Abstract
Although the tobacco production and consumption rate in Bangladesh is very high and a substantial portion of premature deaths is caused by tobacco smoking, the status of heavy metals in tobacco plants has not yet determined. This study, therefore, investigated the concentrations of Cu, Ni, Cd, Pb, Cr, and Zn in tobacco plants and their surrounding agricultural soils in Kushtia District, Bangladesh. The geochemical maps showed a similar spatial distribution pattern of the analyzed metals and identified Shempur, Kharara, Taragunia, and Shantidanga as metal hot spots. Geoanalytical indexes were applied to assess the extent of soil contamination, and the results depicted that the soils of Shempur, Kharara, Taragunia, and Shantidanga were moderately contaminated where Cd contributed the most to contamination degree (C d) in spite of its relative low content. However, other five areas in Kushtia District were suggested as uncontaminated according to both C d and pollution load index (PLI). The hazard quotient (HQ) and hazard index (HI) showed no possible indication of human health risks via ingestion of agricultural soils. This study also determined that human activities such as excess application of commercial fertilizers, animal manures, and metal-based pesticides were the sources of Cu, Ni, Cd, and Cr enrichment in soils and that the metals into tobacco plants were transported from the soils. The present study conclusively suggested that regulation of improper use of agrochemicals and continuous monitoring of heavy metals in tobacco plants are needed to reduce the tobacco-related detrimental health problems in Bangladesh.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terrestrial accumulation of heavy metals and their migration into human body is a matter of present world concern (Rahman et al. 2014a, 2014b; Abuduwailil et al. 2015; Chai et al. 2015). Tobacco plants can accumulate metals from different sources including soils through roots, dry and wet atmospheric deposition on exposed part of the plants in polluted environment, and agricultural treatment during tobacco growing, curing, and processing. It is important to note that biogeochemical cycles of heavy metals in agricultural soils are influenced by anthropogenic activities such as industrial effluents, agricultural practices, municipal wastes, vehicular emissions, and atmospheric deposition of dust and aerosol particulates (Mamat et al. 2014; Cai et al. 2015; Chai et al. 2015). However, accumulated heavy metals in tobacco plants can enter into human body through inhalation and consumption (Saha and Zaman 2013; Mamat et al. 2014; Abuduwaili et al. 2015). A pathway of heavy metal transportation from soil to tobacco plant and ultimately to human body has been depicted in Fig. 1. After entering into human body, they react with body enzymes to slow or to stop the essential physiological reactions. Eventually, they can cause different types of serious problems, e.g., anemia, kidney dysfunction, and brain damage. Exposure to high levels of heavy metals may also destroy testicular tissue and can cause cancer, tumor, and paralysis (Pappas et al. 2006). For instance, Cd is considered as one of the “strong carcinogens” in tobacco smoke that partition preferentially into the smoke phase on combustion. Ni is classified as group 1 “carcinogenic to humans” by the International Agency for Research on Cancer (IARC) (Arain et al. 2008). Due to persistent and non-biodegradable nature of heavy metals, even low dose intake over a long period can lead chronic toxicity (Abuduwaili et al. 2015; Li et al. 2015). High exposure levels of virtually all metals, including essential metals, can pose detrimental effect to human health (Rahman and Islam 2009).
Tobacco is one of the basic agricultural products in Bangladesh since the middle of last century. After the liberation war of Bangladesh in 1971, the tobacco cultivation was enhanced by the direct involvement of British American Tobacco Company (BATC) in three specific areas in Bangladesh, i.e., Kushtia, Rangpur, and Chittagong Hill. Subsequently, tobacco industries created job opportunities in Bangladesh and increased local and foreign income sources. Conversely, tobacco cultivation damages our environment and public health. However, it has been advised that the long-term harmful impacts substantially outweigh the short-term economic benefits (Geist 1999).
Recently, it has been estimated that cigarette smoking is responsible for about 25.0 % of deaths in men and 7.6 % in women in Bangladesh (Wu et al. 2013). Despite the public health problems, a little effort has been taken concerning investigation of heavy metals in tobacco plants grown in Bangladesh. To the best of our knowledge, heavy metal contamination status in tobacco cultivating soils and in the tobacco plants grown in Kushtia District (main tobacco production area in Bangladesh) has not yet determined. Thus, the aims of this study are (1) to investigate the spatial distribution pattern and extent of contamination of six metals (Cu, Ni, Cd, Pb, Cr, and Zn) in soils used for tobacco cultivation, (2) to determine the transfer of metals from soils to plants, and (3) to identify the possible sources of metals.
Materials and methods
Study area
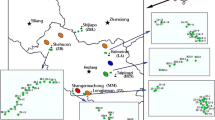
The present study area, Kushtia District (23° 50′ 00″ N and 88° 55′ 00″ E), belongs to the Khulna administrative division and is situated on the western part of Bangladesh (Fig. 2). Kushtia District has an area of 1621.15 km2. It is bounded in the north by Natore and Pabna districts, in the south by Chuadanga and Jhenaidah districts, in the east by Rajbari District, and in the west by West Bengal, India. The main rivers of the study area include Ganges, Gorai, Mathabhanga, Kaligonga, and Kumar. Tobacco cultivation represents one of the most important economic activities of the Kushtia region, covering major portion (∼9000 ha) of the total available land surface (Hossain and Rahman 2013). Many companies (e.g., British American Tobacco Company, Akij Tobacco, Abul Khair, Dhaka Tobacco, and Nasir Tobacco) are directly involved in the production of international standard of tobacco and in the preparation of different brands of cigarette. This research work was conducted in four Kushtia sub-districts (called Upazila) including Bheramara, Mirpur, Daulatpur, and Kushtia Sadar. Samples were collected from Bhabanipur and Baromile under Bheramara sub-district; Kharara and Shempur under Mirpur sub-district; Taragunia, Kollanpur, and Chabana in Daulatpur sub-district; and Shantidanga and Modhupur in Kushtia Sadar sub-district. The locations of sampling sites are shown in Fig. 2.
