Abstract
Lead (Pb) has been highlighted as a major pollutant of both terrestrial and aquatic ecosystems, causing negative impacts to these environments. The concentration of Pb in plants has increased in recent decades, mainly due to anthropogenic activities. This study has as a hypothesis that the species Oxycaryum cubense (Poep. & Kunth) Palla, abundant in aquatic environments, has the potential to be used a phytoremediator. The plants were grown in a hydroponic system with Pb in increasing concentrations (0, 4, 8, 16 and 32 mg l−1) for 15 days. Inductively coupled mass spectrometer (ICP OES) was used to determine the concentration of mineral nutrients and lead. Optical and transmission electron microscopy were used for the analysis of cellular damage induced by lead in roots and leaves. Ultrastructural alterations were observed as disorganization of thylakoids in the chloroplast and disruption of mitochondrial membranes in cells of leaf tissues of plants subjected to increasing Pb concentrations. There was accumulation of Pb, especially in the root system, affecting the absorption and translocation of some mineral nutrients analysed. In roots, there was reduction in the thickness of the epidermis in plants treated with Pb. This species was shown to be tolerant to the Pb concentrations evaluated, compartmentalizing and accumulating Pb mainly in roots. Due to these results, it may be considered a species with phytoremediation capacity for Pb, with potential rizofiltration of this metallic element in contaminated watersheds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead has been highlighted as one of the greatest pollutants of both terrestrial and aquatic ecosystems, and is considered an important environmental pollutant (Kabata-Pendias and Pendias 2000; Shen et al. 2002; Almeida et al. 2007, 2011). The recovery of these Pb contaminated areas can be made through the use of plants, phytoremediation, that are able to accumulate this metal element in their tissues, especially in shoots (Almeida et al. 2007, 2011). Phytoremediation is considered a technique with low cost and high efficiency in situ decontamination and with less disturbance to the environment (Santos and Lenzi 2000; Meuleman et al. 2004). Therefore, it is necessary to use plants that have a good absorption capacity, accelerated growth rate, easy to harvest and high resistance to pollutants (Coutinho and Barbosa 2007). Phytoremediation of contaminated water can be carried out using the rhizofiltration mechanism. This technique is defined as the use of plants to absorb, concentrate and precipitate contaminants in their roots in polluted aqueous sources with low concentration of contaminants (Ghosh and Singh 2005). Rhizofiltration is considered an emerging technology that can provide solutions that are environmentally safe and economically feasible to remediate environments contaminated with Pb (Verma and Dubey 2003).
Plants have several mechanisms at the cellular and tissue level, which may be involved in detoxification and adaptation of the same in environments contaminated by toxic metals, such as the translocation to shoots, root exudation of substances capable of chelating metals; metals' connections to cell walls, production of intracellular compounds and storage in the vacuole and other subcellular compartments to reduce the effects of stress and increase the internal resistance to the toxicity of metals (Almeida et al. 2011).
Herbaceous plants are generally more tolerant to excessive toxic metals than woody species (Eltrop et al. 1991) for presenting more effective physiological and biochemical mechanisms for reducing the toxicity of heavy metals in their tissues. However, with respect to Pb, more studies are needed to better understand the mechanisms of tolerance, uptake, translocation and accumulation by plants. The Cyperaceae family has several species that are tolerant to toxic metals (Kabata-Pendias and Pendias 2000), so it is important to study the tolerance of species belonging to this family. The species Oxycaryum cubense (Poep. & Kunth) Palla, of this family occurs in riparian zones, water courses and wetlands. It is characterized as a perennial species, among species of epiphytic such as floating Salvinia sp., Pistia stratiotes and Eichhornia crassipes, whose roots use absorbent hairs to affix to locations, facilitating their attachment and support (Tur 1971; Neves et al. 2006; Leite et al. 2009), and besides being an amphibious species, it may, in the dry season, settle in the riparian zone (Pott and Pott 2000). The aim of this study was to evaluate the changes caused by Pb in anatomy, ultrastructure of leaf tissue and root and concentration of mineral nutrients in O. cubense plants grown hydroponically with different concentrations of Pb, as well as its phytoremediation capacity for Pb removal in solution.
Material and methods
Plant material and experimental design
Plants of O. cubense of approximately the same size were collected at the “Lagoa Encantada,” a non-impacted region, located in the city of Ilheus, Bahia, Brazil, at 14°46′8″S and 39°01′6″W. After harvest, the plants were transported in plastic buckets with roots immersed in water, until arrival at greenhouse of the Universidade Estadual de Santa Cruz (UESC), where they were placed in plastic trays with a capacity of 30 l, containing nutrient solution (NS) prepared in accordance with Hoagland and Arnon (1950). The plants remained in the NS initially for a period of 30 days. Subsequently, we performed the exchange of NS, maintaining the same concentration, and established the treatment with different concentrations of Pb (0, 4, 8, 16 and 32 mg l−1) in the form of Pb (NO3)2, for a period of 15 days. During this period, the NS were monitored for pH and adjusted to pH 5.8 using NaOH or HCl and maintained under constant aeration. The daily level of NS was also maintained by replacing the volume with deionized water.
