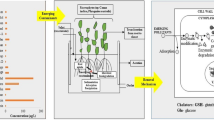
Abstract
Heavy metal contamination is a by-product of rapid urbanization and industrialization. Water contaminated with heavy metals can have serious health consequences for humans and is also a major threat to aquatic biodiversity. Phytoremediation technologies were developed by academicians, researchers, and industrialists due to the disadvantages and economic impacts of conventional treatment methods to treat heavy metal contamination. Nowadays, the major focus of the scientific community is to develop innovative approaches for treating wastewater containing heavy metals to reduce their toxicity levels in order to meet technology-based treatment requirements. In this connection, this study was carried out to assess the ability of three types of wetland macrophytes to remove heavy metals from simulated feed water that contains a mixture of metals using lab-scale vertical flow-constructed wetland (CW) microcosms planted with Canna (C), Typha (T), and Eichhornia (E) in both single and mixed cultures. All three species showed high rhizofiltration capability in the CWs with mixed cultures. The mixed culture (E + C + T) removed 98–99% of Cu, Cd, Ni, Pb, Fe, and 90% of Cr from the feed water in 4 days. Heavy metal uptake and mobility in the plants were also examined during this study. Higher values of bio concentration factor (BCF) indicated that these species can store higher amounts of heavy metals, and higher values of translocation factor (TF) showed that most heavy metals stay below the ground, except Fe, which moves more easily to shoots. This study highlighted the efficacy of Canna (C), Typha (T), and Eichhornia (E) plants in removing heavy metals from the contaminated water and observed that their efficacy has been enhanced in the mixed cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal contamination of water caused by several industries through the discharge of toxic metals into wastewater has become one of the most serious potential health hazards [1,2,3]. Major industry based operations such as mining operations, smelting of metalloferrous ores, surface finishing, electroplating, electrolysis, electro-osmosis, power generation, refining ores, sludge disposal, processing of radioactive materials, manufacturing of electric equipment, paints, making of alloys, batteries, fertilizers, preservatives, leather, and tanning industries, are responsible for the discharge of large quantities of metal-containing wastewater [4,5,6,7]. Some of the metals like Cu, Zn, Fe, and Ni are essential for living organisms in trace quantities, but when they exceed the threshold value, they pose serious health threats, causing damage to the liver, nerves, and bones, and can even be lethal [8,9,10]. Metals like Pb, Hg, Cd, and Ag do not have any essential cellular roles. Due to their toxic effects and non-biodegradable nature, which lead to bioaccumulation in the food chain, it becomes imperative to remove these heavy metals from the wastewater before discharging into freshwater bodies [11,12,13].
Conventional treatment methods like ion exchange, electro-dialysis, electrochemical precipitation, evaporation, solvent extraction, reverse osmosis, chemical reduction, or oxidation show varying efficiency and involve the use of large quantities of chemicals, resulting in the generation of toxic sludge. These methods have high operating costs and are less efficient at removing heavy metal ions from waste water [14,15,16]. Nowadays, a variety of nanomaterials and nanocomposites have also been used for the same purpose, but their preparation and characterization take a longer time, and operation costs are also high [17,18,19,20,21,22,23,24,25]. Hence, there is a need to look for new, efficient, low-cost processes for the removal of heavy metals from water streams that are environmentally friendly and sustainable [26,27,28].
Constructed wetlands (CW) have been emerging as a promising technology in recent years due to their lower construction and operational costs and greater resilience. Constructed wetlands are engineered systems that are specially designed and constructed to utilize the natural processes involving wetland vegetation, soils, and their associated microbial assemblages to assist in treating different types of wastewater [29, 30]. Besides treating wastewater, constructed wetland can also be an attractive natural landscape with wildlife habitats. While field-scale application of constructed wetlands has been well established for the treatment of storm water, municipal sewage, and industrial waste waters of the mining, textile, paper, and pulp industries [31], the efficacy of this technology for the removal of heavy metals from wastewaters needs to be studied adequately. There are a few reports suggesting CWs are capable of metal removal depending upon plant type and metal mobility [32]. Rhizofiltration by the plants in CWs seems to be a promising technique for utilizing plant roots to absorb, concentrate, or precipitate toxic metals from polluted effluents [33]. Removal of heavy metals in such CWs may be mediated through processes like adsorption or precipitation of sulphides and carbonates by the substrate, removal by the plants, or retention in the soil profile or the sediments [34, 35]. The cycling and distribution of metals in constructed wetlands receiving different types of wastewater are found to be influenced by the vegetation and substrate [36].The type of macrophyte used in the CW is considered crucial because of the different mechanisms used by the plants for tolerating or remediating the metals [37, 38]. The capability of accumulating the metals belowground or translocating them into shoots is likely to be the decisive factor in the performance of the CWs, on which very few studies have, however, been carried out [39]. Moreover, the metal removal efficacy of CW using plant combinations is likely to be better as ecosystem complexity leads to better stability and performance. In this regard, the present study was designed and conducted to investigate the removal of six heavy metals by exploiting the rhizofiltration potential of three macrophytes in single and mixed culture in a CW microcosm.