Sample collection, processing, and preservation
Tobacco cultivating agricultural soils
A total of 27 (three samples from each sampling site) surface soils (0 to 15 cm of depth) were collected with a stainless steel spatula from nine sampling stations of Kushtia District and were kept in polyethylene bags. Several studies have investigated the vertical profile of metals in agricultural soils and reported that metal content in surface soil (i.e., 0 to 15-cm depth) is higher (Maldonado et al. 2008; Li et al. 2015). This was attributed to human-induced pollution on agricultural land and higher organic matter content of surface soil. Therefore, the studies worldwide (e.g., Wong et al. 2002; Bhuiyan et al. 2010; Kelepertzis 2014) are using the top soils for monitoring anthropogenic heavy metal contamination and used in this study. However, after air-drying at room temperature, the collected samples were oven-dried at 105 °C in analytical chemistry laboratory, NRCD, Institute of Nuclear Science and Technology, Bangladesh. The dried soil samples were then ground and passed through a 0.2-mm sieve to obtain a homogenous powder. After that, the powder samples were stored in airtight plastic vials inside a desiccator for further analysis.
Accurately weighed amount of 0.25 g of each dried soil sample was digested with a solution of 3:1 concentrated HNO3: 70 % HClO4 (v/v) at 150 °C in an oil bath (Sun et al. 2010). The digested solutions were then diluted to 50 mL with deionized water and passed through Whatman No. 541 filter papers for analyzing heavy metals by atomic absorption spectrophotometer (AAS) (Ahmed et al. 2010).
Tobacco leaves and roots
A total of 27 tobacco leaf samples and 27 tobacco root samples (three samples per site) were collected from the mature tobacco plants at the same nine sampling sites, where the soils were sampled and transported to the analytical chemistry laboratory. Since the tobacco products are manufactured from mature tobacco leaves due to their higher nicotine content, we have chosen only the mature leaves randomly from each plant. In terms of root samples, we have used whole root of each selected tobacco plant. At first, all the leaves and the roots were cleaned under tap water to remove any adhering soil particles and then several times by deionized water. The samples were then cut into small pieces by sharp stainless steel knife. After that, the small pieces of leaves and roots were dried at 75 °C in an electric oven until a constant weight was obtained. The dried samples were ground into fine powdered substances using a mortar and pestle and passed through a 0.2-mm sieve. The resulting powdered samples were preserved in plastic containers and then kept into laboratory desiccator until digestion.
One gram of each sample (both leaf and root) was ashed at 450 °C in a Muffle furnace (ThermolyneTM, Thermo Scientific, USA). Dry ash was treated with 10 mL of concentrated HCl acid and 3 mL of concentrated HNO3 acid and then wet-digested via heating the mixture on hot plate at about 100 to 120 °C. After reaching to near dryness, 2.5 mL of concentrated HCl acid was added and evaporated to dryness. Then, 5 mL of HNO3 (1:1) was used to dissolve the dry ash. The solution was diluted to 50 mL with 10 % HNO3 in a 50-mL volumetric flux and then filtered through Whatman No. 42 filter paper for AAS analysis (He and Singh 1994; Golia et al. 2009).
Heavy metal analysis
The digested solutions of soil, leaf, and root were analyzed for six heavy metals (i.e., Cu, Ni, Cd, Pb, Cr, and Zn) by atomic absorption spectrophotometer (AA-6800, Shimadzu Corporation, Japan) using air-acetylene flame with digital read out system. A detailed description of analytical conditions of AAS for the heavy metal measurement is provided in Online Resource (Table S1). Wako Pure Chemical Industry Ltd., Japan, supplied standard stock solutions of 1000 mg/L for all the analyzed metals. These standard solutions were diluted to desired concentrations for the calibration of AAS. The results were expressed in milligrams per kilogram as dry weight basis. Deionized water was used throughout the study. Prior to analyses, all glassware and containers were thoroughly cleaned and rinsed several times with deionized water and air-dried following standard method (APHA-AWWA-WEF 2005).
Quality control
The correctness of the methodology was confirmed by analyzing blanks and certified reference materials, CRM-277 (trace elements in estuarine sediment) and CTA-VTL-2 (a reference material of Virginia tobacco leaves) by the same procedure used for the soil and tobacco samples respectively. The results of the reference material analysis are provided in Online Resource (Table S2). A good agreement (94 to 105 %) between the certified and measured values was observed. All the samples were analyzed triplicate, and the relative standard deviations (%RSDs) of the sub-samples were less than 9 %, meaning an excellent reproducibility of the analytical procedures and the instrument.