Anatomical analysis
For anatomical analysis, samples were collected from the middle portion of mature leaves and root tips of a plant per experimental unit, totaling five plants per treatment. Samples were fixed in 2.5 % glutaraldehyde in sodium cacodylate buffer 0.1 M, pH 6.8, for 4 h, and dehydrated in an ethanol series (75 %, 85 % and 95 %) and alcohol 1 h each. Later, we started the process of pre-infiltration (historesin+alcohol) and infiltration (historesin pure), 7 days in each case, and finally, samples were included in a historesin Leica Historesin Embedding Kit. Further using RM 2145 Leica microtome, slices of 7 mm thickness were obtained, which were then mounted on glass slides and stained with 1 % toluidine blue. Slides were observed and photographed under an inverted microscope Leica DMI 3000 B, for subsequent measurement of leaf tissues (upper and lower epidermis, mesophyll and aerenchyma) and root (epidermis, exodermis and endodermis) through software Leica Application Suite V3.
Transmission electron microscope (TEM) analysis
The samples were then washed in the same buffer six times, for 10 min and post-fixed in 1 % osmium tetroxide diluted in the same buffer for 2 h and then washed again in the same buffer for the same time period. Dehydration of samples was conducted in order of increasing ethanol concentrations (30 %, 50 %, 70 %, 80 %, 90 % and 100 %) for 15 min at each concentration, and twice in 100 % ethanol. The steps of dehydration and inclusion of samples were done under slow agitation, using proportions of ethanol/LR White of 3:1 for 2 h, 1:1, 3:1 for 2 h overnight. In the samples, we used resin which was changed every 4 h and finally included in LR White in gelatin capsules and placed in an oven at 60 °C for 8 h. Subsequently, ultrathin sections were obtained using an 80-nm-thick, Leica UC6 ultramicrotome. Then, the sections were deposited on copper grids, contrasted with uranyl acetate aqueous solution for 25 min and lead citrate for 30 min. Analyses were performed using Morgani™ 268D TEM (FEI Company), with acceleration voltage of 80 kV, equipped with a CCD camera and controlled by software running under Windows OS. At least four grids with three to five sections for each treatment were observed and photographed. Images where selected that best represented the changes in the ultrastructure of leaf mesophyll cells and of O. cubense root cells, after application of different concentrations of Pb.
Energy dispersive X-ray spectrometry (EDS)
EDS was performed on unstained ultra-thin sections of leaf and root samples prepared as described above for TEM. The ultrathin sections were collected on carbon-coated copper grids. The EDS spectra in these materials were taken in nanoprobe mode that allows the creation of probes in nanometer scale, in this specific case the probe that analyzes the sample was 200 nm in diameter. To test if the Cu signal came from the grid or not we moved the beam along the sample, leaving the region of interest. We found that the peak of copper disappeared, except when we were very close to the edge of the grid. However, in regions distant from the edges, as was the case for our sample, a signal was not found from Cu, except where there is contamination. The EDS spectra was taken from an Si–Li detector mounted in an FEI Tecnai G 20 microscope in Electron Microscopy Center of Universidade Federal de Minas Gerais operating at 80 kV in nanoprobe mode; the probe size was around 0.5 μm diameter.
Inductively coupled plasma mass spectrometer (ICP OES) analysis
At the end of the experimental period, the plants were washed once with deionized water, once with solution of 3 % HCl, and twice with deionized water. Then, the plants were divided into parts (roots and shoots) and placed in an oven at 70 °C until constant mass. Subsequently, the plant parts were ground using a Willy-type mill and placed separately in polypropylene vials for subsequent acid digestion. For this, each sample was weighed in triplicate and placed in 50-ml tubes. Thereafter, 3 ml of concentrated nitric acid (A.P.) was added to each sample. The tubes were then heated using a TECNAL digester block (Model TE-007MP) at 50 °C for 30 min and at 80 °C for 1 h. Soon after, the temperature of the block was adjusted to 130 °C and 1 ml of H2O2 was added three consecutive times at intervals of 20 min. After digestion, the samples were transferred to Falcon tubes and increased in volume to 14 ml with milli-Q water. The analyses of nutrients (P, K, Ca, Mg, S, Mn, Zn and Fe) and Pb in the digested samples were performed by optical emission spectrometry with ICP OES (Varian 710 – ES).
Statistical analysis
A completely randomized experimental design was used with five treatments (4 Pb concentrations+control [no Pb]), five replicates of eight plants per experimental unit (tray), making a total of 200 plants. Results were subjected to analysis of variance (ANOVA) and the regression equations were fitted to the data, in order to relate the various concentrations of Pb and mineral nutrients in plant dry biomass. The choice of the equation that best fitted the data was based on the significance of the effect of regression, the deviations of the regression tested by F test at 5 % probability, and the highest coefficient of determination (R 2).