2 Materials & methods
2.1 Canna indica, typha and eichhorniacrassipes species
Canna indica (Kelly or Canna lily) belongs to the Cannaceae family, mostly found in tropical countries. It is a perennial facultative wetland herb with a well-developed underground rhizome. The plant attains a height of 3 feet. The main advantage of the canna plant is its rapid development rate and high biomass production. Rapidly growing plants with extensive roots enhance nitrification because they are a good fit for nitrifying bacteria, which increases the surface area of the biofilm due to their high biomass and rapid growth rate. Compared to other wetland plants, the canna plant uses three to five times as much water. Moreover, the blooming and attractiveness offer more advantages for its use [40,41,42].
Typha species is a perennial aquatic herb with thick, ribbon-like, structured leaves belonging to the family of Typhaceae. Well-developed aerenchyma provides buoyancy to the plant, and even the dead stalks are capable of transmitting oxygen to the root zone. Typha is a prevalent plant found in wetlands that is used to purify wastewater. This species thrives in the nutrient-rich, saturated soil found in nature, where ammonium is typically a key source of nitrogen. Additionally, it is tolerant of alkaline or saline environments. This species has been utilised to treat a variety of wastewater types due to its rapid growth and excellent adaptation to eutrophic environments [43,44,45,46,47,48,49].
Eichhorniacrassipes (EC) is an aquatic weed commonly found in tropical and subtropical regions. It is a fast-growing plant with a well-developed fibrous root system. EC were utilized in a man-made wetland composed of gravel, sand, and humus layer for wastewater treatment [50,51,52,53,54,55]. The effectiveness of EC was assessed for raw sewage, synthetic medium and groundwater, oily river water, dye wastewater, domestic wastewater, dairy wastewater, and river water, as well as raw wastewater collected from stabilised ponds [56,57,58,59,60,61,62,63].The main mechanisms for reducing contaminant toxicity is found in the root system, which is also where most pollutants are absorbed by plants. The vast surface area of the root system allows it to collect and retain various non-essential pollutants together with the water and nutrients needed for growth. (Fig. 1).
2.2 Preparation of metal containing feedstock
Feed water containing different heavy metals (Cu, Cd, Cr, Ni, Pb and Fe) was prepared by dissolvingcopper nitrate [Cu(NO3)2.3H2O], cadmium chloride (CdCl2), potassium dichromate (K2Cr2O7), nickel sulphate [NiSO4.7H2O], lead nitrate [Pb(NO3)2]and ferric nitrate [Fe(NO3)2.9H2O]in fresh tap water with composition as shown in Table 1. These heavy metal concentrations were selected considering their presence in different industrial waste waters.
2.3 Analytical methods
The concentration of heavy metals in the plants and microcosm effluents before and after the batch treatment in CWs was analyzed by Atomic Absorption Spectrophotometer (AAS) [AA6300 model (Shimadzu make)]after digestion with nitric acid and perchloric acid (9:1) mixture.
In the laboratory, the plants were carefully washed with distilled water, divided into shoots and roots, and dried for 12 h at 120 °C in a hot air oven. The samples were then ground to a fine powder using a silica pestle and mortar. Plant samples were then digested and analyzed on an atomic absorption spectrophotometer [64]. The efficiency of each plant in accumulating heavy metals in their shoots and roots was determined to calculate the bioconcentration and translocation factor.
2.4 Bio-concentration factor and translocation factor
Bio concentration factor (BCF) of the metal was calculated as follows [65]:
-
BCF = Ratio of Concentration of the heavy metal in plant tissue at harvest (mg/kg) and Initial concentration of the heavy metal in effluent (mg/L)
Translocation factor (TF) was calculated by dividing the metal concentration in above ground tissues by that accumulated in root tissues [66].