Statistical analysis and mapping of soil metal concentrations
The ANOVA tests were conducted to evaluate the variation of heavy metals in nine sampling locations and also in three sample categories (soil, root, and leaf). When significant differences were found by ANOVA test, Tukey post hoc tests for multiple comparisons were also performed. The relationship of the metal concentrations among soil, root, and leaf was identified by the Pearson’s correlation coefficient analysis. Principal component analysis (PCA) was employed to determine the sources of heavy metals in tobacco plants. In all cases, the level of significant was set at a 95 % confidence level (α = 0.05). All these statistical analysis were performed using IBM-SPSS Statistics (Version 21) for Windows (IBM, USA). Moreover, the geochemical maps for showing the spatial distribution pattern of the heavy metals in the soil samples were generated using Surfer 11 (Golden Software, Inc., USA).
Transfer factor
In order to quantitative characterization of elemental transfer from soil to plant, the soil-plant partition coefficient or transfer factor (TF) or concentration ratio or biological accumulation coefficient (BAC) is used that expresses a ratio between the elemental concentration in plant parts and in soil (Hope 1995; Dinelli and Lombini 1996; Blanco Rodrıguez et al. 2002). In this study, we assessed the TF values as follows (Khan et al. 2008):
where C root, C leaf, and C soil represent the heavy metal concentration in tobacco root, leaf, and soil samples on dry weight basis, respectively.
Metal contamination assessment
Enrichment factors
To determine the relative degree of metal contamination, heavy metal enrichment factors were calculated by comparing the metal concentration in analyzed soil to geochemical background metal concentration in natural soil following the equation below (Manta et al. 2002):
The background concentrations (in mg/kg, dry wt.) of metals in natural soils were considered as 27 for Cu, 22 for Ni, 0.098 for Cd, 20 for Pb, 35 for Cr, and 68 for Zn (Domingo and Kyuma 1983; Taylor and McLennan 1995).
Contamination factor (C if ) and degree (C d)
The contamination factor (C if ) and degree of contamination (C d) were proposed by Hakanson (1980) to measure the metal pollution in sediment. Many authors, however, have used C if and C d for the metal contamination in soil (Loska et al. 2004; Hashmi et al. 2013). The C if is a single-metal index and was calculated by the following equation:
The C d is a measure of the degree of overall contamination in a particular sampling site and was defined as the sum of all C if . Thus,
where C i0 − 1 is the measured concentration of metal and C in is the standard preindustrial concentration of individual metal determined from various European and American lake sediments by Hakanson (1980). In this study, the background levels of metals in upper Earth’s crust suggested by Taylor and McLennan (1995) were used as C in , since we utilized Hakanson’s formula for soil contamination assessment instead of sediment contamination. The values of C in (in mg/kg) are 25 for Cu, 20 for Ni, 0.098 for Cd, 20 for Pb, 35 for Cr, and 71 for Zn. Four classes of C if were recognized by Hakanson (1980): (i) low contamination factor (if C if < 1), (ii) moderate contamination factor (if 1 ≤ C if < 3), (iii) considerable contamination factor (if 3 ≤ C if < 6), and (iv) very high contamination factor (if C if ≥ 6). Likewise, Hakanson (1980) classified C d into four groups to define the quality of the environment in the following way: (i) low degree of contamination (C d < 8), (ii) moderate degree of contamination (8 ≤ C d < 16), (iii) considerable degree of contamination (16 ≤ C d < 32), and (iv) very high degree of contamination (C d ≥ 32).
Pollution load index
The extent of heavy metal pollution in individual sampling site was also calculated by applying PLI proposed by Tomlinson et al. (1980). The expression of PLI:
where n is the number of metals (in this study n = 6) and C f is the contamination factor for individual pollutant. The value of PLI presents a simple and comparative means for assessing the quality of a sampling site and was classified into four groups in the following ways (Wang et al. 2010): (i) no pollution (if PLI < 1), (ii) moderate pollution (if 1 ≤ PLI < 2), (iii) heavy pollution (2 ≤ PLI < 3), and (iv) extreme pollution (PLI ≥ 3).
Potential human health risk assessment
Earlier research has demonstrated that human health risks are mainly caused by direct oral ingestion of contaminated soils (i.e., entry of soil contaminants through the mouth). Exposure to contaminants via dermal contact and inhalation is negligible (Olawoyin et al. 2012; Chabukdhara and Nema 2013; Li et al. 2015). Therefore, potential non-carcinogenic risks to adult workers were determined by comparing the average daily dose (ADD) (mg/kg/day) of a contaminant via only soil ingestion over a specified time with the respective reference dose (RfD) for a similar exposure period. This ratio is called a hazard quotient (HQ). To evaluate the overall potential for non-carcinogenic risks resulted from more than one contaminant, a hazard index (HI) approach has been developed. The HI is equal to the sum of all HQs. The ADD, HQ, and HI were calculated using the equations below (USEPA 1989; Man et al. 2010; Li et al. 2015):
where C soil is the concentration of heavy metal in soil (mg/kg), IngR represents the ingestion rate of soil (mg/day) and is suggested to be 100 mg/day for adults (Man et al. 2010), EF and ED represent exposure frequency and exposure duration and their values were suggested to be 6 years and 350 days/year, respectively (Man et al. 2010). The average body weight (BW) of an adult is assumed to be 60 kg (Saha and Zaman 2013). The average time (AT) for non-carcinogenic effects was suggested to be 2190 days (Man et al. 2010; Li et al. 2015). CF is the conversion factor (1 × 10−6 kg/mg). The variable i represents the ith heavy metal. The values of RfD for Cu, Ni, Cd, Pb, Cr, and Zn are 0.04, 0.02, 0.001, 0.0035, 0.003, 0.3 mg/kg/day, respectively (USEPA 2014; Li et al. 2015). Any adverse human health effect is unlikely when the value of HQ or HI is lower than one, whereas the value of HQ or HI greater than one may cause adverse human health effects (USEPA 1989).