Results
Symptoms of lead toxicity
The species O. cubense showed chlorosis and brown spots at leaf level after 7 days of treatment with Pb, whose symptoms intensified until the plant harvest period (15 days after treatment application). At the end of the experimental period, plants exposed to 8, 16 and 32 mg Pb l−1 showed signs of necrosis, which were more evident in plants exposed to 16 mg Pb l−1 (Fig. 1).
Anatomical alterations
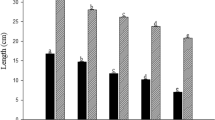
In leaves of plants subjected to treatment with Pb, reduction in leaf mesophyll thickness (Fig. 2) was observed with increasing concentrations of Pb in NS. In roots, there was reduction in the thickness of the epidermis in plants treated with Pb (Fig. 3). Increased thickness could also be demonstrated in the exodermis, possibly through an increase in cell wall thickening (Fig. 3). There was no significant difference (P < 0.05) between the thickness of the endoderm of Pb-treated plants.
Variations of thickness of exodermis (empty square), epidermis (empty triangle) and endodermis (empty circle) evidenced in transversal sections of plant roots of O. cubense exposed to increased concentrations of Pb in nutritive solution for 15 days (n = 3). Regression equations: y = 10.56 + 0.06x (r 2 = 0.72) for the exodermis, y = 10.72 − 0.07x (r 2 = 0.66) epidermis and y = 8.83 for the endodermis
Ultrastructural alterations
The cells in the leaf tissue of the control plant showed normal ultrastructural features with intact organelles, as well as the presence of plastoglobule and starch grains (Fig. 4a,b). Ultrastructural characteristics similar to the control were observed in plants subjected to Pb concentration of 4 mg l−1 (Fig. 4c). The cells of the leaf tissue of plants treated with 8 mg Pb l−1 showed an increase in the formation of plastoglobules (Fig. 4d), disorganization of thylakoids in the chloroplast (Fig. 4d–f) and disruption of mitochondrial membranes (Fig. 4e,f). But, the leaf tissue cells of plants treated with 16 mg Pb l−1 showed disorganization of thylakoids in the chloroplast (Fig. 5a), deposits of electro-dense material in the vacuole (Fig. 5b), deposits of electro-dense material in the cell wall and retraction of the cytoplasm (Fig. 5c), disruption of mitochondrial membranes (Fig. 5d), increase in the formation of plastoglobules (Fig. 5a and e) and deposits of electro-dense material in the intercellular spaces (Fig. 5f). The leaf tissue cells of plants treated with 32 mg Pb l−1 showed an increase in the formation of plastoglobules (Fig. 5g), disorganization of thylakoids in the chloroplast and disruption of mitochondrial membranes (Fig. 5h). The cells in the root tissue showed normal ultrastructural features with intact organelles (Fig. 6).
Electron micrograph of mesophyll cells from plants of O. cubense exposed to increased concentrations of Pb in nutritive solution for 15 days. a, b Control evidencing a normal ultra-structural aspect. c Treatment of 4 mg Pb l−1 that, same as with the control, shows normal ultra-structural characteristics. d–f Treatment of 8 mg Pb l−1, evidencing the increase in numbers of plastoglobule (d), disorganized thylakoids in the chloroplast (d–f) and rupture of the mitochondrial membranes (e, f). Ch chloroplast, M mitochondria, N nucleus, Sg starch granule; thin arrow: plastoglobule; wide arrow: rupture of the mitochondrial membranes. Bars: a–c, f 1 μm; d 0.5 μm, e 2 μm
Electron micrograph of mesophyll cells in leaf of plants O. cubense subjected to Pb concentration of 16 and 32 mg l−1 in nutrient solution for 15 days. a Chloroplast showing disorganized thylakoids, numerous starch grains and presence of plastoglobule. b Deposit of electro-dense material in the vacuole. c Cell with a cytoplasm retracted, and the presence of electro-dense deposits in cell walls, inter-cellular spaces and plasmodesm. d Disruption of mitochondria. e Increasing in size and number of plastoglobule. f Deposit of electro-dense material in cell walls, inter-cellular spaces and plasmodesm. g Chloroplast with many starch grains and presence of plastoglobule. h Degradation of mitochondria and chloroplast showing disorganized thylakoids. Ch chloroplast, M mitochondria, N nucleus, Sg starch granule; thin arrow: plastoglobule; wide arrow: disruption of mitochondrial membranes; white arrow: deposit of electro-dense material; dotted arrow: plasmodesm. Bars: a, b 2 μm; c–h 1 μm
In addition, electro-material deposits were found in the dense vacuoles in the cell wall and intercellular spaces in leaf tissues and presence of Pb was confirmed in these electro-dense material deposits by EDS (Fig. 7a–d). The presence of Pb was observed in root vacuoles and cell walls of plants treated with Pb, confirmed by EDS (Fig. 8a–d).
