Abstract
We aimed at assessing the effects of four wetland plant species commonly used in constructed wetland systems: Typha, Phragmites, Iris and Juncus for removing ibuprofen (IBU) and iohexol (IOH) from spiked culture solution and exploring the mechanisms responsible for the removal. IBU was nearly completely removed by all plant species during the 24-day experiment, whereas the IOH removal varied between 13 and 80 %. Typha and Phragmites were the most efficient in removing IBU and IOH, respectively, with first-order removal rate constants of 0.38 and 0.06 day−1, respectively. The pharmaceuticals were taken up by the roots and translocated to the aerial tissues. However, at the end of the experiment, plant accumulation constituted only up to 1.1 and 5.7 % of the amount of IBU and IOH spiked initially. The data suggest that the plants mainly function by facilitating pharmaceutical degradation in the rhizosphere through release of root exudates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thousands of pharmaceuticals are produced in significant quantities for domestic and agricultural purposes, and most of these are eventually released into freshwater and coastal water systems, where they or their biotransformation products can pose a risk to wildlife and humans. There is no direct evidence documenting adverse effects of pharmaceuticals in the environments on humans. However, renal lesions and alterations of the gills were observed in diclofenac-exposed fish at concentration of 5 μg L−1 (Schwaiger et al. 2004). Therefore, efficient removal of organic micropollutants, including pharmaceuticals, from wastewaters before discharge into the environment is a topic of increasing concern. However, current wastewater treatment plants (WWTPs) are not designed for and effective in eliminating pharmaceuticals and other organic micropollutants. Ibuprofen (IBU) is one of the most consumed pharmaceuticals in the world (Zhang et al. 2011). The removal efficiency of IBU in conventional WWTPs can reach levels above 95 %, but still nanogram per litre level concentrations have been detected in the waters of lakes, rivers and the sea (Buser et al. 1999). Additionally, because of bioaccumulation, microgram per litre level concentrations has been detected in fish bile (Brozinski et al. 2012). The radiocontrast agent iohexol (IOH) is used in diagnostic tests in hospitals and is excreted from the body in the urine in a non-metabolised form. The discharge of IOH deserves concern as high doses are used in hospitals, and conventional WWTPs are inefficient in removing IOH (Ryu et al. 2014).
Constructed wetland system (CW) is a robust and economic wastewater treatment technology, which enables many different kinds of wastewater to be treated in a cost-efficient way. CWs have also been found to be able to remove a variety of pharmaceuticals and other organic micropollutants from polluted water with promising results (Verlicchi and Zambello 2014). Several processes are involved in the removal of pharmaceuticals in CWs, including plant uptake, photodegradation, hydrolysis and microbial degradation. Plants have been shown to play an essential role in the removal of pharmaceuticals from water (Matamoros et al. 2012b), and there are also evidence of direct uptake of pharmaceuticals by several crop plant species (Carvalho et al. 2014; Herklotz et al. 2010; Shenker et al. 2011). Besides direct uptake, plants may facilitate the removal of pharmaceuticals by the release of root exudates into the rhizosphere (Zhang et al. 2011). The root exudates released by plants are a range of low molecular weight carbon-containing compounds, such as sugars and organic acids, which can provide an organic carbon and nutrient source for microorganisms in the rhizosphere (Vanek et al. 2010).
Although several studies have documented a significant removal of pharmaceuticals in CWs, data on the direct uptake of pharmaceuticals by wetland plants are sparse and restricted to a few selected compounds and plant species (Dordio et al. 2011b; Matamoros et al. 2012a, b), and the effect of root exudates on the removal of pharmaceuticals has barely been studied. Hence, the aim of the present study was to assess the plant uptake ability of IBU and IOH by four common emergent wetland plant species, namely cattail (Typha latifolia L.), common reed (Phragmites australis (Cav.) Trin. ex Steud.), yellow flag (Iris pseudacorus L.) and soft rush (Juncus effusus L.) to better understand the removal mechanism of IBU and IOH in plant-based systems. In addition, we wanted to get more knowledge on the potential effects of root exudates for the removal of IBU and IOH. The characteristics of IBU and IOH are shown in Table S1.