Results and discussion
Heavy metal concentrations in soils, roots, and leaves
The concentrations of analyzed heavy metals in tobacco cultivating soils are summarized in Table 1 and decreased in the order of Cr > Zn > Cu > Ni > Pb > Cd. ANOVA showed that the concentrations of each metal in nine sampling locations were significantly different (Online Resource Table S3). The observed significant variations might be due to different types of soils, weathering of rocks in various degrees, and different agricultural activities such as application of manures, chemical fertilizers, and pesticides. The results of post hoc Tukey test for multiple comparisons of metal concentrations are shown in Table 1. For example, concentrations of Cu between Bhabanipur and Baromile were not significantly different (shares similar lowercase letter), whereas between Bhabanipur and Shempur were significantly different (shares different lowercase letters) and so on. Likewise, all the metals were significantly different (each metal shares a different uppercase letter) in Bhabanipur.
The metal concentrations in tobacco roots and leaves are listed in Table 1 and decreased, respectively, in similar fashion of Zn (40.62) > Cu (20.21) > Cr (15.76) > Ni (5.20) > Pb (2.65) > Cd (0.22) and Zn (30.66) > Cu (20.14) > Cr (19.12) > Ni (4.14) > Pb (2.56) > Cd (0.62). The variations in metal concentrations in roots collected from nine sampling locations were statistically significant (at p < 0.05) (Online Resource Table S3). ANOVA also indicated statistically significant differences for metals in leaves (Online Resource Table S3). Multiple comparisons of metal concentrations in roots and leaves based on post hoc Tukey test are also presented in Table 1. Roots and leaves collected from Baromile accumulated lower amount of metals. Whereas higher amount of metals were estimated in roots and leaves from Shempur and Taragunia might be due to the observed higher soil metal concentrations in these areas (Table 1). The mean metal concentrations, except Cd, in roots and leaves were lower than that estimated in soils. The Cd was ranked as leaves (0.62 mg/kg) > roots (0.22 mg/kg) > soils (0.16 mg/kg), which indicates that tobacco plants can readily accumulate high level of Cd (Nnorom et al. 2005). The higher Cd accumulation in tobacco leaves compared to roots was also reported by Clarke and Brennan (1989). Thus, there is an increased possibility of human health hazard from Cd associated with tobacco smoking. However, the tobacco leaves produced in Bangladesh mainly used for the production of different brands of cigarettes by several major companies including British American Tobacco Company, Akij Tobacco, Abul Khair, Dhaka Tobacco, and Nasir Tobacco. Therefore, the metal contents found in our tobacco leaf samples were compared with the cigarette tobacco from Canada (Hammond and O’Connor 2008), USA (Swami et al. 2009), UK (Stephens et al. 2005), India (Verma et al. 2010), Pakistan (Kazi et al. 2009), and China (O’Connor et al. 2010). The measured Cu and Cr concentrations were higher than all other countries. Concentrations of two carcinogenic metals, Ni and Pb, found in our leaf samples were higher than the reported values in Canada, USA, UK, and Pakistan, whereas similar to the values found in India and China. The values of Cd, group 1 carcinogen, in Bangladeshi tobacco leaves were in line with the other countries. Zn concentrations were quite similar to UK and Indian tobacco, while pretty higher than the USA.
Spatial distribution of metals in soils
Spatial distribution is a useful tool for determining hot spot area with high metal concentration. Spatial distribution pattern of Cu, Ni, Cd, Pb, Cr, and Zn in the tobacco farming soils of Kushtia District are presented using contour maps in Fig. 3a–f. Contour maps were generated using Surfer 11 (Golden Software, Inc., USA), where kriging method was used to grid the experimental data (Wei et al. 2009). As Fig. 3a shows, the soils from Shempur and Taragunia contained higher concentrations of Cu, followed by Kharara and Shantidanga. Mid-level Cu concentrations were observed in Modhupur and Kollanpur, while lower concentrations were recorded in soils of Chabana, Bhabanipur, and Baromile. The distribution pattern of Ni (Fig. 3b) was similar to that of Cu, and a significantly strong positive correlation (r = 0.948, p < 0.01) between Cu and Ni were also observed (Online Resource Table S4). The higher concentrations of Cd appeared in Kharara and Shempur under Mirpur sub-district and Taragunia under Daulatpur sub-district, followed by Shantidanga. However, the soil Cd concentrations were reduced by 7 to 12 times compared to hot spot Kharara in the rest of the areas of Kushtia District (Fig. 3c). The contour map of Pb again identified Shempur as a hot spot area, and a similar range of Pb variability was observed in Taragunia and Kharara. Decrease in Pb concentration was marked in other sampling spots (Fig. 3d). As shown in Fig. 3e, f, the distribution pattern of Cr and Zn was similar with higher metal concentrations in Taragunia and Shempur and lower in Baromile and Chabana. Overall, this study identified Shempur and Kharara under Mirpur sub-district, Taragunia under Daulatpur sub-district, and Shantidanga under Kushtia Sadar as hot spot areas for the analyzed metals. The geochemical maps presented in Fig. 3 showed similar spatial distribution patterns of the assessed heavy metals in soils, which means that the same types of input sources controlled the metals’ variability in all the sampling points. Moreover, the Pearson’s correlation analysis (Online Resource Table S4) showed significantly (p < 0.01) strong positive correlations among all the metals, which also suggest that the metals may have been derived from the same source. Possible sources of heavy metals in soils are discussed in “Contamination assessment” section below.
