Abstract
The denitrification potential of the soil horizons between 0- and 90-cm depth of 20 agricultural fields, representative of the most frequent combinations of agricultural crops and soil textures in Flanders (Belgium), and the factors affecting the denitrification potential were studied in the laboratory under controlled conditions. The denitrification potential in the presence of an added soluble C and N source was measured at 15°C after saturation of air-dried soil samples with water. The denitrification potential of the lower horizons was generally negligible compared to the upper horizons. The lower denitrification potential of the deeper horizons could partially be explained by their limited C availability. The denitrification potential of the upper horizons strongly depended on texture. Based on this parameter the soils could be divided into three groups: soils with a high clay content (>30% clay) were characterised by a high denitrification potential (>8.33 µg N g-1 dry soil day-1); soils with medium texture had a medium denitrification potential, between 0.41 and 7.25 µg N g-1 dry soil day-1; and soils with a high sand content (>80% sand) had a low denitrification potential (<2.58 µg N g-1 dry soil day-1). In most cases, extending the saturation period during pre-incubation increased the denitrification potential. Comparison of the denitrification potential of the upper horizons with and without addition of a soluble C source showed that the denitrification potential of the upper horizons of these soils was limited by their percentage of endogenous C. The measured denitrification potentials indicate that denitrification losses in soils high in clay content can be important when NO3 - concentrations are high.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological denitrification is a form of anaerobic respiration during which NO3 - or NO2 - is reduced to gaseous N-oxides (NO and N2O) and N2 (Aulakh et al. 1992). Denitrification is important with respect to N use efficiency and is a process that needs to be considered in N budgets for crop production (Hofman and Van Cleemput 2001). Moreover, N2O is a greenhouse gas and affects the stratospheric ozone layer.
Several factors are known to regulate soil denitrification. Denitrification rates in the field tend to increase with increasing soil water content (e.g. Abbasi and Adams 2000; Hénault and Germon 2000). Denitrification strongly depends on soil NO3 - concentrations and on the availability of organic C such as soil organic matter, crop residues and green manure. Field studies also showed an increased denitrification with increasing fineness of soil texture (Aulakh et al. 1992).
In situ studies of the factors influencing denitrification are difficult because of the extreme temporal and spatial variability of denitrification under field conditions. Measuring subsoil denitrification in situ is especially complex and demanding (Well and Myrold 2002). Spatial variability results from an irregular distribution of denitrifying "hot spots" in the soil. Hot spots are caused by non-homogeneous distribution of available C, soil NO3 - and soil water content (Luo et al. 2000). Even in apparently well-aerated soils hot spots may be present where denitrification occurs. Moreover, under field conditions, variables having a strong effect on denitrification, such as moisture content and temperature, often override the effect of soil variables such as texture and availability of N and C. Knowledge of the impact of the soil variables that regulate denitrification is essential for modelling denitrification and other N processes in the field. The "water filled pore space" (WFPS) is a good indicator of the aeration status of the soil and is related to aeration-dependent processes such as denitrification. Denitrification, independent on soil texture, only occurs if WFPS is >60% and increases with increasing WFPS (Aulakh et al. 1991, 1992).
In this study, the denitrification potential of the soil horizons (up to 90 cm) of 20 agricultural fields representative of the most frequent combinations of agricultural crops and soil textures in Flanders, Belgium, were studied in the laboratory under controlled conditions. The aim of this study was to determine the factors influencing the denitrification potential of these representative soils and to derive a pedotransfer function that can be used to calculate this potential for a large part of the agricultural soils in Flanders.
Materials and methods
Soils
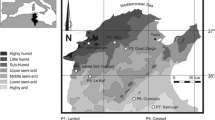
Samples of the soil horizons between 0- and 90-cm depth were collected from 20 agricultural fields in Flanders in the autumn of 2000. These 20 locations were selected on the basis of the most important combinations of soils and crops in Flanders, so as to obtain a sample representative of the majority of agricultural soils in the area.
