm glass fibers, followed by EEM measurements using an F-7000 fluorescence spectrophotometer (Hitachi, Japan). Excitation wavelengths were incrementally increased from 220 to 450 nm at 5 nm intervals, and the emission wavelengths were increased from 240 to 600 nm at 1-nm intervals. The detailed procedure was as described in Yang et al. (2017).
The zeta potential of the supernatant of the sludge samples was measured at 25 °C using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK). The particle size in the sludge samples was analyzed at 25 °C using a Microtrac S3500 particle size analyzer (Microtrac, Germany). The diameters of 10%, 50%, and 90% particles less than the indicated diameters are designated as d25, d50, d75, and d90, respectively.
The viscosity of a 300 mL sludge sample was evaluated using a digital rotational viscometer (NJD-8S, Shanghai Yueping Scientific Instrument Ltd., China). The detection range was 10–200,000 mPa s.
The microstructure of the sludge samples was investigated using a scanning electron microscope (SEM) (Tescan Vega II, Tescans.r.o, Czech). The procedures were detailed by Ding et al. (2018).
The water state in the sludge samples was differentiated by low-field nuclear magnetic resonance (NMR). Sludge samples were vacuum filtered at 0.07 MPa to remove the supernatant to increase the relative percentage of water states potentially linked to the solid phase. The cake (pellet) obtained from the filtration was subject to the T2 measurement using low-field NMR (NIUSTEL-2, MINI20-015V-I System, Niumag Technology Ltd., China). For the measurement, 1–2 mL of the sludge sample was placed in an 18-mm diameter polytetrafluoroethylene cylindrical tube, and the tube was placed in the radio frequency coil to collect CMPG decay signals with 90° and 180° pulses of 8 and 40 μm, respectively, and an s-value of 0.125 s. The data from 8000 echoes were acquired as 16 scan repetitions.
Results and discussion
Single-factor experiment
Single-factor experiments were carried out on the two types of WAS to determine the range of FeCl3 and PAM doses in the RSM design. The dose response curves of FeCl3 on the dewaterability of municipal and citric acid WAS are presented in Fig. 1a and b, respectively. The CST of both the municipal and citric acid WAS gradually decreased as the concentration of FeCl3 increased, but plateaued at 3 and 2 g/L of FeCl3, respectively. The SRF of the municipal WAS was reduced to a minimum at a FeCl3 concentration of 4 g/L, and SRF of the citric acid WAS was reduced to a minimum at an FeCl3 concentration of 3 g/L. The SRF of both the WAS types increased as the FeCl3 concentration was further increased to 6 mg/L (Fig. 1).
The dose response curves of PAM on the dewaterability of municipal and citric acid WAS are presented in Fig. 2a and b, respectively. As the PAM dose increased, both the SRF and CST of the municipal WAS decreased and reached a minimum at a PAM concentration of 10 mg/L, followed by an increase as the PAM dose further increased to 20 mg/L. Both the SRF and CST of the citric acid wastewater decreased as the PAM dose increased to 20 mg/L (Fig. 2). The effects of PAM on the municipal wastewater and citric acid wastewater were quite different. This difference was most likely due to the differences in sludge properties (Wu et al. 2019b). Comparing the basic properties of both types of WAS (Table 1) shows that the MC of municipal WAS was higher than that of the citric acid WAS, and more detailed analyses on the sludge properties are described in the following sections.
RSM design experiment
Taking into consideration the results of the single-factor experiments described in section “Single-factor experiment” as well as the economic use of the coagulants and flocculants, the factors and levels of the three variables of each type of WAS were defined as listed in Table 2. The RSM was designed this way to explore the optimal condition to maximize the reduction of SRF and CST at the lowest cost. A magnetic field up to 80 mT was applied to represent low strength magnetic field. The results for the municipal and citric acid WAS are shown in Tables S1 and S2 in the supplementary materials.