Contamination assessment
The results of EFs of all analyzed metals in all sampling points are shown in Table 2. The average order of metal EFs was as follows: Cr (1.73) > Cd (1.64) > Ni (1.34) > Cu (1.10) > Zn (0.67) > Pb (0.55). Based on the EF analysis, there is no accepted pollution ranking system or categorization of degree of pollution. However, EFs can only indicate the natural or anthropogenic origin of metals. The values of EFs < 1.0 indicate a possible mobilization or depletion of metals, and the values close to unity suggest the Earth crust origin, whereas EFs > 1.0 indicate the anthropogenic origin of metals (Bhuiyan et al. 2010). In this study, the EFs for Pb and Zn were less than 1.0, indicating a natural source of these metals in the studied soils. On the other hand, Cr, Cd, Ni, and Cu showed EF values greater than 1.0, indicating anthropogenic origin of them. Higher EF values for Cd along with Cu, Ni, and Cr were observed in Shempur, Kharara, Taragunia, and Shantidanga. The geochemical maps (Fig. 3) also identified these areas as hot spots in terms of analyzed metals. Since the present study areas were not industrially developed, the use of waste irrigation water would not be a relevant cause for Cu, Ni, Cd, and Cr enrichment in the soils. An alternative cause of metal enrichment could be various agricultural activities. Cu is considered as a marker element of agricultural activities, and several authors have reached similar conclusion that increased accumulation of Cu in soil is related to high use of fertilizers, manures, and Cu-based pesticides (Facchinelli et al. 2001; Sanghi and Sasi 2001). It has also been reported that the use of organic materials such as animal manures, biosolids, or composts acts as sources of heavy metals including Ni and Cr in the agricultural field (Chander and Brookes 1993; Valsecchi et al. 1995; McBride and Cherney 2004). Cd is found predominantly in phosphate fertilizers due to its presence as impurities in all phosphate rocks (Nziguheba and Smolders 2008). Several authors documented that increased amount of Cd accumulates in agricultural soils due to the application of high doses of phosphate fertilizers with high Cd concentration over a long period (Peris et al. 2008; Atafar et al. 2010; Cai et al. 2012). Zn may derive in agricultural soils from both natural and anthropogenic sources (Micó et al. 2006; Sun et al. 2013). In this study, Zn and Pb variability was controlled by the natural sources (EFs < 1). Overall, the long agricultural history coupled with excess use of fertilizers, manures, and pesticides in tobacco cultivating land of Kushtia District (especially Shempur, Kharara, Taragunia, and Shantidanga) was assumed to result in higher accumulation of Cu, Ni, Cd, and Cr compared to background concentrations.
The contamination factor (C if ) and the degree of contamination (C d) were also used to estimate the extent of metal pollution in the examined agricultural soils, and the results are presented in Table 2. The C if values of metals analyzed ranged from 0.52 to 1.56 for Cu, 0.90 to 1.87 for Ni, 0.31 to 3.88 for Cd, 0.31 to 1.23 for Pb, 1.21 to 2.15 for Cr, and 0.36 to 0.86 for Zn. Analysis of the data depicted an order of mean C if in soils: Cr (1.73) > Cd (1.64) > Ni (1.47) > Cu (1.19) > Zn (0.64) > Pb (0.55). On the basis of mean C if , the soil was classified as moderately contaminated (1 ≤ C if < 3) with Cu, Ni, Cd, and Cr, while slightly contaminated (C if < 1) with Pb and Zn. Kharara (3.88) and Taragunia (3.37) showed considerable (3 ≤ C if < 6) Cd contamination.
The values of C d ranged from 3.87 (Bhabanipur) to 10.43 (Taragunia) with an average of 7.23. Shempur (10.28), Kharara (10.36), Taragunia (10.43), and Shantidanga (8.57) exhibited moderate degree of contamination (8 ≤ C d < 16), while low contamination levels (C d < 8) were observed in other five areas. It was found that Cd contributed the most to C d of the soils collected from Shempur (27 %), Kharara (38 %), Taragunia (32 %), and Shantidanga (26 %). The second contributor in these four areas was Cr, followed by Ni, Cu, Zn, and Pb.
PLI values of studied nine sampling stations were varied from 0.55 to 1.59, with a mean value of 1.06 (Table 2). Again, Shempur, Kharara, Taragunia, and Shantidanga were determined as moderately polluted (1 ≤ PLI < 2) areas with the highest PLI (1.59) in Shempur. The results of C d also identified these four areas as moderately contaminated (Table 2). However, other sites with PLI between 0.54 and 0.89 were classified as unpolluted (PLI < 1).