Soil texture was determined by the combined sieve and pipette method (De Leenheer 1959). The total C and N contents were measured using a wet oxidation method (Walkley and Black 1934) and the modified Kjeldahl analysis, respectively. The pHKCl was measured in a 1:2.5 suspension using a glass electrode. Bulk density (BD) and moisture content at field capacity (pF 2 for sand soils and pF 2.7 for silt and clay soils) and at saturation (pF 0.0) of the different layers were measured on undisturbed soil cores. Table 1 summarises the crops grown at or just before the time of sampling and some physical and chemical characteristics of the soil horizons (up to 90 cm) of the 20 selected fields.
Denitrification potential
After sampling, the soils were air-dried, sieved (2-mm) and stored at room temperature until use. For each soil horizon, six 200-ml flasks were filled with 20 g dry soil, rewetted to a moisture level of 75% of field capacity and pre-incubated at 15°C for 7 days. To reproduce a sudden increase of moisture content, e.g. after a heavy downpour, the soil in three flasks was saturated just before the denitrification measurement (i.e. on day 7). To simulate a longer period of high moisture content, the soil of the other three flasks of the same horizon was saturated on day 4 of the pre-incubation. The denitrification potentials upon addition of NO3 --N and glucose-C, saturated on day 4 and 7, will hereafter be referred to as D4NC and D7NC, respectively. The denitrification potentials upon addition of only NO3 --N saturated on day 7, will hereafter be referred to as D7N.
Luo et al. (1998) showed that temporal changes in denitrification potential of soils due to differences in sampling time can be eliminated by the addition of NO3 --N and soluble C. Therefore, the denitrification potential of the soil horizons was measured with the addition of both a NO3 --N and soluble C source. At the end of the pre-incubation 50 µg NO3 --N g-1 dry soil (as KNO3) and 300 µg glucose-C g-1 dry soil were added just before the start of the measurement.
In an additional experiment the denitrification potential of the upper horizon saturated on day 7 was measured upon addition of 50 µg NO3 --N g-1 dry soil but without C addition (D7N). The aim of this approach was to quantify the influence of the percentage of endogenous C in the upper horizons on the denitrification potential.
The denitrification potential was measured with the acetylene (C2H2) inhibition technique. Anaerobic conditions during the incubation were obtained by evacuating the flasks and adding helium. Next, 10% of the helium was removed from each bottle and replaced by acetylene. In the presence of 10% (v/v) acetylene the formation of N2O during nitrification and the reduction of N2O to N2 during denitrification is inhibited (Yoshinari et al. 1977). To prevent the use of C2H2 as C source and the depletion of NO3 --N (e.g. Warland and Thurtell 2000), the N2O-N concentration measurements in the headspace were limited to a 7-h period after the application of C2H2. The gas samples (1 ml) were taken by gas-tight syringes after 1.5, 3, 4.5, 6 and 7 h incubation. The N2O-N concentrations were immediately measured with a gas chromatograph (Shimadzu GC-14B). The gas chromatograph was equipped with an electron capture detector and two packed columns (1 and 2 m respectively; Porapack Q, 80/100 mesh). The operating conditions were as follows: carrier gas N2 (55 ml min-1), injector temperature 105°C, column and oven temperature 35°C and detector temperature 250°C. The chromatograms were calibrated using N2O standard gas (25±1.5 µl l-1 in He). The denitrification potential was calculated as the slope of the regression line fitted to the data and expressed as µg N g-1 dry soil day-1.
Results
Denitrification potentials of the entire profile
The denitrification potentials of the lower soil horizons were generally negligible compared to the upper horizons (Table 2, Fig. 1). The median of the A horizons (A or Ap horizon) and second horizons (B or AC horizon) for treatment D4NC were 3.63 and 0.18 µg N g-1 dry soil day-1, respectively (i.e. D4NC of the second horizon was on average only 7.1% of D4NC of the upper horizon). The median of the A horizons and second horizon for treatment D7NC was 2.00 and 0.06 µg N g-1 dry soil day-1, respectively (i.e. D7NC of the second horizon was on average 4.9% of D7NC of the upper horizon). Still deeper in the profile the denitrification potentials were even smaller. The median for treatment D4NC and D7NC of the C horizons was 0.06 and 0.01 µg N g-1 dry soil day-1, respectively, which was only on average 3.1% and 2.7% of D4NC and D7NC of the A horizons, respectively.