The optimized treatment conditions for the municipal WAS used FeCl3 4 g/L, PAM 5.32 mg/L, and a magnetic field 80 mT, and the modeled output of the SRF and CST was 2.75 × 1011 m/kg and CST was 7.3 s, which decreased by 82.9% and 43.4%, respectively. The model results were confirmed by conducting experiments at the optimal conditions in triplicate, and the resulting SRF was (3.09 ± 0.57) × 1011 m/kg, and CST was 7.5 ± 0.2 s, which were close to the modeled values. At these conditions, the pH of the treated WAS was measured to be 4.50 ± 0.23.
The optimized conditions for citric acid WAS used were FeCl3 3 g/L, PAM 20 mg/L, and a magnetic field strength of 80 mT, and the modeled output for the SRF and CST was 4.56 × 1012 m/kg and 12.2 s, which decreased by 70.4% and 68.3%, respectively. The model results were confirmed by conducting the experiments at optimal conditions in triplicate, and the resulting SRF was (4.90 ± 0.54) × 1012 m/kg, and CST was 12.0 ± 0.4 s, which were also close to the modeled values. At these conditions, the pH of the treated WAS was measured to be 6.33 ± 0.10.
In this study, the optimum FeCl3 dose for the combined conditioning of municipal WAS was 4 g/L (approximately 125 mg/g dry solid (DS)), which was higher than the dose of 100 mg/g DS reported in Niu et al. (2013) that only used FeCl3. However, the solid contents in the municipal WAS used in this study was approximately 3.20% and was much higher than the 1.21% content in Niu et al. (2013). In another study of a sewage sludge with comparable solid contents to the present result, using only cationic PAM at 20 mg/g DS barely changed the SRF (Lu et al. 2017). Therefore, the conditioning results using a magnetic field combined with coagulation/flocculation obtained in this study was promising.
The ANOVA analysis of the BBD experiments on both WAS types are shown in Tables S3 and S4. The SRF of both WAS types and the CST of the citric acid WAS were significantly affected by the FeCl3 dose. The effect of PAM dose on both types of WAS was insignificant. The influence of the magnetic field strength on the CST of the municipal WAS was significant. It has been reported that the enhancement of coagulation by a static magnetic field depended on the coagulant, and iron-based coagulants may not be affected (Zielinski et al. 2018). The variation of the effects of the magnetic field strength combined with coagulation on the dewaterability of both WAS types also revealed the differences in their properties. Therefore, detailed analysis of the WAS properties was conducted subsequently.
EPS analysis
Figure 3 shows the changes of the protein and polysaccharide contents in the EPS of the two types of WAS after treatment at their respective optimal condition. It was clear that the compositions of the two WAS types were quite different. The polysaccharide and protein contents in the EPS of the municipal WAS were much lower than those of the citric acid WAS. This was because citric acid production uses crops as raw material, which results in high concentrations of organics in the wastewater and WAS. In municipal WAS, the polysaccharide and protein contents were predominantly in TB-EPS, while in the citric acid WAS, the polysaccharide and protein were predominantly in S-EPS. Protein was the major component in the EPS. The protein concentration increased in the S-EPS but decreased in TB-EPS in both treated WAS types. This suggested that at the optimal treatment conditions, the TB-EPS or/and the intracellular material of both types of WAS had been disrupted. By treating the waste at the optimal conditions, the pH of municipal and citric acid WAS decreased from 7.51 to 4.50 and from 6.85 to 6.33, respectively. This result supported that the addition of FeCl3 decreased the pH (Oriekhova and Stoll 2014). The disruption of EPS may have been caused by the hydrolysis of the FeCl3, which has also been seen in previous studies where lowering the pH reduced the bounded EPS and intracellular material contents (Wang et al. 2017; Wang et al. 2018; Wu et al. 2019b).