Evidence of Pb accumulation of the mesophyll cells of leaves of plants O. cubense subjected to Pb concentration of 32 mg l−1 in nutrient solution for 15 days. a Electron micrograph showing the presence of deposit of electro-dense material in the cell wall and intercellular spaces. Bars: 1 µm. b EDS spectra from leaf cell wall taking at 80 kV in nanoprobe mode. This result shows clearly the presence of Pb. c Electron micrograph showing the presence of deposit of electro-dense material in the vacuole. Bars: 0.5 µm. Ch chloroplast, M mitochondria, V vacuole; white arrow deposit of electro-dense material. d EDS spectra from leaf (vacuolo) taking at 80 kV in nanoprobe mode. This result shows clearly the presence of Pb
Evidence of Pb accumulation in root tissue of O. cubense subjected to Pb concentration of 32 mg l−1 in nutrient solution for 15 days. a Electron micrograph analysis in TEM microscope FEI-TECNAI-G2 showing the presence of deposit of electro-dense material in the cell wall and intercellular spaces. Bars: 1 μm. b EDS spectra from root cell wall taking at 80 kV in nanoprobe mode. This result shows clearly the presence of Pb. c Electron micrograph analysis in TEM microscope FEI-TECNAI-G2 showing the presence of deposit of electro-dense material in the vacuole. Bars: 0.5 μm. M mitochondria, V vacuole; white arrow deposit of electro-dense material. d EDS spectra from root (vacuolo) taking at 80 kV in nanoprobe mode. This result shows clearly the presence of Pb
Absorption of Pb, Mg, Ca, P, S and K
At 15 days of application of Pb, it was observed that this metal element mainly accumulated in the root system, whose accumulation increased proportionally to the concentration of metal in NS (Fig. 9). The accumulation of Pb in roots represented 93 % of total Pb uptake by plants. At the root, the concentration of Mg, Ca, P, S and K decreased by 47 %, 58 %, 22 %, 16 % and 67 %, respectively, until concentration of 16 mg Pb l−1, and 34 %, 38 %, 16 %, 3 %, and 36 % in concentration corresponding to 32 mg Pb l−1 (Fig. 10a–e). In the shoots, the concentrations of Mg and Ca decreased by 5 % and 11 %, respectively, with increasing concentration of Pb in solution (Fig. 10a,b). Moreover, both the concentration of P and the concentration of S reduced 4 % to a concentration of 16 mg Pb l−1 and increased by 15 % and 6 %, respectively, at the concentration of 32 mg Pb l−1 (Fig. 10c,d). The concentration of K decreased by 5 % until the concentration of 16 mg Pb l−1 and 1 % in the concentration corresponding to 32 mg Pb l−1 (Fig. 10e).
Variations in the concentration of Pb in roots (filled circle) and shoot (empty circle) of plants O. cubense exposed to increasing concentrations of Pb in nutrient solution for 15 days, n = 3, mean ± SE (p ≤ 0.05, by t-test). Regression equations: y = 3560.98 (1 − exp(−0.22x) (r 2 = 0.92) for root and y = 28.36 + 6.69x − 0.11x 2 (r 2 = 0.65) to shoot
Variations in the concentration of Mg, Ca, P, S and K in roots (filled circle) and shoot (empty circle) of plants O. cubense exposed to increasing concentrations of Pb in nutrient solution for 15 days, n = 3, mean ± SE (P ≤ 0.05, t-test). Regression equations: a ŷ = 0.38 − 0.02x + 0.0004x 2 (r 2 = 0.80) to root and ŷ = 3.07 − 0.004x (r 2 = 0.51) to shoot; b ŷ = 2.03 − 0.12x + 0.003x 2 (r 2 = 0.77) for root and ŷ = 4.90 − 0.01x (r 2 = 0.77) for the shoot; c ŷ = 3.90 − 0.09x + 0.002x 2 (r 2=0.24) for root and ŷ = 14.27 − 0.13 0.006 + x 2 (r 2 = 0.75) for shoots; d ŷ = 2.42 − 0.04x + 0.001x 2 (r 2 = 0.78) for root and ŷ = 3.01 − 0.02x + 0.0008x 2 (r 2 = 0.86) to shoot; e ŷ = 10.02 − 0.73x + 0.02x 2 (r 2 = 0.61) for root and ŷ = 42.81 − 0.25x + 0.007x 2 (r 2 = 0.52) to shoot
Absorption and translocation of micronutrients Fe, Zn and Mn
At the root, there was a 17 % increase in the concentration of Fe until the concentration of 16 mg Pb l−1, then decreasing by 27 % in plants subjected to concentration of 32 mg Pb l−1 (Fig. 11a). A reduction could be shown for the concentrations of Zn and Mn at 60 % and 80 %, respectively, at the concentration of 16 mg Pb l−1, and 30 % to 70 %, respectively, at a concentration corresponding to 32 mg Pb l−1 of NS (Fig. 11b,c). In the shoots, Fe and Zn concentrations decreased, 14 % and 9 %, respectively, with increasing concentration of Pb in NS (Fig. 11a,b).