Materials and methods
Experimental setup
The four plant species: T. latifolia, P. australis, I. pseudacorus and J. effuses, were grown from seeds in a greenhouse. After germination, individual seedlings were potted in 0.7-L pots containing a mixture of sand and commercial sphagnum compost. When plants were approximately 200-mm tall, the soil was carefully washed from the roots. The plants were rinsed with Milli Q water, then divided in homogenous groups and acclimatised for 10 days in the hydroponic culture solution in a growth chamber (Bio 2000S, Weiss Umwelttechnik GmbH, Germany) with the same environmental conditions as those of the experiment (see later). Nine plants of similar size of each species (6.2 ± 1.9 g fresh mass) were selected and distributed at random between the treatments: (i) planted control (n = 3), (ii) 10 mg L−1 of IBU with plants (n = 3), (iii) 10 mg L−1 of IOH with plants (n = 3), (iv) 10 mg L−1 of IBU without plants (n = 5) and (v) 10 mg L−1 of IOH without plants (n = 5). The plants were mounted in the lids of 0.7-L glass vessels containing 0.5 L of the culture solution. The glass vessels were covered with aluminium foil to avoid photodegradation. The plants were held in position in the lids of the vessels by slices of soft polyethylene foam. Care was taken that all roots were covered by the culture solution. The basic culture solution was prepared according to Smart and Barko (1985) from Milli Q water and reagent grade salts, and additional nutrients were added using a commercial liquid fertiliser (Plant Nutrition+, Tropica, Egaa, Denmark) (full composition detailed in supplementary material). Water lost by evapotranspiration and sampling from the vessels along the experiment were replaced by addition of Milli Q water. The growth chamber was programmed at a day/night cycle of 25:22 °C, 16:8 h light and 70:80 % relative air humidity. The photon flux density at the base of the plants was approximately 400 μmol m−2 s−1 (PAR).
Sampling
Sampling of the culture solution in the vessels was conducted at day 0, 0.5, 1, 2, 3, 4, 5, 7, 10, 14, 18, 21 and 24 of the experiment. The time frame selected was decided based on the behaviour of the two compounds under investigation. Ibuprofen is a fast degrading compound, so intensive sampling campaigns were conducted at the beginning of the experiment. On the other hand, as iohexol is considered more recalcitrant, a longer experimental period was selected to follow the evolution of this compound. Before each sampling event, the culture solution in the vessels was thoroughly mixed by injecting and ejecting solution inside the vessels several times using a glass syringe. Then, 1 mL of the culture solution from each vessel was collected, filtered through a 0.45 μm pore size mixed cellulose ester filter (KX Syringe Filter, UK) and analysed for IOH and IBU.
At the end of the experiment, 350 mL of the culture solutions were collected for root exudate analysis. These samples were concentrated to 50 mL using a Multievaporator (Syncore Polyvap, BÜCHI, Denmark) and then analysed by HPLC.
The total plant fresh mass, the maximum root length and the maximum leaf length of each individual plant were measured at days 0, 3, 7, 10, 14, 18, 21 and 24 to monitor plant growth; day 0 and day 24 were selected to calculate fresh mass production and change in maximum root length and maximum leaf length. When measuring these parameters, the plants were carefully removed from the vessels. At the end of the experiment, the plants were removed from the vessels and rinsed with Milli Q water. Plant tissue from the control group (not spiked) was also collected to analyse the pharmaceutical background levels. The plants were separated into roots, stems and leaves, frozen and lyophilised for dry mass determination. The freeze-dried material was finely ground in a mill grinder and then stored at −20 °C until analysis of IBU and IOH.
Analysis of IOH and IBU
The water samples and plant extracts were analysed for IOH and IBU by a HPLC system equipped with a diode array detector (DAD) (Ultimate 3000, Thermo Scientific, Denmark). Description of the plant tissue extraction, HPLC procedures and the analytical material are thoroughly described in the supplementary material.