-
TF = Ratio of Heavy metal accumulated in above ground tissues of plant (mg/kg) and Heavy metal accumulatedin below ground tissuesof plant (mg/kg)
3 Results and discussion
3.1 Heavy metal removalin the CW microcosms as influenced by planted macrophytes
Removal of different heavy metals in the CW microcosm was found to vary significantly, depending upon the type of macrophyte planted and also on their heterogeneity. Changes in concentration of different metals at the outflow of different batch treatments are depicted in Figs. 2, 3, 4, 5, 6 and 7. Removal efficiency under different batch treatments is shown in Table 2.
Concentration of Cu (Fig. 2) gradually decreased from initial value of 10.5 mg/L to 0.12, 0.13, 0.033 and 0.027 mg/L in CW reactors planted with (C), (T), (E) and (E + C + T), respectively in just 4 days of treatment. CWs planted with (E) and (E + C + T) showed substantial decrease of 99% of the metal by the end of 96 h (Table 2).
Cd concentration in the microcosm effluent gradually decreased from 6.15 mg/L to 0.56, 0.45, 0.10 and 0.08 mg/L in CWs planted with (C), (T), (E) and (E + C + T), respectively (Fig. 3) showing maximum removal of 98%-99% again with (E) & (E + C + T) plant species (Table 2).
Concentration of Ni metal (Fig. 4) after 96 h of treatment gradually reduced from initial concentration of7.76 mg/L to 0.19, 0.26 for (C) and (T) whereas no Ni was detected in the outlet water in case of (E) and (E + C + T) CW microcosms showing 100% removal efficiency (Table 2).
In case of Cr (Fig. 5) the metal concentration gradually decreased from 10.24 mg/L to 2.85, 2.99, 1.33 and 0.99 mg/L for (C), (T), (E) and (E + C + T) respectively, with per cent removal efficiencies of 72%, 70%, 87% & 90%, respectively (Table 2).
Figure 6 depicts the gradual decline in Pb concentration from 27.61 mg/L to 0.17, 0.19, 0.15 and 0.28 mg/L in the outlet of (C), (T), (E) and (E + C + T) microcosm, respectively, with 96%, 95%, 97% &99% removal efficiency (Table 2).
For Fe (Fig. 7) the initial concentrationin feed water was 4.40 mg/L which gradually reduced to0.16, 0.19, 0.10and 0.075 mg/L for CWs planted with (C), (T), (E) and (E + C + T) with maximum removal efficiency (99%) for all theplanted CW microcosms (Table 2).
The results showed that all the planted CW microcosms have high removal efficiency for all the metals, which increased with an increase in the retention period of the feedwater up to 96 h in the CW. Removal of the metals increased, and there was almost complete removal by the end of 96 h of treatment in most CW microcosms. The CW microcosms planted with each of the three species individually exhibited very good metal removal capacity, exceeding 70%. However, CWs planted with Eichhornia alone or in mixed culture showed outstanding metal-removing efficiency, i.e., complete removal of Ni and 98–99% removal of other studied metals. While most of the metals could be removed by CW microcosms with single species in the range of 88–99%, the removal of Cr was relatively less (70–87%). The removal increased to 90% when mixed cultures were used. CWs planted with canna and typha showed relatively less removal of various metals, particularly Cr and Cd (Table 2). A significant decrease in the concentration of cadmium and lead in municipal sludge by using Canna species has been observed by Shugeng et al. [67]. The accumulation of heavy metals by Typhalatifolia, Limnocharisflava, and Thaliageniculata plants was also demonstrated by Anning et al. [68] using constructed wetland.
The removal efficiency of the metals by the planted CWs was maximum for Ni and minimum for Cr. This indicates that the overall micro-ecosystem structure and function that develop in the CW microcosms vary with soil type and substrate. Waste water composition also plays a role in influencing the removal of pollutants in CWs, besides that of the plant species growing in the CW. When all three macrophytes were grown together (E + C + T), 99% to 100% removal of the metals was achieved. Mixed species in the CW microcosm showed a synergistic effect rather than an additive, indicating possibly modified and improved interactions in the mixed rhizosphere, resulting in better metal removal efficiency.
In order to test the significance of differences in heavy metal removal, a two-way ANOVA was applied, as shown in Table 3.