Variations in the concentration of Fe, Zn and Mn in roots (filled circle) and shoot (empty circle) of plants O. cubense exposed to increasing concentrations of Pb in nutrient solution for 15 days, n = 3, mean ± SE (P ≤ 0.05, t-test). Regression equations: a ŷ = 1,402.84 + 41.94x − 1.68x 2 (r 2 = 0.56) for root and ŷ = 194.87 − 0.81x (r 2 = 0.72) for shoot; b ŷ = 56.07 − 3.64x + 0.09x 2 (r 2 = 0.84) for root and ŷ = 78.30 − 0.22x (r 2 = 0.64) for shoot; c ŷ = 221.91 − 17.45x + 0.39x 2 (r 2 = 0.90) for root and ŷ = 380.66 for the shoot
Discussion
Symptoms of lead toxicity
The visual symptoms of toxicity presented by the species O. cubense are common in plants exposed to toxic metals (Kabata-Pendias and Pendias 2000; Souza et al. 2011; Santana et al. 2012), and can is related to a nutritional imbalance, as lead can change the selectivity of the plasma membrane toward root tissue cells, affecting the absorption, transport and allocation of nutrients in various parts of plants, resulting in an imbalance of mineral nutrition due to the disturbed uptake of cations such as K, Ca, Mg, Zn, Cu and Fe in the root system (Kerbauy 2004; Sharma and Dubey 2005).
Anatomical alterations in O. cubense
Thickness reduction in the abaxial epidermis in plants O. cubense has also been demonstrated in Plantago major plants treated with Pb (Kosobrukhov et al. 2004). The tolerance to Pb presented by O. cubense may be related to the root anatomy; in particular the presence of exodermis, which according to Ranathunge et al. (2011), is a major apoplastic barrier. In plant species in which the exodermis is present, ionic selectivity occurs much closer to the periphery of the root, preventing the concentration of potentially toxic substances in the cortex (Enstone et al. 2003). Deng et al. (2009) assessed the tolerance of ten aquatic species to Pb, Zn and Fe, and observed that the most tolerant species had more suberin and lignin in root tissues, and had sclerenchyma fibers with thick walls and dense cells in the outer layers of the cortex. Therefore, the thick root exodermis of O. cubense may constitute a barrier to the transport of Pb and to a possible site of accumulation of the metal, because its thickness increases with increasing Pb concentration to which the species is subjected. Gomes et al. (2011) suggested that the thickening of the exodermis and endodermis in Brachiaria decumbens could be a strategy of the plant to minimize the translocation of metals. The thickening of the cell wall protects the plant cells from the harmful effects of toxic metals (Krzesłowska 2011), providing greater retention area of these metal elements and thus decreasing its translocation into the shoot (Gomes et al. 2011).
Ultrastructural alterations
Lead caused damage at the chloroplast level in the mesophyll cells of O. cubense plants treated with concentrations from 8 mg Pb l−1. Damage caused by Pb to the chloroplasts was also observed in Potamogaton crispus (Hu et al. 2007) and Solanum licopersicum (Zhao et al. 2011). The deposition of electro-dense material in the intercellular spaces, cell wall and the vacuole of leaf mesophyll cells of plants subjected to higher Pb concentration was also observed in Lemna minor plants subjected to treatment with Pb (Samardakiewicz and Wozny 2000). This electro-dense material deposition in intercellular spaces and cell walls may result from increased apoplastic transport of Pb (Tung and Temple 1996). According to Sharma and Dubey (2005), Pb is accumulated mainly in the intercellular spaces, cell wall and vacuoles of leaf mesophyll cells. Moreover, deposition and accumulation in the cell wall is considered as the main strategy for resistance of plants to Pb (Piechalak et al. 2002). Therefore, the immobilization of Pb in the cell wall and its detoxification by immobilization in vacuoles are important processes that influence plant tolerance and protects cell metabolism against toxic effects of this metal (Seregin and Ivanov 2001).
The plastoglobule are existing structures within the chloroplasts that are attached to thylakoids (Austin et al. 2006), functioning as a reservoir of lipids (Kessler et al. 1999), and serving as an active site of synthesis and recycling proteins under stress conditions (Ytterberg et al. 2006). Therefore, the increased formation of plastoglobules in leaf mesophyll cell chloroplasts of the plants treated from 8 mg Pb l−1 could be a plant response to the toxicity of this metal. According to Bréhélin and Kessler (2008), the plastoglobules are related to plant responses to stress. The increase in size and number of these structures is common in plants subjected to environmental conditions that cause oxidative stress to the photosynthetic apparatus (Austin et al. 2006), like Pb (Verma and Dubey 2003). Oxidative stress is related to the excessive formation of reactive oxygen species (ROS) such as superoxide (O2 −), hydrogen peroxide (H2O2) and hydroxyl radicals (OH−) (Zimmermann and Zentgraf 2005), which, in turn, may be derived from normal cellular metabolism or also by disrupting the electron transport system in chloroplasts and mitochondria during stress conditions (Breusegem et al. 2001). Moraes (2011) observed increased plastoglobules with increasing Pb concentrations in seedlings of Solanum lycopersicon and suggested that this increase may represent a defence mechanism to prevent possible damage to the photosynthetic apparatus. A similar effect was also observed in Glycine max treated with Pb (Weryszko-Chmielewska and Chwill 2005) and Zea mays treated with Cd (Rascio et al. 1993).