Change in N2O-N concentration (µg N g-1 dry soil) of soils saturated on day 4 and day 7 with addition of a C and N source (respectively D4NC and D7NC) as a function of time in a helium atmosphere enriched with 10% acetylene (in the headspace of sealed flasks) from the soil horizons of field 1, 3 and 15, (clay, silt and sand soil, respectively). Error bars represent ±1 standard deviation
The Pearson correlation coefficients between denitrification potentials and the main soil properties of all soil horizons of the 20 fields were calculated (Table 3). The denitrification potentials showed the best correlation with the % N [Pearson correlation of 0.77 and 0.80 (P <0.001) between % N and D4NC and D7NC, respectively]. The % C was also strongly correlated with both D4NC and D7NC [Pearson correlation of 0.63 and 0.67 (P <0.001) between % C and D4NC and D7NC, respectively]. The strong correlation between denitrification potential and the N and C content of the soil horizons follows logically from the strong decrease in N and C content with depth.
Although soil N and C determined the denitrification potential to a large extent, the soil texture was also significantly correlated to the denitrification potential [Pearson correlation coefficient of 0.49 and 0.43 (P <0.001) between % clay with D4NC and D7NC, respectively].
Saturation of the soils on day 4 instead of just before measurement (day 7) increased the denitrification potential in the upper horizons, except for fields 6, 11 and 14 (Table 2, Fig. 2). In general D4NC values were also larger than D7NC in the lower horizons.
Box plots of the denitrification potentials of the soils saturated on day 4 and day 7 with addition of a C and N source (respectively D4NC and D7N) of the texture groups of the 20 experimental agricultural fields (group 1: >30% clay, field 1 and 2; group 2: <30% clay and <80% sand, field 3–14; group 3: >80% sand, field 15–20)
Denitrification potential of the upper horizons
Because the denitrification potential of the lower horizons was in general very small compared to that of the upper horizon, the relation between soil properties and denitrification potential of the upper horizons has been investigated in more detail. Soil texture had a very strong influence on the denitrification potential of the upper horizons. The Pearson correlation coefficient between the percentage of clay and D4NC, D7NC and D7N of the upper horizons was 0.85, 0.80 and 0.88 (P <0.001), respectively (Table 3). Based on this parameter the soils of the upper horizons could be divided in three groups. Group 1 were the soils with a high clay content (>30% clay) in the upper horizons. The soils of group 1, only fields 1 and 2, were characterised by very large denitrification potentials (8.33–22.43 µg N g-1 dry soil day-1). The second group of soils had a medium texture (<30% clay and <80% sand) and included fields 3–14. The denitrification potentials varied between 0.41 and 7.25 µg N g-1 dry soil day-1 (average value for D4NC, D7NC and D7N within this group was 3.95, 3.39 and 2.14 µg N g-1 dry soil day-1, respectively). The soils of group 3 had a high sand content (>80% sand, fields 15–20) and small denitrification potentials of 0.15–2.58 µg N g-1 dry soil day-1. The average value for D4NC, D7NC and D7N within this group was 1.62, 1.13 and 0.45 µg N g-1 dry soil day-1, respectively. Figure 2 shows the box plots for D4NC, D7NC and D7N of the upper horizon of each group. The denitrification potentials (D4NC, D7NC and D7N) of the upper horizons of group 1 were significantly different from those of group 2 and 3 (P <0.001). The differences between groups 2 and 3 were only significant at P <0.05, for D4NC and D7N but not for D7NC (P =0.053).
The Pearson correlation coefficient between % N and D4NC, D7NC and D7N of the soil of the upper soil horizons was also significant at the 0.001 level. Within the three different textural groups identified, the soil N content was significantly correlated with D7NC and D7N in group 2 (P <0.05 and P <0.01, respectively) but not with D4NC, whereas in group 3 no significant correlation was found between the soil N content and the denitrification potential.