The concentrations of IBU and IOH in the culture solution and the plant samples were used to calculate the bioaccumulation factors (BAFs), defined as the ratio of compound concentration in the plant roots to the final concentration in the culture solution, and the translocation factors (TFs), defined as the ratio of compound concentration in the plant aerial parts to the concentration in the plant roots.
A first-order kinetic model was used to describe the removals of IBU and IOH from the culture solutions:
where C 0 and C are the concentrations of IBU and IOH at time zero and time t, respectively, and k is the first-order removal rate constant.
The half-life of IBU and IOH was calculated as follows:
where t ½ is the half-life for the removal of IBU and IOH and k is the first-order removal rate constants.
Root exudates
The concentrations of low molecular weight organic carbon compounds, organic acids and sugars, in the culture solution, were analysed using a HPLC system. The following organic acids were targeted: oxalic, maleic, tartaric, malic, malonic, quinic, succinic, lactic, formic, acetic, fumaric, propionic, isobutyric, butyric and gallic acids. The analysed sugars were raffinose, maltose, lactose, glucose, mannose, fructose and arabinose. A detailed description of the HPLC procedures and the analytical material are thoroughly described in the supplementary material.
The organic carbon content (expressed as mass of carbon; μg C) of the culture solutions was calculated from the concentrations of organic acids and sugars in solution as analysed with the HPLC multiplied with the solution volume. The true organic C content in the solution was higher than calculated here, as we only considered organic acids and sugars.
Statistical analysis
Statistical analyses were carried out using the SPSS software (statistical software, version 20.0, SPSS, Inc., Chicago, IL, USA). One-way ANOVA and post hoc Tukey’s HSD tests were used to compare the plant growth parameters in the different treatments, as well as the concentrations of IBU and IOH in the plant tissue, the BAF and TF and the total mass of IBU and IOH in the plant tissue of the different plant species at the 5 % significance level. Pearson correlation analyses were used to evaluate the relationships between the IBU and IOH removal rate constants and the plant dry mass and the organic carbon contents in the culture solution.
Results and discussion
Plant growth
We selected an initial concentration of IBU and IOH in the experiment (10 mg L−1) that is much higher than the usual concentration levels of these compounds found in surface waters in order to be able to study the possible uptake by the plants. It is a common practice to use high concentrations of the target compound studied to asses, for instance, phytotoxicity of the compound (Iori et al. 2012; Kotyza et al. 2010) or, as in the present study, the uptake of IBU and IOH by the plants. The high initial concentrations of IBU and IOH were needed in order to obtain concentration levels in the plants above the limits of detection by the analytical technique used.
Plants’ exposure to IBU and IOH significantly affected the growth of T. latifolia (fresh biomass) and J. effusus (maximum root length; p < 0.05). P. australis was affected only by IOH (leaf length; p < 0.05). I. pseudacorus was the only of the tested plant species which was not significantly affected by IBU and IOH (Table 1).
High concentrations of pharmaceuticals are known to be able to affect plant growth. Kotyza et al. (2010) found yellowing and desiccation of shoots when P. australis was exposed to 0.2 mM (i.e. 41 mg L−1) of IBU, a concentration four times higher than the concentration used in this study. The effects of IBU were minor in our study, as only the root growth of J. effusus and the fresh mass of T. latifolia was significantly affected by IBU, and there were no effects of IBU on the other species. IOH, however, had more effects on plant development. However, considering that the real concentration levels of pharmaceuticals in surface waters are at least three orders of magnitude lower (ng L−1 to μg L−1) than the concentrations used in this experiment and the presence of substrate in subsurface flow CWs will influence the bioavailability of pharmaceuticals to the plants, it is not expected that phytotoxic effects will occur in plant-based systems used for phytoremediation of these compounds (Carvalho et al. 2014).