The EEM contours of the EPS layers of the two WAS are shown in Figs. S1 and S2. Consistent with the protein analysis results, the EEM contours showed that the fluorescence intensity of the proteinaceous substances in the S-EPS of both types of WAS increased after treatment. In the S-EPS of the municipal WAS, the intensity in region III decreased while the intensity in region IV increased after treatment, suggesting that fulvic acid was likely degraded into soluble microbial byproducts by the acidification (Fig. S1). The intensity of the proteinaceous substances in the TB-EPS of both types of WAS decreased, which aligned with the results of the protein analysis in EPS.
Particle size, zeta potential, and viscosity
The changes in particle size, zeta potential, and viscosity of the municipal and citric acid WAS after conditioning at their respective optimal condition are shown in Tables 3, 4, and 5. After conditioning, the particle size and viscosity decreased while the absolute value of zeta potential increased in the municipal WAS, and the trend was opposite for the citric acid WAS. The strong acidification in the municipal WAS disrupted the sludge particles into smaller ones (Shao et al. 2009), which did not significantly affect the ionization of the anionic groups, and slightly decreased the viscosity. In general, other than the sludge particles, the zeta potential and viscosity of the municipal WAS did not change greatly. These results suggested that flocculation did not play the primary role for the enhanced dewaterability of the municipal WAS. Instead, in accordance with the dramatic decrease in pH from 7.51 to 4.50, the hydrolysis of the FeCl3 that caused the acidification also likely weakened and destroyed the biological flocs and enhanced the dewaterability (Wang et al. 2019). Relevant studies showed that the destruction of EPS structure was conducive to forming smaller particles and denser flocculent structures in the sludge (Lin et al. 2019). However, due to the significant differences in properties, the mechanism of the enhanced dewaterability of the citric acid WAS was likely different from that seen in the municipal WAS. The particle size of the citric acid WAS was originally much smaller than that of municipal WAS and increased after treatment, which suggested that sludge flocs and small fragments including the dissociated parts of the EPS regrouped. The zeta potential of the citric acid WAS significantly decreased, showing that the extent of ionization of the anionic groups decreased. Through electron neutralization, the colloidal particles were destabilized and collided with each other to re-aggregate into large particles, and other colloidal particles settled together during the process of floc settlement (Xiao et al. 2017). The viscosity of the citric acid WAS was originally much higher than that of municipal WAS and further increased after treatment. Therefore, the enhanced dewaterability of the citric acid WAS was primarily due to the coagulation process. In general, the citric acid WAS was sticky, had smaller particle sizes, had higher organic contents, and was more likely to flocculate.
Water state analysis
Water exists in sludge in various forms and can be divided into four states: free water, interstitial water, vicinal water, and hydration water. The water state in the WAS depends on the binding strength between the water molecules and the solid phase. In NMR analysis, a longer T2 generally indicates a lower binding energy and higher mobility of the water (Rudi et al. 2008). The release of bound water is generally key to evaluating the dewatering efficiency of the sludge dewatering process with different pretreatment methods (Mowla et al. 2013). Reducing the amount of bound water in the sludge is the key step to achieve deep dewatering of sludge (Vaxelaire and Cezac 2004; Zhang et al. 2014). Figure 4 shows the T2 distribution of the water spectra in the sludge cake before and after WAS conditioning. Compared to other water states in the sludge, the interstitial water showed the most prominent peak (Wu et al. 2019a). At the optimal conditions, the T2 peak of the interstitial water in the municipal WAS decreased from 3–30 to 1–20 ms, and the T2 peak of the interstitial water in the citric acid WAS decreased from 4–100 to 0.6–5 ms. Apparently, after conditioning, the interstitial water peak in the citric acid WAS left shifted much more than that of the municipal WAS. As stated earlier, the mechanisms of dewaterability enhancement were different due to the difference in sludge properties. It has been reported that the amount interstitial water is governed by the micro- and macro-capillary structure of the bio-flocs (Cheng et al. 2018). As the effects of coagulation and flocculation dominated in the citric acid WAS conditioning, the flocs became denser and thus increased the trapping strength of the interstitial water.