Human health risks from soil heavy metals
The mean values of HQs for Cu, Ni, Cd, Pb, Cr, and Zn were 1.19 × 10−3 (ranged from 5.17 × 10−4 to 1.56 × 10−3), 2.35 × 10−3 (1.44 × 10−3 to 2.99 × 10−3), 2.57 × 10−4 (4.79 × 10−5 to 6.07 × 10−4), 5.06 × 10−3 (2.86 × 10−3 to 1.12 × 10−2), 3.23 × 10−2 (2.25 × 10−2 to 4.0 × 10−2), and 2.42 × 10−4 (1.38 × 10−4 to 3.24 × 10−4), respectively. Thus, the HQ values for all examined metals were less than 1, indicating no probable health risks due to workers’ exposure to soil heavy metals in Kushtia District. The combined effects of all contaminants (average HQ value was 0.0414 with minimum and maximum of 0.0275 and 0.0568, respectively) were also lower than the threshold value of 1, again representing no appreciable risk of deleterious effects over a lifetime.
Transfer factors and plant metal source identification
The transfer factor, which is a function of both soil and plant properties, is used to determine the relative abundance of heavy metals in soil and plant samples. Plant is an important component of our ecosystems as it transfers elements from abiotic to biotic environment. Transfer of metals from soils to plants is one of the key components of human exposure to toxic metals via food chain. For human health risk assessment, it is necessary to establish mathematical model to predict soil-to-plant metal transfer and a large dataset on soil-to-plant TF is required for better prediction of the model. For soil-to-plant transfer concept, the presence of linear relationship between soil and plant metal concentrations is considered as an essential prerequisite (Sheppard and Evenden 1988; Bunzl et al. 2000). Positive linear relationships were obtained for Cu (slope = 0.36, r 2 = 0.73), Ni (slope 0.29, r 2 = 0.85), Cd (slope = 2.18, r 2 = 0.82), Pb (slope = 0.32, r 2 = 0.82), Cr (slope = 0.89, r 2 = 0.87), and Zn (slope = 1.06, r 2 = 0.87) (Fig. 4). This study, therefore, confirmed the linearity assumption for every analyzed metal and reflected the dominant control of soils on the metals in tobacco plants. The values of TF for the analyzed heavy metals at each sampling location are presented in Table 3. Higher values of TF mean relatively poor metal retention in soils or greater efficiency of plants to absorb metals, whereas lower TF values reflect strong metal sorption capacity of soil colloids (Alloway and Ayres 1997). Kloke et al. (1984) reported that usually Pb and Cr fixed tightly in soils, while Cd, Zn, and Cu absorbed readily by plant. This study also showed similar results where TFroot/soil values were in the order of Cd > Zn > Cu > Cr > Pb > Ni and TFleaf/soil values were in the following order: Cd > Cu > Zn > Cr > Pb > Ni (Table 3). The TF value of Cd was the highest at every sampling location, which indicates that Cd is more mobile than other metals.
Relationship of heavy metal concentrations (in mg/kg, dry weight basis) between soils and tobacco plants. Graphs a to f show the relationship for Cu, Ni, Cd, Pb, Cr, and Zn, respectively. Gray lines represent regression bands with 95 % confidence level. Metal concentration in tobacco plant is the average of tobacco root and leaf metal concentration
PCA coupled with Pearson’s inter-element correlation coefficients provide information on heavy metals sources (Mamat et al. 2014; Cai et al. 2015; Chai et al. 2015; Molla et al. 2015). According to the values of Pearson’s correlation coefficients (Online Resource Table S4), all the plant metals were highly positively correlated (p < 0.01, p < 0.05), indicating same origin or controlling factor of heavy metals in analyzed tobacco plants. In addition, the results of PCA identified one principal component with eigenvalue higher than 1 that explained 78.51 % of the total variance (Table 3). Since both the Pearson’s correlation analysis and PCA identified one contributing source of metals in tobacco and positive linear relationships (Fig. 4) were also observed between soil and plant metals, we therefore assumed soil as the main source of accumulated heavy metals in studied tobacco plants.
Conclusions and recommendations
The spatial distribution of heavy metals in soils revealed that Shempur, Kharara, Taragunia, and Shantidanga were the areas with higher metal concentrations. EF analysis highlighted the anthropogenic origin of Cr, Cd, Ni, and Cu and pointed out natural source of Pb and Zn. Contamination assessment using various indexes such as C f, C d, and PLI showed that tobacco cultivating soils in Kushtia District were moderately contaminated with the analyzed metals and Cd was identified as the main contributor. However, from human health point of view, both HQ values (for individual metals) and HI (for combined heavy metals) values were lower than unity, indicating no possible health risks for the workers via ingestion pathway of the analyzed soils. The TFleaf/soil values decreased in the order of Cd > Cu > Zn > Cr > Pb > Ni, indicating higher Cd accumulation capability of tobacco plants and also the possibility of Cd-induced human health hazards through tobacco consumption. The utilization of higher amount of commercial fertilizers, animal manures, and metal-based pesticides to accelerate the production of tobacco plants might be the cause of metals’ load in agricultural soils and eventually in the tobacco leaves. Therefore, to reduce the human health risk from tobacco consumption, it is advisable to impose regulation on the improper use of agrochemicals in tobacco farmlands, as well as to implement continuous monitoring of heavy metals both in soils and tobacco plants. Health officials and regulators in Bangladesh should establish safe level of heavy metals in tobacco to mitigate the metal contamination.