Removal performance
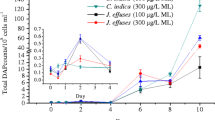
IBU in the solution were nearly completely removed by the four plant species during the 24-day experiment but at different rates (Fig. 1). The half-life for the removal of IBU in T. latifolia, P. australis, I. pseudacorus and J. effusus were 2.3, 6.1, 4.3 and 5.5 days, respectively, and the first-order removal rate constants were 0.38, 0.17, 0.26 and 0.18 day−1, respectively (Table 2). For IOH, P. australis was the only of the four species that was able to remove the compound with a good (80 %) efficiency (Fig. 1). IOH was recalcitrant to the other plant species as the removal rates were only 15 % for T. latifolia, 13 % for I. pseudacorus and 31 % for J. effusus. The first-order removal rate constants of IOH were 0.06 and 0.007 day−1, and the half-lives were 13.3 and 79.3 days for P. australis and J. effusus, respectively (Table 2).
IBU is regarded as a biodegradable compound (Matamoros et al. 2012b). Our results concerning IBU removal are in agreement with the findings of Dordio et al. (2011a) who reported that Typha could remove over 99 % of IBU during a 21-day experiment with a first-order removal rate constant of 0.768 day−1. Matamoros et al. (2012b) found removal rate constants for IBU ranging between 0.016 and 0.043 day−1 in microcosm systems planted with the free-floating plants Salvinia molesta and Lemna minor and the submerged aquatic plants Ceratophyllum demersum and Elodea canadensis. The removal rate constants obtained for IBU in our experiment (from 0.17 to 0.38 day−1) for the four emergent wetland plant species and by Dordio et al. (2011a) for Typha are one order of magnitude higher than the rates reported for the free-floating and submerged aquatic plant species by Matamoros et al. (2012b). This reported difference in removal ability between the different life forms of plants can partly be explained by differences in experimental conditions in the experiments as the temperature was lower (18 °C) and the ratios of plant biomass to culture solution volume was lower in the experiments of Matamoros et al. (2012b) compared to the present experiment. Thus, the higher biomass of the emergent plants relative to the culture solution volume seems to explain the higher removal rate constants of IBU in the emergent plants in comparison with the free-floating and submerged aquatic plants. This statement is supported by the fact that, in the present study, the first-order removal rate constants for IBU were significantly correlated with the dry mass of the four plant species (r = 0.68, p < 0.05).
In contrast to IBU, IOH was relatively recalcitrant. Matamoros et al. (2012b) analysed the removal kinetics of seven organic micropollutants (diclofenac, triclosan, naproxen, IBU, caffeine, clofibric acid and 2-methyl-4-chlorophenoxyacetic acid (MCPA)) and found that the first-order removal rate constants were compound dependent, with MCPA being the most recalcitrant compound (k = 0.004 day−1) and triclosan the most easily removed compound (k = 0.315 day−1). Hence, the differences in removal rate constants between compounds were attributed essentially to their different physicochemical characteristics (Matamoros et al. 2012b).
The removal pathways for IBU and IOH in the hydroponic systems could theoretically be attributed to abiotic processes (photodegradation, volatilisation and hydrolysis) and biotic processes (plant uptake and microbial degradation). However, in the present study, photodegradation and volatilisation were not expected to occur due to the low volatility of the pharmaceuticals studied and the fact that the vessels were light protected. Additionally, as pharmaceuticals are usually designed for oral intake to avoid hydrolysis (Andreozzi et al. 2003), biotic processes are likely the major pathways for the removal of IBU and IOH in the present study. This is consistent with the fact that no removal was observed in the chemical controls.
Plant uptake of IBU and IOH
The plants took up IBU and IOH from the solution and translocated it to the aerial tissues (Fig. 2). For IBU, I. pseudacorus had the highest IBU concentrations in the tissue, with a concentration of 19.2 μg g−1 dry mass (DM) in the aerial tissues and 20.2 μg g−1 DM in the roots (Fig. 2). For IOH, J. effusus had the highest concentrations in both aerial tissues (79 μg g−1 DM) and roots (311 μg g−1 DM) (Fig. 2). In the plant tissue from the control group (not spiked with pharmaceuticals), IBU and IOH were not detected.