Absorption of Pb and nutrients
The highest accumulation of Pb in the roots occurred due to the presence of exodermis which is a major apoplastic barrier (Ranathunge et al. 2011), minimizing the translocation of toxic metals (Gomes et al. 2011) and establishing a possible retention site of the metal, which consequently prevents its translocation to the shoot. Moreover, the endodermis acts as an additional barrier for the translocation of this metallic element (Jones et al. 1973; Verma and Dubey 2003), also helping to reduce its translocation from roots to shoots (Kabata-Pendias and Pendias 2000). Therefore, the roots can sequester metals, preventing or reducing translocation to the leaves (Patra et al. 2004). Similar results were also observed in Oryza sativa (Kim et al. 2002) and Brassica oleracea (Sinha et al. 2006).
Lead is absorbed by the roots via cation transporters (Kim et al. 2002), and absorption by the plant increases as levels of exogenous Pb are raised (Patra et al. 2004). This statement is consistent with the results for O. cubense. The increase in P concentrations in plant shoots exposed to higher concentrations of Pb, may be due to direct interference of Pb in the metabolism of P (Gopal and Rizvi 2008), or may be related to Zn deficiency which promotes the loss of membrane integrity, consequently, contributing to the absorption and transport of P to the shoot (Kerbauy 2004). In addition, Zn deficiency also inhibits the redistribution of P from roots to shoots (Kerbauy 2004), which explains the fact that this element decreases its concentration in roots of plants treated with Pb as compared to the control. Moreover, the increased concentration of P in shoots may favour the precipitation of Pb as lead phosphate. This Pb precipitation occurs mainly in the cell wall and in the vacuole of plants and constitutes a strategy for Pb detoxification (Zheng et al. 2012). These same authors reported the presence of P with all Pb deposits in cell walls and vacuoles in the cytoplasm Lespedeza chinensis and L. davidii subjected to treatment with Pb and, in some cases, also noted the presence of Ca and S in these sites. With respect to K concentrations, reduction in root cation concentrations may possibly be explained by competitive inhibition between K and divalent cations present in high concentrations in nutrient solution (Walker et al. 1977).
The Ca concentration decreased both in the shoots and in roots of plants treated with Pb. This result was also found by Kibria et al. (2009), where the concentration of Ca decreased in shoots and roots of Amaranthus gangeticus and roots of the species A. oleracea exposed to different concentrations of Pb. Similar results were found in Zea mays treated with Pb (Huang and Cunninghan 1996). A transport route of Pb to root tissue cell’s interior through the communication channels of the membrane, such as Ca channels (Huang and Cunningham 1996; Kim et al. 2002). This implies an inhibition in absorption of Ca due to blockage of the channels by Pb or by competitive transport with Pb with Ca through these channels (Huang and Cunningham 1996). Kim et al. (2002) observed in roots of Oryza sativa treated with Pb that the presence of competing cations such as Ca could change the amount of Pb absorbed from the roots. The highest concentration of Ca in the roots when subjected to 32 mg Pb l−1 compared to plants in 16 mg Pb l−1 may be a strategy of the plant to try to reduce the phytotoxicity and increase the plant's tolerance to the metal. Antosiewicz (1995) noted that in populations of Silene, Pb tolerance was associated with high concentrations of Ca and high tolerance to Ca deficit. Malkowski et al. (2002) also observed an increase in Ca concentration with increasing NS with Pb concentrations with Zea mays seedlings and linked this increase to the constitutional tolerance of the species to Pb. According to Antosiewicz (1995), Ca regulates plant tolerance to Pb because this ion is involved in the immobilization of Pb in the cell wall, being an important factor in decreasing the toxicity of this metal. But, this is an additional factor in the regulation of Pb toxicity and is not a common regulating mechanism. The results for Mg showed that the addition of Pb to the NS reduced the concentration of Mg in shoots and roots. Kim et al. (2002) proposed that toxic metals can enter cells through root tissue Mg2+ transporters, and can therefore compete for the same carriers. The higher concentration of S in roots subjected to 32 mg Pb l−1 compared to 16 mg Pb l−1, as well as increasing the concentration of this element in shoots of plants under higher concentration of Pb, can be related to the detoxification process of Pb, since S is a glutathione precursor which, in turn, is a precursor of phytochelatins, which function in the detoxification of toxic metals (Kerbauy 2004). Therefore, Pb induces the biosynthesis of phytochelatins (Grill et al. 1987). According to Piechalak