Microscopic morphology
In order to further observe the changes in the sludge structure before and after the combined treatment with a magnetic field and added reagents for coagulation/flocculation, the microscopic morphology of the sludges was analyzed with SEM to observe their surface shape. The SEM results are shown in Fig. 5. For the municipal WAS, the raw sludge was flaky, but the structure was compact and continuous (Fig. 5a). In Fig. 5b, after conditioning, the sludge became more porous and loose. The reason could be that due to the acidification effect, in that as EPS was destroyed, the interstitial water was released, and the particle size was reduced (Zhang et al. 2019). As seen in Fig. 5c, the structure of the raw citric acid WAS was compact with voids and smoother than municipal WAS. After conditioning, the sludge was even more compacted with smaller voids (Fig. 5d). Due to the effects of coagulation/flocculation, the compression of the electric double layer by the added conditioner and the bridging adsorption between particles reduced the electrostatic repulsion, and the sludge particles were destabilized and coalesced together (Wang et al. 2017; Guo et al. 2018).
The effect of the magnetic field on the sludge dewaterability was complex. It has been reported that the application of a magnetic field affects the stability and wettability of solids and leads to accelerated aggregation and precipitation of the particles (Krzemieniewski et al. 2004; Kney and Parsons 2006). However, contradictory results have also been reported where the coagulation rate was reduced or not affected by the application of a magnetic field (Higashitani et al. 1992; Zielinski et al. 2018). Research found that the applied magnetic field may intensify the formation of free radicals, which may oxidize the organic particles (Krzemieniewski et al. 2004). As shown in this study, the application of magnetic field did not significantly enhance the coagulation in the citric acid WAS but did affect the CST of municipal WAS. The variability in behavior was due to many factors, including types of materials in the particles, the particle size fractions, types of coagulants, etc. (Zaidi et al. 2013).
Conclusion
In this study, the combination of an applied magnetic field and coagulation/flocculation reagent addition was used to enhance the dewaterability of municipal and citric acid WAS. The doses of FeCl3, PAM, and the strength of the magnetic field were optimized. For municipal WAS, at the optimal conditions of FeCl3 4 g/L, PAM 5.32 mg/L, and a magnetic field strength of 80 mT, the SRF and CST decreased by 82.9% and 43.4%, respectively. For citric acid WAS, at the optimal conditions of FeCl3 3 g/L, PAM 20 mg/L, and a magnetic field strength of 80 mT, the SRF and CST decreased by 70.4% and 68.3%, respectively. It is shown that the magnetic field significantly affected the capillary suction time (CST) in municipal WAS but did not affect the specific resistance filtration (SRF) and CST of citric acid WAS. By analyzing the EPS composition, particle size, zeta potential, viscosity, water state in the sludge cake, and microscopic morphology, it was found that the effect of enhanced of dewaterability in the different WAS types may be due to different mechanisms. For municipal WAS, both the particle size and viscosity decreased, which was opposite to the effects in the citric acid WAS. The results indicated that coagulation played the major role in improving the dewaterability of the citric acid WAS, and acidification was likely the main cause for the enhanced dewaterability of the municipal WAS.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
American Public Health Association (2005) Standard methods for the examination of water and wastewater, 21th edn. American Public Health Association, Washington, D.C.
Angumeenal AR, Venkappayya D (2013) An overview of citric acid production. LWT-Food Sci Technol:367–370
Bennamoun L (2012) Solar drying of wastewater sludge: a review. Renew Sust Energ Rev 16:1061–1073
Cai C, Liu H, Wang M (2017) Characterization of antibiotic mycelial residue (AMR) dewatering performance with microwave treatment. Chemosphere 174:20–27
Cheng SS, Tang YQ, Zhang T, Song YK, Wang XH, Wang HH, Wang HT, Tan MQ (2018) Approach for monitoring the dynamic states of water in shrimp during drying process with LF-NMR and MRI. Dry Technol 36:841–848
Ding N, Peng C, Ren Y, Liu Y, Wang P, Dong L, Liu H, Wang D (2018) Improving the dewaterability of citric acid wastewater sludge by Fenton treatment. J Clean Prod 196:739–746
Guo XX, Qian X, Wang YL, Zheng HL (2018) Magnetic micro-particle conditioning-pressurized vertical electro-osmotic dewatering (MPEOD) of activated sludge: role and behavior of moisture and organics. J Environ Sci 74:147–158
Higashitani K, Okuhara K, Hatade S (1992) Effects of magnetic fields on stability of non-magnetic ultrafine colloidal particles. J Colloid Interface Sci 152:125–131
Higashitani K (1996) Effects of magnetic field on stability of non-magnetic colloidal particles. Proceedings of the 2nd International Meeting on Anti-Scale Magnetic Treatment, Cranfield University, UK.