The ANOVA results showed that each of the two factors (CW type and treatment time) and their interactions played a very important role in determining heavy metal removal efficiency. The P value was found to be higher (0.08, 0.42, and 0.82) for Cu, Cd, and Ni heavy metals due to the factor interaction, which was greater than 0.05, i.e., non-significant, while for Cr and Pb, differences due to factor interactions were found to be highly significant (P < 0.0001) and for Fe significant (P < 0.005).
3.2 Metal accumulation by plants
The accumulation of heavy metals in the belowground (rhizome/roots) and aboveground (stem, leaf) biomass is shown in Table 4, 5, 6 and 7. Most of the heavy metals in the present study tended to accumulate more in the belowground than in the aboveground biomass, which may be due to direct contact of the former with the metal-containing feed water in the CW. Metal accumulation in the aboveground parts of the plants would occur by translocation. The translocation ability of each heavy metal is found to be different, as revealed by varying TF values. Similar results of higher metal content in the roots than the top organs of plants were also reported by Lesage et al. [69] using CW technology.
3.2.1 Accumulation and mobility of heavy metals by canna indica
Table 4 shows the accumulation of different heavy metals in Canna Indica. Belowground biomass shows greater heavy metal accumulation for the heavy metals except Fe. Aboveground plant parts accumulated 4.3, 0.4, 0.3, 2.2, and 2.4 mg/kg of Cr, Cd, Cu, Pb, and Ni, respectively. Belowground plant parts accumulated much greater concentrations (72.4, 22.0, 22.4, 12.6, and 12.4 mg/kg) of the above metals, respectively. Translocation factors (TF) of the metals in the plant species indicate metal mobility. As shown in Table 4, TF was found to be less than 1 (0.01–0.2) for all these metals, which confirmed that the belowground plant biomass of the wetland macrophytes is the major metal accumulator.
The trend of accumulation of Fe was, however, very different, showing a large accumulation of 199.2 mg/kg in the shoots as opposed to just 23.2 mg/kg in the belowground parts with a high TF of 8.6. Thus, the mobility of Fe was quite high in this plant species.
The bioconcentration factor (BCF), indicating the capability of metal uptake by the plants from the medium, showed wide variations for various metals (Table 4). The BCF of Fe was exceptionally high (50.5), indicating the specific Fe-accumulating nature of this species. The BCF was greater than 1.0 (except for Pb), which indicates the distinct metal-accumulating nature of Canna Indica.
3.2.2 Accumulation and mobility of heavy metals in typhaLatifolia
In the case of CWs planted with Typha (Table 5), again, the metals accumulated in the aboveground parts were much less, i.e., 8.2, 3.0, 2.8, 286.2, 11.9, and 2.2 mg/kg, than those in the belowground plant parts, i.e., 42.0, 53.9, 20.4, 13.0, 43.6, and 38.3 mg/kg for Cr, Cd, Cu, Fe, Pb, and Ni, respectively. More than 1 BCF value was obtained for all the heavy metals studied. This indicates the high capability of Typha plants for heavy metal accumulation. The BCF for various metals in Typha was higher than that in Canna, ranging from 2 to 9.3, indicating better accumulation.
The accumulation trend of Fe was different from that of other metals, like that in Canna. It showed even higher accumulation (286.2 mg/kg) in aboveground parts with a high BCF of 68. While the mobility of all other studied heavy metals in Typha plant species was low (TF < 1), Fe showed high mobility. This may be because of the formation of “iron plaque” in the rhizosphere by the release of oxygen from roots [70, 71]. Iron oxyhydrides cause metal accumulation in the iron plaque, and thus the metal is directly sorbed from the solution by the roots of the macrophytes.
3.2.3 Accumulation and mobility of heavy metals in eichhorniaCrassipes
As compared to Canna and Typha, the accumulation of various metals in the shoots of Eichhornia was much higher, i.e., 52.3, 44.5, 61.5, 82.9, 57.5, and 25.5 mg/kg for Cr, Cd, Cu, Fe, Pb, and Ni, respectively. Like the other two macrophytes, Eichhorniaroots also accumulated higher amounts of heavy metals, i.e., 98.2, 104.5, 11.7, 104.9, and 52.7 Cr, Cd, Pb, and Ni, respectively, than their shoots. The mobility of these metals was low, as indicated by TF < 1 in Eichhornia, just like the other two species. Fe, however, showed greater mobility. However, in this case, accumulation in shoots (16.6 mg/kg) and TF (5.0) was lower than that in the other two species (Table 6). The values of BCF for the heavy metals in Eichhornia showed the highest metal accumulation capability for Cd and Fe, i.e., 24.2 and 22.6, respectively, followed by Cu (16.4), Cr (14.7), and Ni (10.1), whereas minimum BCF (5.9) was observed for Pb. Eichhornia showed maximum TF and BCF for all the metals (except Fe) amongst the three macrophytes used in the CW microcosms.