et al. (2002), plants of Vicia faba, Pisum sativum and Phaseolus vulgaris treated with Pb in NS were induced to synthesize glutathione. Metal ions are accumulated and detoxified by phytochelatins, which are synthesized from glutathione during their exposure to toxic metals (Yadav 2010). According to Nascimento and Xing (2006) compartmentalization in vacuoles and in the cytoplasm and chelation are among the most significant proposed mechanisms related to accumulation of metals in plants. Furthermore, phytochelatins are associated with precipitation of intracellular and extracellular Pb as phosphates, carbonates and sulfates (Salt et al. 1998). According to Zheng et al. (2012) S may favour the sequestration of Pb in the form of PbSO4. The decrease in the concentration of Zn in roots and shoots treated with Pb, is consistent with the results found by Kibria et al. (2009), for A. gangeticus where Zn decreased with increasing concentration of Pb in the shoots and roots. With respect to Mn, the reduction in the concentration of this element in roots is in agreement with the results found by Sinha et al. (2006) in Brassica oleracea and by Kibria et al. (2009) in roots of A. oleracea and A. gangeticus. This reduction in the concentration of Mn as a function of increasing concentrations of Pb in NS, demonstrates the existence of antagonism between these two elements (Kabata-Pendias and Pendias 2000). Iron concentrations in shoots decreased with increasing Pb concentrations in NS. Similar results were found by Huang and Cuningham (1996) in Zea mays. The root already showed an increase in the concentration of Fe up to 16 mg Pb l−1, later decreasing by 32 mg Pb l−1. This reduction in the concentration of Fe in roots exposed to higher concentrations of Pb in solution is probably due to its blocking the entry of cations in the plant's root system (Sharma and Dubey 2005). In studies conducted by Sinha et al. (2006), a decrease in the Fe concentration was found in B. Oleracea roots.
Conclusion
In summary, the increase in thickness of exodermis in roots, forming plastoglobules in chloroplasts and changes in the concentration of mineral nutrients, especially P, S and Ca may be considered important strategies developed in O. cubense plants to reduce the toxic effect of Pb. Thus, the species can be characterized as tolerant to the concentrations of Pb evaluated. In addition, Pb is compartmentalized and accumulated primarily in the roots. Therefore, it can be considered a species with phytoremediation capacity for Pb, with potential rizofiltration of this metallic element in contaminated watersheds. To better understand the influence of Pb on cellular metabolism, further research into molecular analysis and oxidative stress will need to be conducted in the future.
References
Almeida AAF, Valle RR, Mielke MS, Gomes FP (2007) Tolerance and prospection of phytoremediator woody species of Cd, Pb, Cu and Cr. Braz J Plant Physiol 19:83–98
Almeida AAF, Mielke MS, Gomes FP, Gomes LMC, Mangabeira PAO, Valle RR (2011) Phytoremediation of Cd, Pb and Cr by woody plants. In: Golubev IA (ed) Handbook of phytoremediation. Nova Science Publishers, New York, pp 529–550
Antosiewicz DM (1995) The relationships between constitutional and inducible Pb-tolerance and tolerance to mineral deficits in Biscutelia laevigata and Silene inflate. Environ Exp Bot 35:55–69
Austin JR, Frost E, Vidi PA, Kessler F, Staehelin LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contains biosynthetic enzymes. Plant Cell 18:1693–1703
Bréhélin C, Kessler F (2008) The plastoglobule: a bag full of lipid biochemistry tricks. Photochem Photobiol 84:1388–1394
Breusegem FV, Vranová E, Dat JF, Inzé D (2001) Review: the role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Coutinho HD, Barbosa AR (2007) Fitorremediação: Considerações Gerais e Características de Utilização. Silva Lusitana 15:103–117
Deng H, Ye ZH, Wong MH (2009) Lead, zinc and iron (Fe2+) tolerances in wetland plants and relation to root anatomy and spatial pattern of ROL. Environ Exp Bot 65:353–362
Eltrop L, Brown G, Joachim O, Brinkman NK (1991) Lead tolerance of Betula and Salix in the mining area of Mechernich/Germany. Plant Soil 131:275–285
Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to the environment. Plant Growth Regul 21:335–351
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3:1–18
Gomes MP, Marques TCLLSM, Nogueira MOG, Castro EM, Soares AM (2011) Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci Agric 68:566–573
Gopal R, Rizvi AH (2008) Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 70:1539–1544
Grill E, Winnacker EL, Zenk MH (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci 84:439–443
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. California Agricultural Experiment Station, Berkeley, p 32
Hu JZ, Shi GX, Xu QS, Wang X, Yuan QH, Du KH (2007) Effects of Pb2+ on the active oxygen-scavenging enzyme activities and ultrastructure in Potamogeton crispus leaves. J Plant Physiol 54:414–419