BAF and TF of IBU and IOH in the four plant species were examined (Table S4). BAF is a measurement of the plant uptake ability, and the TF is a measurement of the translocation ability of the compounds in the plants. For IBU, root concentrations in I. pseudacorus and P. australis were 100 to 200 times higher than the final IBU concentrations in the culture solutions and significantly higher than the BAF of T. latifolia and J. effusus (p < 0.05). T. latifolia had the highest TF (p < 0.05) and hence the highest ability to translocate IBU from roots to the aerial plant parts. For IOH, P. australis and J. effusus had significantly higher BAF than T. latifolia and I. pseudacorus (p < 0.05), while there were no significant differences in the TF among the plant species (Table S4).
The existing studies on plant uptake of IBU are not consistent. Winker et al. (2010) found no detectable IBU in ryegrass fertilised with IBU spiked urine, and Cortés et al. (2013) did not detect IBU in soybean and wheat fertilised by IBU containing sewage sludge. However, IBU was taken up in lettuce and carrot after growth in soil irrigated with IBU-contaminated ground and reclaimed water (Calderón-Preciado et al. 2013). These studies were, however, carried out with plants growing in a soil matrix, which may have affected the bioavailability of IBU to the plants. The results of the present study, however, clearly documents that IBU can be taken up by the roots of plants and be translocated from the roots to the aerial tissues. To the best of our knowledge, this is the first time that plant uptake and translocation of IBU are reported for wetland plants. On the other hand, for crop plants, the translocation of selected xenobiotics has been studied in two leafy vegetable species: lettuce and collards, among others (Dodgen et al. 2013). Here, Dodgen et al. (2013) found that the TF for bisphenol A, diclofenac sodium, naproxen and 4-nonylphenol were lower in lettuce than in collards. Differences in uptake and translocation of the same compound between different plant species are attributed to differences in the physiological characteristics of the plants, as it has been shown that neutral compounds are more likely to be taken up and translocated in plant tissues with high lipid content (Collins et al. 2011). Hence, different plant species are likely to possess different BAF and TF of the same compound.
We have not been able to find any literature documenting plant uptake of IOH. Polar, highly water-soluble organic compounds such as IOH are, however, believed to be readily taken up by plant roots and translocated to the shoots (Dettenmaier et al. 2008). Trapp (2009) demonstrated that neutral polar compounds had a high potential for plant uptake and, furthermore, that neutral polar compounds can be readily translocated to the leaves with the transpirational water, resulting in higher concentrations in the leaves than in the roots. Dettenmaier et al. (2008) proposed to use the transpiration stream concentration factor (TSCF = 11 / (11 + 2.6logKow)) as a proxy to estimate the ability of an organic compound to be passively transported from the roots to the shoot. Using this formula, the TSCF for IOH is 0.994. Compounds or elements actively taken up by plants generally have TSCF values greater than 1.0 (N, P and K), while compounds and elements that move into the plants at the same rate as water have a TSCF value of 1. Hence, the TSCF for IOH suggests that IOH is transported within the plants at the same rate as water. Uptake and translocation of more lipophilic compounds with TSCF values lower than 1 will be low, as the uptake relies on the traditional processes of transport through the lipid cell membranes of the roots. The significantly higher concentrations of IOH in the plant tissues compared to the concentrations of IBU observed in the present study may be explained by the differences in the TSCF between the two compounds and also differences in the bioavailability of the two compounds. The high molecular weight of IOH may contribute to explain the higher IOH concentration in the roots than in the aerial tissues as the high molecular weight and the molecular structure of IOH may result in some size exclusion limitations along the water transport pathway. This is in agreement with Fismes et al. (2002) who demonstrated that the translocation ability of polycyclic aromatic hydrocarbons within the plant tissue was dependent on the molecular weight.
The experiment was conducted in a hydroponic system to study to what extent the plant uptake contributes to the IBU and IOH removal in the system. It should be noted that in the system, the plant uptake is conditioned by the bioavailability of IBU and IOH, as plants were only subjected to the culture solution instead of substrate in subsurface flow CWs. In real CWs where the substrate is present, the sorption of IBU and IOH to the substrate needs to be considered. Especially for IBU, it is lipophilic and tends to be adsorbed by substrate in comparison with hydrophilic compound IOH.