References
Abuduwaili J, Zhang ZY, Jiang FQ (2015) Assessment of the distribution, sources and potential ecological risk of heavy metals in the dry surface sediment of Aibi Lake in northwest China. PLoS One 10:e0120001
Abuduwailil J, Zhaoyong Z, Fengqing J (2015) Evaluation of the pollution and human health risks posed by heavy metals in the atmospheric dust in Ebinur Basin in Northwest China. Environ Sci Pollut Res:1-14 doi:DOI 10.1007/s11356-015-4625-1
Ahmed MK, Bhowmik AC, Rahman S, Haque MR (2010) Heavy metal concentration in water, sediments, freshwater mussels and fishes of the river shitalakhya, bangladesh. Asian J Water Environ Pollut 7:77–90
Alloway BJ, Ayres DC (1997) Chemical principles of environmental pollution. Blackie Academic & Professional, London
APHA-AWWA-WEF (2005) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Arain MB et al (2008) Hazardous impact of toxic metals on tobacco leaves grown in contaminated soil by ultrasonic assisted pseudo-digestion: multivariate study. J Hazard Mater 155:216–224
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392
Blanco Rodrıguez P, Vera Tome F, Lozano J (2002) About the assumption of linearity in soil-to-plant transfer factors for uranium and thorium isotopes and 226Ra. Sci Total Environ 284:167–175
Bunzl K, Albers B, Schimmack W, Belli M, Ciuffo L, Menegon S (2000) Examination of a relationship between 137Cs concentrations in soils and plants from alpine pastures. J Environ Radioact 48:145–158
Cai L et al (2015) Multivariate and geostatistical analyses of the spatial distribution and source of arsenic and heavy metals in the agricultural soils in Shunde, Southeast China. J Geochem Explor 148:189–195
Cai L et al (2012) Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotoxicol Environ Saf 78:2–8
Chabukdhara M, Nema AK (2013) Heavy metals assessment in urban soil around industrial clusters in Ghaziabad, India: probabilistic health risk approach. Ecotoxicol Environ Saf 87:57–64
Chai Y, Guo J, Chai S, Cai J, Xue L, Zhang Q (2015) Source identification of eight heavy metals in grassland soils by multivariate analysis from the Baicheng–Songyuan area, Jilin Province, Northeast China. Chemosphere 134:67–75
Chander K, Brookes P (1993) Residual effects of zinc, copper and nickel in sewage sludge on microbial biomass in a sandy loam. Soil Biol Biochem 25:1231–1239
Clarke B, Brennan E (1989) Differential cadmium accumulation and phytotoxicity in sixteen tobacco cultivars. JAPCA 39:1319–1322
Dinelli E, Lombini A (1996) Metal distributions in plants growing on copper mine spoils in Northern Apennines, Italy: the evaluation of seasonal variations. Appl Geochem 11:375–385
Domingo LE, Kyuma K (1983) Trace elements in tropical Asian paddy soils: I. Total trace element status. Soil Sci Plant Nutr 29:439–452
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114:313–324
Geist HJ (1999) Global assessment of deforestation related to tobacco farming. Tob Control 8:18–28
Golia EE, Dimirkou A, Mitsios IK (2009) Heavy‐metal concentration in tobacco leaves in relation to their available soil fractions. Commun Soil Sci Plant Anal 40:106–120
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hammond D, O’Connor R (2008) Constituents in tobacco and smoke emissions from Canadian cigarettes. Tob Control 17:i24–i31
Hashmi MZ, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y (2013) Risk assessment of heavy metals pollution in agricultural soils of siling reservoir watershed in Zhejiang Province, China. Bio Med Res Int 2013:1–10
He QB, Singh BR (1994) Crop uptake of cadmium from phosphorus fertilizers: I. Yield and cadmium content. Water Air Soil Pollut 74:251–265
Hope BK (1995) A review of models for estimating terrestrial ecological receptor exposure to chemical contaminants. Chemosphere 30:2267–2287
Hossain MM, Rahman MM (2013) A socioeconomic analysis on tobacco cultivation in Kushtia District of Bangladesh. Soc Sci 2:128–134
Kazi TG, Jalbani N, Arain MB, Jamali MK, Afridi HI, Shah AQ (2009) Determination of toxic elements in different brands of cigarette by atomic absorption spectrometry using ultrasonic assisted acid digestion. Environ Monit Assess 154:155–167
Kelepertzis E (2014) Accumulation of heavy metals in agricultural soils of Mediterranean: insights from Argolida basin, Peloponnese, Greece. Geoderma 221–222:82–90
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Kloke A, Sauerbeck DR, Vetter H (1984) The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In: Changing metal cycles and human health. Springer, pp 113-141
Li P, Lin C, Cheng H, Duan X, Lei K (2015) Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol Environ Saf 113:391–399
Loska K, Wiechuła D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30:159–165
Maldonado V, Rubio Arias H, Quintana R, Saucedo R, Gutierrez M, Ortega J, Nevarez G (2008) Heavy metal content in soils under different wastewater irrigation patterns in Chihuahua, Mexico. Int J Environ Res Public Health 5:441–449
Mamat Z, Yimit H, Ji RZA, Eziz M (2014) Source identification and hazardous risk delineation of heavy metal contamination in Yanqi basin, northwest China. Sci Total Environ 493:1098–1111