The denitrification potential of the upper soil horizons without addition of a soluble C source was on average lower than that with addition of a soluble C source (Table 2, Fig. 2). This difference between D7NC and D7N, however, was not significant. This indicates that the denitrification potentials of these agricultural soils were limited by their C percentage.
Based on the results of the 20 representative fields obtained here, we derived a pedotransfer function that could be used to determine denitrification potentials of other agricultural soils in Flanders. Since the denitrification potentials of the lower horizons are very small, the pedotransfer function was limited to the denitrification potential of the upper horizons only. As mentioned earlier single linear regression of the soil properties and D4NC, D7NC and D7N of the upper horizon determined percentage of clay as the main factor influencing the denitrification potential of the upper horizons of Flemish soils. Multiple linear regression improved the correlation slightly but the coefficients of the second factor were not significant. Because C percentage of the upper horizons was shown to limit the denitrification potential, the pedotransfer function was based on D7N:
with kdenit, pot the potential denitrification constant (µg N g-1 dry soil day-1) determined under the conditions as for D7 N. The high denitrification potentials of soils 1 and 2, with high clay content, have a large influence on the pedotransfer function. The 95% prediction interval which determines the limits within which the kdenit, pot of other agricultural soils will be found with 95% certainty is broad. For instance the kdenit, pot of an agricultural soil with 20% clay will be, with 95% certainty, 3.09±2.75 µg N g-1 dry soil day-1.
Discussion
Factors influencing the denitrification potential
Since several methods have been used for measuring denitrification potentials in the laboratory (denitrification potentials are measured with or without addition of N and/or C, under widely differing moisture content conditions, at different temperatures and on fresh or air-dried soil samples prior to rewetting), it is difficult to compare denitrification potentials measured by different scientists. Nevertheless, the values found here are of the same order of magnitude as those reported by Patten et al. (1980), Luo et al. (1996) and Stevens et al. (1998). Other research consistently demonstrated a decrease in denitrification potential with depth. The denitrification potentials for the 30- to 60-, 60- to 90- and 90- to 120-cm horizons with addition of NO3 --N and after saturation, measured by Van Dyck (1996), were 28%, 5% and 1% of the 0- to 30-cm horizon, respectively. The denitrification potentials found by Colbourn et al. (1984), with addition of a soluble N source, also decreased with depth (the denitrification potentials of the 10- to 20-, 20- to 40- and 40- to 60-cm layers were 90%, 16% and 7% of the 0- to 10-cm layer, respectively). The ratios between denitrification potential of the upper and lower horizons found in our experiments were in the same range. The contribution of lower soil horizons towards gaseous N losses was also found to be low under field circumstances (Aulakh et al. 1984).
The measured denitrification potential was closely correlated with the percentage of N and C. Drury et al. (1991) also found a strong relationship between the denitrification activity and organic C and microbial biomass C. The importance of the percentage of C is confirmed by the close relation between total denitrification rates and the anaerobic CO2 production as measured by Swerts et al. (1996) and Drury et al. (1998). Addition of NO3 --N and a soluble C source instead of only NO3 --N increased the denitrification potential, but the difference was not significant. Luo et al. (1996, 1998) also found that the denitrification potential of rewetted soil increased with addition of C.
According to Jarvis and Hatch (1994) and Luo et al. (1998), addition of glucose-C as well as NO3 --N to different soil layers increased the denitrification potential of the deeper layers more than that of the upper layers. However, even with addition of both a soluble C and NO3 - source, denitrification in the lower horizons remained at least one order of magnitude smaller than in the upper horizons, showing that the decrease in the size/activity of the denitrifying community rather than a limitation of substrate is the reason for this difference, as suggested by Luo et al. (1998). Parkin and Meisinger (1989) also found that the numbers of denitrifying bacteria decreased exponentially with increasing soil depth (to 150 cm) in a well-drained silt loam.