Mass balance of IBU and IOH
In general, only small percentages (<1.1 and <5.7 % for IBU and IOH, respectively) of the amounts of IBU and IOH added to the culture solution initially were found in the plant tissues (Fig. 3). The highest mass uptake was observed for IBU (1.1 %) for I. pseudacorus and for IOH (5.7 %) for J. effusus.
IBU was nearly completely removed from the culture solutions, but the amount of IBU accumulated in the plant tissues constituted only 0.3 to 1.1 % of the amount added to the culture solutions. For IOH, removals ranged between 13 and 80 %, while the amount of IOH accumulated in the plant tissues constituted 1.5 to 5.7 % of the amount added to the culture solutions. The very high removal of IBU from the culture solutions combined with the low amount of IBU that was accumulated in the plant tissue suggest that the majority of the IBU in the systems have been transformed along the time, either by microbial degradation in the culture solutions or internally in the plants after uptake. Similar results can be found in Matamoros et al. (2012b), who detected metabolites of ibuprofen from solutions in plant-based microcosms. However, in the present study, it was not possible to quantify the relative importance of microbial degradation processes and plant uptake for the removal of IBU. For IOH, due to the lower removal in the culture solutions and higher accumulation in the plant tissues, plant uptake seems to contribute significantly to the removal of IOH in the plant-based systems.
The BAF and TF are essential parameters when evaluating candidate plant species for application in phytoremediation. A good candidate species should have a high BAF as well as a high TF. The contaminant mass uptake and the calculated BAF and TF give, however, complementary information. To make a qualified evaluation of the phytoremediation ability of a particular plant species, both the contaminant mass uptake, which is the product of the biomass and the tissue contaminant concentration, and the ability of the plants to extract the contaminant from the water at very low ambient concentrations, i.e. the BAF, and to translocate the contaminant into the aboveground harvestable tissue, i.e. the TF, need to be taken into account. The BAF and TF are normally used as predictors of plant uptake ability when obtaining biomass is unprocurable. In the present study, the high TF of IBU in T. latifolia, the high BAF of IBU in I. pseudacorus and the high BAF of IOH in J. effusus correlated with the corresponding high mass uptake of IBU and IOH, respectively. Moreover, no significant difference was observed between the TF of IOH among plant species, while the contaminant mass uptake of T. latifolia and J. effusus were significantly higher than those of P. australis and I. pseudacorus due to the higher biomass.
Root exudates
Figure 4 shows the concentrations of the organic acids in the culture solution spiked with IBU and IOH as well as in the plant control. Twelve of the 15 organic acids analysed were detected in the culture solutions (oxalic, butyric and gallic acids were not detected in any of the solutions). Propionic and isobutyric acid were only detected in the plant control, and maleic, tartaric, malonic and quinic acid were both detected in the IBU and IOH treatments. None of the sugars were detected in the culture solutions because the concentrations were lower than LOQ. The composition of organic acids clearly differed between the plant control and the IBU and IOH treatments. These differences may partly be due to the observed phytotoxicity of IBU and IOH, as the phytodetoxification processes of the plants may include the release of root exudates. Also, damaged and degrading root cells release a cocktail of organic compounds to the surrounding medium. Although the plants used in the experiment were cleaned carefully prior to setup in the hydroponic cultures, the root systems were not axenic, and microorganisms associated with the roots of the plants may also have released organic compounds into the culture solutions.
It is known that root exudates, mainly low molecular weight carbon-containing compounds, can fuel microbial processes in the rhizosphere and facilitate the microbial degradation of contaminants (Zhai et al. 2013). Zhang et al. (2011) demonstrated that root exudates may play a direct role in the degradation of certain pharmaceuticals and/or increase the bioavailability of pharmaceuticals by acting as surfactants or transporters. Additionally, Gujarathi et al. (2005) found high removals of antibiotics by filtered root exudates from Helianthus annuus, suggesting the involvement of root secreted enzyme(s)/metabolite(s) in antibiotic degradation.