The tendency to have a higher concentration of metals in the belowground parts as compared to the aboveground parts has also been observed by some other workers [39, 72]. Though Eichhornia has been reported as an obnoxious aquatic weed, its remarkable metal removal capability in constructed wetlands cannot be undermined.
3.2.4 Accumulation and mobility of heavy metals in mixed culture macrophytes (Canna + Typha + Eichhornia)
In the case of CWs planted with mixed culture (Canna + Typha + Eichhornia), the metals accumulated in the aboveground parts were 34.8, 11.0, 17.3, 270.9, 33.8, and 9.7 mg/kg, as compared to those in the belowground plant parts (43.2, 40.8, 24.2, 22.9, 60.0, and 26.7 mg/kg for Cr, Cd, Cu, Fe, Pb, and Ni, respectively). Greater than 1 BCF value was obtained for all the heavy metals, which shows the high capability of plants in mixed culture for heavy metal accumulation (Table 7). A similar trend for Fe as in Typha was also found in mixed culture macrophytes, with a higher accumulation of 270.9 mg/kg in the aboveground parts with a high BCF of 66.8 and TF of 11.8. TF was found to be less than 1 for other heavy metals, which depicts the ability of the belowground part to be a major accumulator of heavy metals.
3.3 Tolerance of the plants to different metals
While the metal removal efficacy of different wetland species planted in the CWs in single and mixed cultures has direct applied value, it is also important to assess the tolerance of the plant species to the metals in terms of biomass and chlorophyll content of the leaves. The biomass and total leaf chlorophyll content of the macrophytes before and after the batch treatment with feed water are depicted in Fig. 8. After 4 days of treatment, singly cultured Canna and Eichhornia plants showed a slight increase in biomass and total leaf chlorophyll content, whereas Typha showed a decline.
Variation in Biomass (a) and Chlorophyll (b) content of plants species before and after 4 days of batch treatment with feed water having metal concentration as shown in Table 1
The mixed culture showed an increase in chlorophyll as well as biomass, indicating their tolerance to the metals. Moreover, in mixed cultures, even Typha showed better tolerance, indicating the positive effects of the mixed culture. The tolerance of the plants to metals in the feed water is very important for the long-term operation of CWs planted with such species. The plants used in this study show quite good tolerance, particularly when grown in mixed cultures. The present study shows that plant species used in the CW have a major role in the removal of heavy metals from the feedstock, with varying capabilities of metal uptake and translocation. Though none of the three species used in the CW microcosm are documented metal hyper accumulators, they show distinct metal accumulating tendencies as indicated by high BCF.
4 Conclusion
Nowadays, constructed treatment wetlands have developed into a dependable wastewater treatment system that may be used for storm water runoff, sewage, industrial, and agricultural wastewaters, as well as landfill leachate. The same processes that remove pollution from natural wetlands also remove it from manmade wetlands, although under more regulated circumstances. Phytoremediation is an effective way to treat wastewater and it has been shown that aquatic plants with the right traits reduce waste-related contaminants. In this connection, the study has been conducted in microcosms suggests that Canna, Typha, and Eichhornia can be used as CW macrophytes for the treatment of wastewater containing heavy metals, particularly when grown in mixed culture. All these species are found to be good metal accumulators, with major sequestration in the belowground parts. Amongst various metals, Fe tended to show better mobility in shoots. The rhizofiltration of metals is improved remarkably in the mixed culture of the three macrophytic species, when almost complete removal of these metals is achieved in a period of just four days.
Data availability
Data provided as supplementary information.