Man YB et al (2010) Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world's most populated city. Environ Int 36:570–576
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300:229–243
McBride M, Cherney J (2004) Molybdenum, sulfur, and other trace elements in farm soils and forages after sewage sludge application. Commun Soil Sci Plant Anal 35:517–535
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872
Molla M, Saha N, Salam S, Rakib-uz-Zaman M (2015) Surface and groundwater quality assessment based on multivariate statistical techniques in the vicinity of Mohanpur, Bangladesh. Int J Environ Health Eng 4:18
Nnorom IC, Osibanjo O, Oji-Nnorom CG (2005) Cadmium determination in cigarettes available in Nigeria. Afr J Biotechnol 4:1128–1132
Nziguheba G, Smolders E (2008) Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci Total Environ 390:53–57
O'Connor RJ et al (2010) Cigarettes sold in China: design, emissions and metals. Tob Control 19:i47–i53
Olawoyin R, Oyewole SA, Grayson RL (2012) Potential risk effect from elevated levels of soil heavy metals on human health in the Niger delta. Ecotoxicol Environ Saf 85:120–130
Pappas R, Polzin G, Zhang L, Watson C, Paschal D, Ashley D (2006) Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem Toxicol 44:714–723
Peris M, Recatalá L, Micó C, Sánchez R, Sánchez J (2008) Increasing the knowledge of heavy metal contents and sources in agricultural soils of the European Mediterranean region. Water Air Soil Pollut 192:25–37
Rahman MS, Islam MR (2009) Effects of pH on isotherms modeling for Cu (II) ions adsorption using maple wood sawdust. Chem Eng J 149:273–280
Rahman MS, Saha N, Molla AH (2014a) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71:2293–2308
Rahman MS, Saha N, Molla AH, Al-Reza SM (2014b) Assessment of anthropogenic influence on heavy metals contamination in the aquatic ecosystem components: water, sediment, and fish. Soil Sediment Contam Int J 23:353–373
Saha N, Zaman M (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ Monit Assess 185:3867–3878
Sanghi R, Sasi K (2001) Pesticides and heavy metals in agricultural soil of Kanpur, India. Bull Environ Contam Toxicol 67:0446–0454
Sheppard S, Evenden W (1988) The assumption of linearity in soil and plant concentration ratios: an experimental evaluation. J Environ Radioact 7:221–247
Stephens WE, Calder A, Newton J (2005) Source and health implications of high toxic metal concentrations in illicit tobacco products. Environ Sci Technol 39:479–488
Sun C, Liu J, Wang Y, Sun L, Yu H (2013) Multivariate and geostatistical analyses of the spatial distribution and sources of heavy metals in agricultural soil in Dehui, Northeast China. Chemosphere 92:517–523
Sun Y, Zhou Q, Xie X, Liu R (2010) Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. J Hazard Mater 174:455–462
Swami K, Judd CD, Orsini J (2009) Trace metals analysis of legal and counterfeit cigarette tobacco samples using inductively coupled plasma mass spectrometry and cold vapor atomic absorption spectrometry. Spectrosc Lett 42:479–490
Taylor SR, McLennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun 33:566–575
USEPA (1989) Risk assessment guidance for superfund. United States Environmental Protection Agency, Washington, EPA/540/1-89/002
USEPA (2014) Integrated risk information system (IRIS). United States Environmental Protection Agency, Washington DC
Valsecchi G, Gigliotti C, Farini A (1995) Microbial biomass, activity, and organic matter accumulation in soils contaminated with heavy metals. Biol Fertil Soils 20:253–259
Verma S, Yadav S, Singh I (2010) Trace metal concentration in different Indian tobacco products and related health implications. Food Chem Toxicol 48:2291–2297
Wang X, He M, Xie J, Xi J, Lu X (2010) Heavy metal pollution of the world largest antimony mine-affected agricultural soils in Hunan province (China). J Soils Sediments 10:827–837
Wei B, Jiang F, Li X, Mu S (2009) Spatial distribution and contamination assessment of heavy metals in urban road dusts from Urumqi, NW China. Microchem J 93:147–152
Wong S, Li X, Zhang G, Qi S, Min Y (2002) Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut 119:33–44
Wu F et al (2013) A prospective study of tobacco smoking and mortality in Bangladesh. PLoS One 8:e58516
Acknowledgments
The authors sincerely thank the authority of Bangladesh Atomic Energy Commission for providing laboratory facilities for analyzing soil and tobacco plant samples by conventional techniques. The authors also thank Islamic University, Bangladesh, for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Zhihong Xu
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 22 kb).
Rights and permissions
About this article
Cite this article
Saha, N., Rahman, M.S., Jolly, Y.N. et al. Spatial distribution and contamination assessment of six heavy metals in soils and their transfer into mature tobacco plants in Kushtia District, Bangladesh. Environ Sci Pollut Res 23, 3414–3426 (2016). https://doi.org/10.1007/s11356-015-5575-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5575-3