In the IBU treatment, the total organic carbon content ranged from 149 to 436 μg C, while in the IOH treatment, the total organic carbon content ranged from 18 to 428 μg C. Interestingly, a significant correlation was observed between the carbon content and the removal rate constants of IOH in P. australis and J. effusus (r = 0.887, p < 0.05). T. latifolia and I. pseudacorus are not shown in Fig. S1 (right panel) because of the poor fit with the kinetic model. The low removal rate constants for IOH by T. latifolia and I. pseudacorus are, however, consistent with the low organic carbon concentrations in the solutions. Regarding IBU, no significant correlation was found between the organic carbon content and the IBU removal rate constants (Fig. S1).
The depletion of IBU and IOH from the culture solutions could be described by a first-order kinetic model suggesting that the mechanism of IBU and IOH removal was biotic processes, i.e. plant uptake and microbial degradation. It is, however, not possible to quantify their individual contribution for the depletion of the compounds. The removal rate constants of IBU and IOH were significantly correlated with the plant dry mass and the carbon content from organic acids in the root exudates, respectively, which suggests that the driving force behind the depletion of IBU from the culture solutions may have been the plants, and the driving force behind the depletion of IOH may have been the microorganisms. The fact that the amount of IBU accumulated in the plant tissues was low at the end of the experiment may result from the metabolisation of IBU within the plant tissue and/or in the culture solution, as has been shown by Matamoros et al. (2012b) for microcosm systems with free-floating and submerged aquatic plants. For IOH, the highly water-soluble properties of the compound enable it to be taken up by plants, and accordingly, we also found higher concentrations of IOH in the plant tissues than of IBU. However, the significant correlation of the carbon content from the root exudates and the removal rate constants for IOH indicates that microorganisms may play a more important role for the removal of IOH than the mass taken up by the plants. Further studies are needed to better understand the removal mechanisms of IBU and IOH in plant-based systems, especially the formation of metabolites as a consequence of microbial degradation in the culture solution as well as metabolisation in the plant tissues.
Conclusions
Ibuprofen was nearly completely removed from solution by all plant species during the 24-day experiment, whereas removal of IOH varied between 13 and 80 %. Typha and Phragmites were the most efficient species for the removal of IBU and IOH, respectively, but plant accumulation constituted only up to 1 and 6 % of the initial amounts of IBU and IOH in solution. We were, however, not able to quantify the relative importance of microbial degradation and plant uptake for IBU and IOH removal. Formation of metabolites as a consequence of both plant activity and microbial degradation need further study.
References
Andreozzi R, Raffaele M, Nicklas P (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50:1319–1330
Brozinski JM, Lahti M, Meierjohann A, Oikari A, Kronberg L (2012) The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant. Environ Sci Technol 47:342–348
Buser HR, Poiger T, Müller MD (1999) Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ Sci Technol 33:2529–2535
Calderón-Preciado D, Matamoros V, Savé R, Muñoz P, Biel C, Bayona J (2013) Uptake of microcontaminants by crops irrigated with reclaimed water and groundwater under real field greenhouse conditions. Environ Sci Pollut Res 20:3629–3638
Carvalho PN, Basto MCP, Almeida CMR, Brix H (2014) A review of plant–pharmaceutical interactions: from uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ Sci Pollut Res 21:1–35
Collins CD, Martin I, Doucette W (2011) Plant uptake of xenobiotics. In: Schröder P, Collins CD (eds) Organic xenobiotics and plants. Springer, Netherlands, pp 3–16
Cortés JM, Larsson E, Jönsson JÅ (2013) Study of the uptake of non-steroid anti-inflammatory drugs in wheat and soybean after application of sewage sludge as a fertilizer. Sci Total Environ 449:385–389
Dettenmaier EM, Doucette WJ, Bugbee B (2008) Chemical hydrophobicity and uptake by plant roots. Environ Sci Technol 43:324–329