References
N.A.A. Qasem, R.H. Mohammed, D.U. Lawal, NPJ Clean Water. (2021). https://doi.org/10.1038/s41545-021-00127-0
Y. Fei, Y.H. Hu, Chemosphere (2023). https://doi.org/10.1016/j.chemosphere.2023.139077
M. Nazaripour, M.A.M. Reshadi, S.A. Mirbagheri, A. Bazargan, J. Environ. Manag. (2021). https://doi.org/10.1016/j.jenvman.2021.112322
M.J. Mehta, R.A. Christian, N.J. Mistry, M. Mukhopadhyay, Int. J. Chem. Eng. 2, 4 (2010)
M. K. Abd Elnabi, N.E. Elkaliny, M.M. Elyazied, S.H. Azab, S. A. Elkhalifa, S. Elmasry, M S. Mouhamed, E.M. Shalamesh, N.A Alhorieny, A.E Abd Elaty, I.M Elgendy, Toxics. (2023). https://doi.org/10.3390/toxics11070580
C.C. Femina, P. Kamalesh, K. Senthil, R. Gayathri, Ind. Eng. Chem. Res.. (2023). https://doi.org/10.1021/acs.iecr.3c00709
Y. Fei, Y.H. Hu, J. Mater. Chem. (2022). https://doi.org/10.1039/D1TA06612A
A.V. Jamode, M. Rao, B.S. Chandak, V.S.B.S Jamode A.V.J. Parwate, Ind. Pollut. Control. (2003). https://doi.org/10.1039/D1TA06612A
M.A. Zoroddu, J.C.G. Aaseth, S. Medici, M. Peana, V.M. Nurchi, J. Inorg. Biochem. (2019). https://doi.org/10.1016/j.jinorgbio.2019.03.013
K. Jomova, M. Makova, S.Y. Alomar, S.H. Alwasel, E. Nepovimova, K. Kuca, C.J. Rhodes M. Valko, Chem. Biol. Interact. (2022). https://doi.org/10.1016/j.cbi.2022.110173
D. Nies, Appl. Microbiol. Biotechnol. (1999). https://doi.org/10.1007/s002530051457
S.B. Schmidt, O. Vatamaniuk, A. Schneider, Front Plant Sci. (2023). https://doi.org/10.3389/fpls.2023.115624
R.Singh, N.Gautam, A.Mishra, R.Gupta, Indian J Pharmacol. (2011). https://doi.org/10.4103/0253-7613.81505
S.A. Razzak, M.O. Faruque, Z. Alsheikh, L.M. Alsheikh, D. Alkuroud, A. Alfayez, S.Z. Hossain, M.M. Hossain, Environ. Adv. (2022). https://doi.org/10.1016/j.envadv.2022.100168
F. Fu, Q. Wang, J. Environ. Manag. (2011). https://doi.org/10.1016/j.jenvman.2010.11.011
A. Gopinath, K. Krishna, C. Karthik, Modern Age Waste Water Problems. (2020). https://doi.org/10.1007/978-3-030-08283-3_15
S.S. Kolluru, S. Agarwal, S. Sireesha, I. Sreedhar, S.R. Kale, Process Saf. Environ. Prot. (2021). https://doi.org/10.1016/j.psep.2021.04.025
T.P. Fato, D.W. Li, L.J. Zhao, K. Qiu, Y.T. Long, ACS Omega (2019). https://doi.org/10.1021/acsomega.9b00731
P. Mondal, A. Nandan, S. A. kumar, N. A. Siddiqui, S. Raja, A.K. Kola, D. Balakrishnan, Environ. Res. (2023). https://doi.org/10.1016/j.envres.2023.116071.