The longer period of saturation in D4NC compared to D7NC generally increased the denitrification potential, but the difference was not significant. The increased moisture content up to saturation in the D4NC treatment probably stimulated the growth of the denitrifying microbial community in the days prior to the measurement so that with addition of a soluble C and N source the denitrifying enzymes were promoted. The higher correlation between percentage of clay and D4NC as compared to D7NC (Table 3) showed that the influence of the percentage of clay was more pronounced under conditions which were more favourable for denitrification. Dendooven et al. (1996) did not find a significant change in total denitrification losses after conditioning a soil to saturation for 96 h instead of 6 h and draining to field capacity but the N2O/N2 balance was reduced. Bergsma et al. (2002) also found a reduction in the N2O/N2 balance as a result of changing soil moisture content but no change in total denitrification losses. This need for an adaptation of the denitrifying community is also evidenced by measurements in the field. Goulding et al. (1993) and Abbasi and Adams (2000) found that the peak of denitrification losses in clayey soils in the field appeared only 4 days after heavy rain.
Relation between denitrification potential and land use
Bijay-Singh et al. (1988) found that the denitrification potentials of pasture soils were higher than arable soils and that this was correlated with the higher percentage of total C and water soluble C of the pasture soils. Gödde and Conrad (2000) identified three groups of soils based on the influence of soil properties on the denitrification potential. The first group were acidic forest soils and the second group acidic agricultural soils. Neutral agricultural soils formed the third group. Because of the small differences in soil pH and the overriding effect of soil texture on the denitrification potential, a relationship between land use (pasture, arable crops and horticultural crops) and denitrification potential can only be expected to be found within the three textural groups identified earlier. The higher percentage of C and N of the upper horizons of the pastures (on average 3.06% C and 0.23% N for the pastures and on average 1.51% C and 0.13% N for arable fields) resulted in higher denitrification potentials of the pastures within each textural groups.
Relation between denitrification potential and denitrification losses in the field
The denitrification potentials were converted to kg N ha-1 day-1, using the bulk density as measured in the field. The values of D4NC, D7NC and D7N of the upper horizons of the 20 fields on a per hectare basis varied between 3 and 82, 2 and 52 and 0 and 30 kg N ha-1 day-1, respectively. These values seem extremely high but interpreting these results in kg N ha-1 day-1 one has to bear in mind that the denitrification potentials were measured on rewetted soil at saturation and in the presence of a soluble C and/or N source. Measuring the denitrification potential on air-dried and rewetted soil had the advantage that the soils could be homogenised prior to the measurements and that the measured denitrification potential was independent of the length of the storage period. The storage time of wet soil is known to affect the measured denitrification potential (e.g. Dendooven and Anderson 1995; Luo et al. 1996). However, air-drying the soil also had a stimulating effect on the denitrification potential. Patten et al. (1980) and Bijay-Singh et al. (1988) indeed showed that denitrification potential after addition of NO3 --N of dried and rewetted soils was higher than the denitrification potentials of field-moist soil and this was correlated with the higher amount of water-soluble C after drying. Moreover, the water content in agricultural fields reaches saturation only in exceptional conditions and the denitrification potential was measured under conditions of complete anaerobiosis during the incubation by evacuating the flasks and adding helium.
Field measurements in other experimentations confirm that the denitrification potentials can not be directly extrapolated to losses in the field. The background denitrification (i.e. without additional supply of N) in the field is very low. If other factors like water status are not limiting, N fertilisation results in brief peaks of denitrification (Kaiser et al. 1996; Abbasi and Adams 2000). Most field studies show that no more than 35 kg N ha–1 year-1 is lost through denitrification in Flanders (Van Cleemput et al. 1994; Vermoesen et al. 1996) and in other countries with a temperate climate (Nieder et al. 1989). Field denitrification losses can amount to 60–70 kg N ha-1 year-1 on poorly drained clay soils (Van Cleemput 1998). The measured peaks of denitrification in the field were much higher than the average denitrification rates and were one order of magnitude smaller than D7N (Ryden 1983; Thompson et al. 1987; de Klein and Van Logtestijn 1996). However, in exceptional situations very favourable to denitrification, namely after incorporation of harvest residues or green manure, peaks of denitrification were in the same order of magnitude as the denitrification potentials after addition of NO3 --N and a soluble C source (Schloemer 1991; Beauchamp et al. 1996). This showed that only when conditions in the field are optimal, can denitrification losses measured in situ be comparable to potential rates under controlled conditions.