Kney AD, Parsons SA (2006) A spectrophotometer-based study of magnetic water treatment: assessment of ionic vs. surface mechanisms. Water Res 40(3):517–524
Krzemieniewski M, Debowski M, Janczukowicz W, Pesta J (2003) Effect of sludge conditioning by chemical methods with magnetic field application. Pol J Environ Stud 12:595–605
Krzemieniewski M, Debowski M, Janczukowicz W, Pesta J (2004) Effect of the constant magnetic field on the composition of dairy wastewater and domestic sewage. Pol J Environ Stud 13:45–53
Liang JL, Huang JJ, Zhang SW, Yang X, Huang SS, Zheng L, Ye MY, Sun SY (2019) A highly efficient conditioning process to improve sludge dewaterability by combining calcium hypochlorite oxidation, ferric coagulant re-flocculation, and walnut shell skeleton construction. Chem Eng J 361:1462–1478
Lin F, Zhu X, Li J, Yu P, Luo Y, Liu M (2019) Effect of extracellular polymeric substances (EPS) conditioned by combined lysozyme and cationic polyacrylamide on the dewatering performance of activated sludge. Chemosphere 235:679–689
Lu Y, Zheng GY, Wu WZ, Cui CH, Zhou LX (2017) Significances of deflocculated sludge flocs as well as extracellular polymeric substances in influencing the compression dewatering of chemically acidified sludge. Sep Purif Technol 176:243–251
Montgomery DC (1991) Design and analysis of experiments. Wiley, New York
Mowla D, Tran HN, Allen DG (2013) A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 58:365–378
Niu M, Zhang WJ, Wang DS, Chen Y, Chen RL (2013) Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants. Bioresour Technol 144:337–343
Okada I, Ozaki M, Matijevi E (1991) Magnetic interactions between platelet-type colloidal particles. J Colloid Interface Sci 142:251–256
Oriekhova O, Stoll S (2014) Investigation of FeCl3 induced coagulation processes using electrophoretic measurement, nanoparticle tracking analysis and dynamic light scattering: importance of pH and colloid surface charge. Colloids Surf A Physicochem Eng Aspects 461:212–219
Pambou YB, Fraikin L, Salmon T, Crine M, Leonard A (2016) Enhanced sludge dewatering and drying comparison of two linear polyelectrolytes co-conditioning with polyaluminum chloride. Desalin Water Treat 57:27989–28006
Rudi T, Guthausen G, Burk W, Reh CT, Isengard HD (2008) Simultaneous determination of fat and water content in caramel using time domain NMR. Food Chem 106:1375–1378
Shao L, He P, Yu G, He P (2009) Effect of proteins, polysaccharides, and particle sizes on sludge dewaterability. J Environ Sci 21:83–88
Skinner SJ, Studer LJ, Dixon DR, Hillis P, Rees CA, Wall RC, Cavalida RG, Usher SP, Stickland AD, Scales PJ (2015) Quantification of wastewater sludge dewatering. Water Res 82:2–13
Vaxelaire J, Cezac P (2004) Moisture distribution in activated sludges: a review. Water Res 38:2215–2230
Wang HF, Ma YJ, Wang HJ, Hu H, Yang HY, Zeng RJ (2017) Applying rheological analysis to better understand the mechanism of acid conditioning on activated sludge dewatering. Water Res 122:398–406
Wang HF, Hu H, Wang HJ, Zeng RJ (2018) Impact of dosing order of the coagulant and flocculant on sludge dewatering performance during the conditioning process. Sci Total Environ 643:1065–1073
Wang S, Ma C, Zhu Y, Yang Y, Du G, Li J (2019) Deep dewatering process of sludge by chemical conditioning and its potential influence on wastewater treatment plants. Environ Sci Pollut Res 26:33838–33846