S. Zahmatkesh, M.H. Keshteli, A. Bokhari, S. Sundaramurthy, B. Panneerselvam, Y. Rezakhani, Environ. Res. (2023). https://doi.org/10.1016/j.envres.2022.114652
Y. Sun, T. Wang, Water (2023). https://doi.org/10.3390/w15071320
M. Hnamte, A.K. Pulikkal, Chemosphere (2022). https://doi.org/10.1016/j.chemosphere.2022.135869
A.O. Adeola, P.N. Nomngongo, Polymers (2022). https://doi.org/10.3390/polym14122462
E.C. Umejuru, T. Mashifana, V. Kandjou, M.A. Beni, H. Sadeghifar, M. Fayazi, H.K. Maleh, N.T. Sithole, Environ. Res. (2023). https://doi.org/10.1016/j.envres.2023.116073
H.E. Al-Hazmi, J. Łuczak, S. Habibzadeh, Md.S. Hasanin, A. Mohammadi, A. Esmaeili, S.J. Kim, M.K. Yazdi, N. Rabiee, M. Badawi, Md.R. Saeb, Chemosphere (2024). https://doi.org/10.1016/j.chemosphere.2023.140578
K.G. Karthikeyan, M.T. Meyer, Sci. Total. Environ. (2006). https://doi.org/10.1016/j.scitotenv.2005.06.030
H. Ahmed, N.A. Sobhy, M.M. Hefny, F.M. Abdel-Haleem, M.A. El-Khateeb, J. Environ. Public Health (2023). https://doi.org/10.1155/2023/7419015
N. D. Nnaji, H. Onyeaka, T. Miri. C. Ugwa, SN Appl. Sci (2023). https://doi.org/10.1007/s42452-023-05351-6
T. Saeed, M.K. Alam, M.J. Miah, N. Majed (2021). https://doi.org/10.1016/j.indic.2021.100146
G. Yu, G. Wang, T. Chi, C. Du, J. Wang, P. Li, Y. Zhang, S. Wang, K. Yang, Y. Long, H. Chen, Sci. Total. Environ. (2022). https://doi.org/10.1016/j.scitotenv.2022.153516
S. Khan, I. Ahmad, M.T. Shah, S. Rehman, A. Khaliq, J. Environ. Manag. (2009). https://doi.org/10.1016/j.jenvman.2009.05.026
M.P. Barya, A. Kumar, T.K. Thakur, Ecohydrology (2022). https://doi.org/10.1002/eco.2424
K. Rawat, M.H. Fulekar, B. Pathak, Intl. J. Inno. Biosci. 2, 193–199 (2012)
C. Ezeah, C. Reyes, J. Gutiérrez, J. Geoscience. Environ. Protection 3, 1–14 (2015). https://doi.org/10.4236/gep.2015.33001
K.L. Allende, T.D. Fletcher, G. Sun, J. Chem. Eng. (2012). https://doi.org/10.1016/j.cej.2011.10.069
J. Vymazal, (ed.). Wetlands - nutrients, metals and mass cycling. - Backhuys Publishers, Leiden p. 376 (2003)
D.E. Salt, M. Blaylock, N.P. Kumar, V. Dushenkov, B.D. Ensley, I. Chet, I. Raskin, Nat. Biotechnol. (1995). https://doi.org/10.1038/nbt0595-468
A.J.M. Baker, J. Proctor, Plant Syst. Evol. (1990). https://doi.org/10.1007/BF00937765
A.K. Yadav, R. Abbassi, N. Kumar, S. Satya, T.R. Sreekrishnan, B.K. Mishra, J. Chem. Eng. (2012). https://doi.org/10.1016/j.cej.2012.09.039
R. Pinninti, V. Kasi, L.P. Sallangi, S.R. Landa, M. Rathinasamy, C. Sangamreddi, P.R. Dandu, Int. J. Phytoremediation 24, 684–694 (2022)
H. Zhu, Q.W. Zhou, B.X. Yan, Y.X. Liang, X.F. Yu, Y. Gerchman, X.W. Cheng, Water Sci. Technol. 77, 829–837 (2018)
Y. Wu, T. He, C. Chen, X. Fang, D. Wei, J. Yang, R. Zhang, R. Han, Int. J. Environ. Res. Public Health 16, 802 (2019)
R. Brändle, J. Pokorný, J. Květ, H. Čížková, Folia Geobot. (1996). https://doi.org/10.1007/BF02803989
L. Steinbachová-Vojtíšková, E. Tylová, A. Soukup, H. Novická, O. Votrubová, H. Lipavská, H. Čížková, Environ. Exp. Bot. (2006). https://doi.org/10.1016/j.envexpbot.2005.06.003
J.B. Grace, J.S. Harrison, Can. J. Plant Sci. (1986). https://doi.org/10.4141/cjps86-051
C.S. Akratos, V.A. Tsihrintzis, Ecol. Eng. 29, 173–191 (2007)
H. Wu, J. Zhang, H.H. Ngo, W. Guo, Z. Hu, S. Liang, J. Fan, H.A. Liu, Bioresour. Technol. 175, 594–601 (2015)
M. Maddison, T. Mauring, K. Remm, M. Lesta, U. Mander, Estonia. Ecol. Eng. 35, 258–264 (2009)
M.M. Aslam, S. Hassan, M. Baig, Int J Agri Biol. 12, 796–798 (2010)
R.K. Trivedy, S. Thomas, Asian J Microbiol Biotechnol. Environ. Sci. 6, 157–162 (2004)