Conclusions
The denitrification potentials as determined from laboratory measurements on rewetted soil under controlled conditions should be regarded as relative values allowing the ranking of soils with respect to their denitrification risk under field conditions. However, to translate potential denitrification to denitrification rates under field conditions correction factors for soil water content, NO3 --concentration and temperature are necessary. Only under extreme conditions, can denitrification losses measured in the field be of the same order of magnitude as denitrification potentials measured in the laboratory. The denitrification potentials obtained in our study indicate that after N fertilisation and under conditions of high soil water content denitrification losses can be very high, especially in fields with a clay texture. The denitrification potentials of the subsoil are much lower than the denitrification potentials of the surface horizons. However, subsoil denitrification in the field will be possible when the amount of leached NO3 --N and carbon to the subsoil is high. Since in most cases saturation of the soils on day 4 of the pre-incubation instead of day 7 just before the measurement increased the denitrification potential, the highest denitrification losses will occur during longer periods of high moisture content.
References
Abbasi MK, Adams WA (2000) Gaseous N emission during simultaneous nitrification-denitrification associated with mineral N fertilisation to a grassland soil under field conditions. Soil Biol Biochem 32:1251–1259
Aulakh MS, Rennie DA, Paul EA (1984) Gaseous losses from soils under zero-till as compared with conventional-till management systems. J Environ Qual 13:130–136
Aulakh MS, Doran JW, Walters DT, Power JF (1991) Legume residue and soil water effects on denitrification in soils of different textures. Soil Biol Biochem 23:1161–1167
Aulak MS, Doran JW, Mosier AR (1992) Soil denitrification—significance, measurement, and effects of management. Adv Soil Sci 18:1–57
Beauchamp EG, Bergstrom DW, Burton DL (1996) Denitrification and nitrous oxide production in soil fallowed or under alfalfa or grass. Commun Soil Sci Plant Anal 27:87–99
Bergsma TT, Robertson GP, Ostrom NE (2002) Influence of soil moisture and land use history on denitrification end-products. J Environ Qual 31:711–717
Bijay-Singh, Ryden JC, Whitehead DC (1988) Some relationship between denitrification potential and fractions of organic carbon in air-dried and field-moist soils. Soil Biol Biochem 20:737–741
Colbourn P, Iqbal MM, Harper IW (1984) Estimation of the total gaseous nitrogen losses from clay soils under laboratory and field conditions. J Soil Sci 35:11–22
De Leenheer L (1959) Procedures of analyses at the Centre for Soil Research (in Dutch). Centre for Soil Research, Ghent
Dendooven L, Anderson JM (1995) Maintenance of denitrification potential in pasture soil following anaerobic events. Soil Biol Biochem 27:1251–1260
Dendooven L, Duchateau L, Anderson JM (1996) Gaseous products of the denitrification process as affected by the antecedent water regime of the soil. Soil Biol Biochem 28:239–245
Drury CF, McKenney DJ, Findlay WI (1991) Relationships between denitrification, microbial biomass and indigenous soil properties. Soil Biol Biochem 23:751–755
Drury CF, Oloya TO, McKenney DJ, Gregorich EG, Tan CS, van Luyk CL (1998) Long-term effects of fertilization and rotation on denitrification and soil carbon. Soil Sci Soc Am J 62:1572–1579
Gödde M, Conrad R (2000) Influence of soil properties on the turnover of nitric oxide and nitrous oxide by nitrification and denitrification at constant temperature and moisture. Biol Fertil Soils 32:120–128
Goulding KWT, Webster CP, Powlson DS, Poulton PR (1993) Denitrification losses of nitrogen-fertilizer applied to winter-wheat following ley and arable rotations as estimated by acetylene inhibition and N-15 balance. J Soil Sci 44:63–72
Hénault C, Germon JC (2000) NEMIS, a predictive model of denitrification on the field scale. Eur J Soil Sci 51:257–270