Dodgen L, Li J, Parker D, Gan J (2013) Uptake and accumulation of four PPCP/EDCs in two leafy vegetables. Environ Pollut 182:150–156
Dordio A, Ferro R, Teixeira D, Palace AJ, Pinto AP, Dias CM (2011a) Study on the use of Typha spp. for the phytotreatment of water contaminated with ibuprofen. Int J Environ Anal Chem 91:654–667
Dordio AV, Belo M, Martins Teixeira D, Palace Carvalho AJ, Dias CMB, Picó Y, Pinto AP (2011b) Evaluation of carbamazepine uptake and metabolization by Typha spp., a plant with potential use in phytotreatment. Bioresour Technol 102:7827–7834
Fismes J, Perrin-Ganier C, Empereur-Bissonnet P, Morel JL (2002) Soil-to-root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J Environ Qual 31:1649–1656
Gujarathi NP, Haney BJ, Park HJ, Wickramasinghe SR, Linden JC (2005) Hairy roots of Helianthus annuus: a model system to study phytoremediation of tetracycline and oxytetracycline. Biotechnol Prog 21:775–780
Herklotz PA, Gurung P, Heuvel BV, Kinney CA (2010) Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 78:1416–1421
Iori V, Pietrini F, Zacchini M (2012) Assessment of ibuprofen tolerance and removal capability in Populus nigra L. by in vitro culture. J Hazard Mater 229:217–223
Kotyza J, Soudek P, Kafka Z, Vaněk T (2010) Phytoremediation of pharmaceuticals—preliminary study. Int J Phytoremediation 12:306–316
Matamoros V, Arias CA, Nguyen LX, Salvadó V, Brix H (2012a) Occurrence and behavior of emerging contaminants in surface water and a restored wetland. Chemosphere 88:1083–1089
Matamoros V, Nguyen LX, Arias CA, Salvadó V, Brix H (2012b) Evaluation of aquatic plants for removing polar microcontaminants: a microcosm experiment. Chemosphere 88:1257–1264
Ryu J, Oh J, Snyder SA, Yoon Y (2014) Determination of micropollutants in combined sewer overflows and their removal in a wastewater treatment plant (Seoul, South Korea). Environ Monit Assess 186:3239–3251
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol 68:141–150
Shenker M, Harush D, Ben-Ari J, Chefetz B (2011) Uptake of carbamazepine by cucumber plants—a case study related to irrigation with reclaimed wastewater. Chemosphere 82:905–910
Smart RM, Barko JW (1985) Laboratory culture of submersed freshwater macrophytes on natural sediments. Aquat Bot 21:251–263
Trapp S (2009) Bioaccumulation of polar and ionizable compounds in plants. In: Devillers J (ed) Ecotoxicology modeling. Springer, US, pp 299–353
Vanek T, Podlipna R, Fialova Z, Petrova S, Soudek P (2010) Uptake of xenobiotics from polluted waters by plants. In: Fatta-Kassinos D, Bester K, Kümmerer K (eds) Xenobiotics in the urban water cycle. Springer, Netherlands, pp 431–444
Verlicchi P, Zambello E (2014) How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci Total Environ 470:1281–1306
Winker M, Clemens J, Reich M, Gulyas H, Otterpohl R (2010) Ryegrass uptake of carbamazepine and ibuprofen applied by urine fertilization. Sci Total Environ 408:1902–1908
Zhai X, Piwpuan N, Arias CA, Headley T, Brix H (2013) Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol Eng 61:555–563
Zhang DQ, Tan SK, Gersberg RM, Sadreddini S, Zhu J, Tuan NA (2011) Removal of pharmaceutical compounds in tropical constructed wetlands. Ecol Eng 37:460–464
Acknowledgments
This work was performed within the Center for Advanced Water Purification funded by the Aarhus University Research Foundation (AUFF). The PhD fellowships of Yang Zhang and Tao Lv are supported by the China Scholarship Council (CSC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Ibuprofen was nearly completely removed by all wetland plant species.
• Iohexol was recalcitrant with only 13 to 80 % removal depending on plant species.
• Both compounds were taken up by the roots and translocated to the aerial tissues.
• Removal was due to microbial degradation and plant uptake.
• Root exudates may play an important role for the degradation of iohexol.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 402 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Lv, T., Carvalho, P.N. et al. Removal of the pharmaceuticals ibuprofen and iohexol by four wetland plant species in hydroponic culture: plant uptake and microbial degradation. Environ Sci Pollut Res 23, 2890–2898 (2016). https://doi.org/10.1007/s11356-015-5552-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5552-x