Y. Kim, D.L. Giokas, P.G. Chung, D.R. Lee, Water Sci. Technol. 48, 115–123 (2003)
S. Rezania, M. Ponraj, M.F.M. Din, S. Chelliapan, F.M. Sairan, Desalin Water Treat. (2016). https://doi.org/10.1080/19443994.2014.967305
S. Rezania, M.F.M. Din, S.M. Taib, F. A. Dahalan, A.R. Songip, L. Singh, H. Kamyab, Int J Phytorem. (2016). https://doi.org/10.1080/15226514.2015.1130018
M. Kumari, B.D. Tripathi, Ecol. Eng. 62, 48–53 (2014)
A. Valipour, V.K. Raman, Y.H. Ahn, Water (2015). https://doi.org/10.3390/w7010329
P.M. Ayyasamy, S. Rajakumar, M. Sathishkumar, K. Swaminathan, K. Shanthi, P. Lakshmanaperumalsamy, S. Lee, Desalination (2009). https://doi.org/10.1016/j.desal.2008.05.008
Z. Ismail, S.Z. Othman, K.H. Law, A.H. Sulaiman, R. Hashim, Clean: Soil, Air, Water 43(4), 521–531 (2015)
C.O. Akinbile, M.S. Yusoff, Int. J. Phytorem. 14(3), 201–211 (2012)
R.A. Shah, D.M. Kumawat, N. Singh, K.A. Wani, Int J Sci Nat. 1(2), 172–178 (2010)
A. Lopes, M.T.F. Piedade, Environ. Sci. Pollut. Res. 21, 13503–13511 (2014)
N.T. Loan, P.M. Nguyen, N.T.N. Anh, Environ. Eng. Manag. J. 13(8), 2031–2038 (2014)
G.R. Munavalli, P.S. Saler, Water. Sci. Technol. 5(4), 713–722 (2009)
P. Moyo, L. Chapungu, B. Mudzengi, Adv. Appl. Sci. Res. 4(4), 55–62 (2013)
APHA, Standard methods for the examination of water and wastewater, 21st edn. (American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, 2005)
A. Zayed, S. Gowthaman, N. Terry, J. Environ. Qual. (1998). https://doi.org/10.2134/jeq1998.00472425002700030032x
F. Y. Wu, E. J. Sun, Environ Prot. (1998). http://140.112.114.62/handle/246246/177102.
S. Li, K. Zhang, S. Zhou, L. Zhang, Q. Chen, Waste Manag. (2009). https://doi.org/10.1016/j.wasman.2008.12.007
A.K. Anning, P. E. Korsah, P. Addo-Fordjour, Int J Phytoremediation. (2013). https://doi.org/10.1080/15226514.2012.716098
T.Y. Yeh, Removal of metals in constructed wetlands: Review, practice periodical of hazardous, toxic, and radioactive waste management, 12(2), 96 (2008). https://doi.org/10.1061/(ASCE)1090-025X(2008)12:2(96)
K.R. Reddy, W.H. Patrick Jr., C.W. Lindau, Limnol. Oceanogr. (1989). https://doi.org/10.4319/lo.1989.34.6.1004
M. L. Otte, C. C. Kearns, M. O. Doyle, Bull Environ Contam Toxicol. (1995). https://doi.org/10.1007/BF00212403
S.W. Liao, W.L. Chang, J. Aquat. Plant. Manag. 4, 60–68 (2004)
Acknowledgements
Authors wish to truly thank the management of SGT University, Gurugram, Haryana, Guru Gobind Singh Indraprastha University, New Delhi, Amity University, Haryana, Presidency University, Bangalore, Karnataka and Veer Surendra Sai University of Technology, Odisha for providing the necessary facilities to conduct and submit the research work for publication.
Funding
None.
Author information
Authors and Affiliations
Contributions
Simranjeet Singh: Conceptualization, Methodology, Writing, Original draft.
Anubha Kaushik: Analysis, Review and original draft.
Anjaneyulu Bendi: Analysis, Writing, Editing and supervision.
Anu Chetal: Writing, original draft.
D.S.Ramakrishna: Review, original draft Editing.
P. Lakshmi Praveen: Analysis, Final draft, Supervision.
Corresponding authors
Ethics declarations
Consent for publication
Author is agreed to publish the article in this journal.
Conflicts of interest
Author declares no conflicts of interest, financial or otherwise.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Kaushik, A., Bendi, A. et al. Constructed wetlands as bioeconomic solutions: rhizofiltration with macrophytes for heavy metal removal. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00675-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00675-4