Wang W, Luo Y, Qiao W (2010) Possible solutions for sludge dewatering in China. Front Environ Sci Eng 4:102–107
Wei H, Gao B, Ren J, Li A, Yang H (2018) Coagulation/flocculation in dewatering of sludge: a review. Water Res 143:608–631
Wu BR, Zhou K, He YP, Chai XL, Dai XH (2019a) Unraveling the water states of waste-activated sludge through transverse spin-spin relaxation time of low-field NMR. Water Res 155:266–274
Wu W, Zhou Z, Yang J, Chen G, Yao J, Tu C, Zhao X, Qiu Z, Wu Z (2019b) Insights into conditioning of landfill sludge by FeCl3 and lime. Water Res 160:167–177
Xia C, Yue Q, Song F, Liu X, Gao B, Zhang T, Li Q, Wang Y (2016) A study on the deep dewatering of urban dewatered-sewage sludge by aluminum chloride. Desalin Water Treat 57:545–552
Xiao KK, Chen Y, Jiang X, Yang Q, Seow WY, Zhu WY, Zhou Y (2017) Variations in physical, chemical and biological properties in relation to sludge dewaterability under Fe (II) - oxone conditioning. Water Res 109:13–23
Xue Q, Chen YJ (2014) Study on dewaterability of municipal sludge conditioning by physical conditioners with ultrasonic and magnetic field application. Desalin Water Treat 52:6396–6402
Yang Z, Sun YX, Ye T, Shi N, Tang F, Hu HY (2017) Characterization of trihalomethane, haloacetic acid, and haloacetonitrile precursors in a seawater reverse osmosis system. Sci Total Environ 576:391–397
Zaidi NS, Sohaili J, Muda K, Sillanpaa M (2013) Magnetic field application and its potential in water and wastewater treatment systems. Sep Purif Rev 43(3):206–240
Zhang H, Yang J, Yu W, Luo S, Peng L, Shen X, Shi Y, Zhang S, Song J, Ye N, Li Y, Yang C, Liang S (2014) Mechanism of red mud combined with Fenton’s reagent in sewage sludge conditioning. Water Res 59:239–247
Zhang W, Wang H, Li L, Li D, Wang Q, Xu Q, Wang D (2019) Impact of molecular structure and charge property of chitosan based polymers on flocculation conditioning of advanced anaerobically digested sludge for dewaterability improvement. Sci Total Environ 670:98–109
Zielinski M, Rusanowska P, Debowski M, Hajduk A (2018) Influence of static magnetic field on sludge properties. Sci Total Environ 625:738–742
Funding
This work was financially supported by National Natural Science Foundation of China (41861124004) and China Postdoctoral Science Foundation (2019M650493).
Author information
Authors and Affiliations
Contributions
ND designed the study and wrote the manuscript; XW conducted the single-factor and BBD experiments and analyses, and protein and polysaccharide analyses of EPS; LJ conducted particle size, zeta potential, and viscosity analyses; JZ conducted including EEM analysis of EPS; YG conducted SEM analysis; LD provided advice on the design of the research; and HL conducted the data analysis of NMR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1607 kb)
Rights and permissions
About this article
Cite this article
Ding, N., Wang, X., Jiang, L. et al. Enhancement of sludge dewaterability by a magnetic field combined with coagulation/flocculation: a comparative study on municipal and citric acid–processing waste-activated sludge. Environ Sci Pollut Res 28, 35728–35737 (2021). https://doi.org/10.1007/s11356-021-13278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13278-x