Hofman G, Van Cleemput O (2001) Gaseous N losses from field crops. Acta Hortic 563:155–162
Jarvis SC, Hatch DJ (1994) Potential for denitrification at depth below long-term grass swards. Soil Biol Biochem 26:1629–1636
Kaiser EA, Eiland F, Germon JC, Gispert MA, Heinemeyer O, Hénault C, Lind AM, Maag M, Saguer E, Van Cleemput O, Vermoesen A, Webster C (1996) What predicts nitrous oxide emissions and denitrification N-loss from European soils? Z Pflanzenernaehr Bodenkd 159:541–547
Klein CAM de, Van Logtestijn RSP (1996) Denitrification in grassland soils in the Netherlands in relation to irrigation, N-application rate, soil water content and soil temperature. Soil Biol Biochem 28:231–237
Luo J, White RE, Ball PR, Tillman RW (1996) Measuring denitrification activity in soils under pasture: optimizing conditions for the short-term denitrification enzyme assay and effects of soil storage on denitrification activity. Soil Biol Biochem 28:409–417
Luo J, Tillman RW, White RE, Ball PR (1998) Variation in denitrification activity with soil depth under pasture. Soil Biol Biochem 30:897–903
Luo J, Tillman RW, Ball PR (2000) Nitrogen loss through denitrification in a soil under pasture in New Zealand. Soil Biol Biochem 32:497–509
Nieder R, Schollmayer G, Richter J (1989) Denitrification in the rooting zone of cropped soils with regard to methodology and climate—a review. Biol Fertil Soils 8:219–226
Parkin TB, Meisinger JJ (1989) Denitrification below the crop rooting zone as influenced by surface tillage. J Environ Qual 18:12–16
Patten DK, Bremner JM, Blackmer AM (1980) Effects of drying and air-dry storage of soils on their capacity for denitrification of nitrate. Soil Sci Soc Am J 44:67–70
Ryden JC (1983) Denitrification loss from a grassland soil in the field receiving different rates of nitrogen as ammonium nitrate. J Soil Sci 34:355–365
Schloemer S (1991) Denitrification losses from a horticultural soil as affected by incorporation of fresh plant residues. Z Pflanzenernaehr Bodenkd 154:265–269
Stevens RJ, Laughlin RJ, Malone JP (1998) Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol Biochem 30:1119–1126
Swerts M, Merckx R, Vlassak K (1996) Denitrification, N2-fixation and fermentation during anaerobic incubation of soils amended with glucose and nitrate. Biol Fertil Soils 23:229–235
Thompson RB, Ryden JC, Lockyer DR (1987) Fate of nitrogen in cattle slurry following surface application or injection to grassland. J Soil Sci 38:689–700
Van Cleemput O (1998) Subsoils, chemo- and biological denitrification, N2O and N2 emissions. Nutr Cycl Agroecosyst 52:187–194
Van Cleemput O, Vermoesen A, De Groot C-J, Van Ryckeghem K (1994) Nitrous oxide emissions out of grassland. Environ Monit Assess 31:145–152
Van Dyck B (1996) Denitrification in the root zone beneath the plough layer (in Dutch). Thesis, Faculty of Agricultural and Applied Biological Sciences, Ghent University, Ghent
Vermoesen A, Van Cleemput O, Hofman G (1996) Long-term measurements of N2O emissions. Energy Conserv Manage 37:1279–1284
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Warland JS, Thurtell GW (2000) A micrometeorological method for in situ denitrification measurements using acetylene inhibition. Agric For Meteorol 103:387–391
Well R, Myrold DD (2002) A proposed method for measuring subsoil denitrification in situ. Soil Sci Soc Am J 66:507–518
Yoshinari T, Hynes R, Knowles R (1977) Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol Biochem 9:177–183
Acknowledgements
Financial support for this research from the VLM, Afdeling Mestbank (Brussels, Belgium) is gratefully acknowledged (project VLM 2000/1). We thank S. Vlerick, R. Degryse and S. Schepens for their skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D'Haene, K., Moreels, E., De Neve, S. et al. Soil properties influencing the denitrification potential of Flemish agricultural soils. Biol Fertil Soils 38, 358–366 (2003). https://doi.org/10.1007/s00374-003-0662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-003-0662-x