Huang JW, Cunninghan SD (1996) Lead phytoextraction: species variation in lead uptake and translocation. New Phytol 134:75–84
Jones LHP, Clement CR, Hopper MJ (1973) Lead uptake from solution by perennial ryegrass and its transport from roots to shoots. Plant Soil 38:403–414
Kabata-Pendias A, Pendias H (2000) Trace elements in soils and plants. CRC Press, Boca Raton
Kerbauy GB (2004) Fisiologia vegetal. Guanabara Koogan, Rio de Janeiro
Kessler F, Schnell D, Blobel G (1999) Identification of proteins associated with plastoglobules isolated from pea (Pisum sativum L.) chloroplasts. Planta 208:107–113
Kibria MG, Islam M, Osman KT (2009) Effects of lead on growth and mineral nutrition of Amaranthus gangeticus L. and Amaranthus oleracea L. Soil Environ 28:1–6
Kim YY, Yang YY, Lee Y (2002) Pb and Cd uptake in rice roots. Physiol Plant 116:368–372
Kosobrukhov A, Knyazeva I, Mudrik V (2004) Plantago major plants responses to increase content of lead in soil: growth and photosynthesis. Plant Growth Regul 42:145–151
Krzeslowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33:35–51
Leite KRB, França F, Scatena VL (2009) Anatomia de espécies anfíbias de Cyperaceae de lagoas do semi-árido, BA, Brasil. Acta Bot Bras 23:786–796
Malkowski E, Kita A, Galas W, Karcz W, Kuperberg JM (2002) Lead distribution in corn seedlings (Zea mays L.) and its effect on growth and the concentrations of potassium and calcium. Plant Growth Regul 37:69–76
Meuleman AFM, Beltman B, Schefer RA (2004) Water pollution control by aquatic vegetation of treatment wetlands. Wetl Ecol Manag 12:459–471
Moraes CL (2011) Alterações bioquímicas, fisiológicas e ultraestruturais em sementes e plantas de tomate expostas ao chumbo. Dissertation, Universidade Federal de Pelotas
Nascimento CWA, Xing B (2006) Phytoextraction: a review on enhanced metal availability and plant accumulation. Sci Agric 63:299–311
Neves EL, Leite KRB, França F, Melo E (2006) Plantas aquáticas vasculares em uma lagoa de planície costeira no município de Candeias, Bahia. Sitientibus 6:24–29
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Piechalak A, Tomaszewska B, Baralkiewicz D, Malecka A (2002) Accumulation and detoxification of lead ions in legumes. Phytochem 60:53–162
Pott VJ, Pott A (2000) Plantas aquáticas do pantanal. Embrapa, Brasília
Ranathunge K, Schreiber L, Franke R (2011) Suberin research in the genomics era—new interest for an old polymer. Plant Sci 180:399–413
Rascio N, Vecchia FD, Ferritti M, Merlo L, Ghisi R (1993) Some effects of cadmium on maize plants. Arch Environ Contam Toxicol 25:244–249
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Samardakiewicz S, Wozny A (2000) The distribution of lead in duckweed (Lemna minor L.) root tip. Plant Soil 226:107–111
Santana KB, Almeida AAF, Souza VL, Mangabeira PAO, Silva DC, Gomes FP, Dutruch L, Loguercio LL (2012) Physiological analyses of Genipa americana L. reveals a tree with ability as phytostabilizer and rhizofilterer of chromium ions for phytoremediation of polluted watersheds. Environ Exp Bot 80:35–42
Santos MC, Lenzi E (2000) The use of aquatic macrophytes (Eichhornia crassipes) as a biological filter in the treatment of lead contaminated effluents. Environ Technol 21:615–622
Seregin IV, Ivanov VB (2001) Physiological aspects of cadmium and lead toxic effects on higher plants. Russian J Plant Physiol 48:523–544
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Shen Z-G, Li X-D, Wang C-C, Chen H-M, Chua H (2002) Lead phytoextraction from contaminated soil with high-biomass plant species. J Environ Qual 31:1893–1900
Sinha P, Dube BK, Srivastava P, Chatterjee C (2006) Alteration in uptake and translocation of essential nutrients in cabbage by excess lead. Chemosphere 65:651–656
Souza VL, Almeida AAF, Lima SGC, Cascardo JCM, Silva DC, Mangabeira PAO, Gomes FP (2011) Morphophysiological responses and programmed cell death induced by cadmium in Genipa americana L. (Rubiaceae). BioMetals 24:59–71
Tung G, Temple PJ (1996) Histochemical detection of lead in plant tissues. Environ Toxicol Chem 15:906–914
Tur NM (1971) Nuevos casos de epifitismo acuático. Bol Soc Arg Bot 13:243–249
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Walker WM, Miller JE, Hassett JJ (1977) Effect of lead and cadmium upon the calcium, magnesium, potassium, and phosphorus concentration in young corn plants. Soil Sci 124:145–151
Weryszko-Chmielewska E, Chwill M (2005) Lead-induced histological and ultrastructural changes in the leaves of soybean (Glycine max (L.) Merr.). Soil Sci Plant Nutr 51:203–212
Yadav SK (2010) Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African J Bot 76:167–179
Ytterberg AJ, Peltier JB, Wijkk JV (2006) Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol 140:984–997
Zhao S, Ye X, Zheng J (2011) Lead-induced changes in plant morphology, cell ultrastructure, growth and yields of tomato. J Biotechnol 10:10116–10124
Zheng L, Peer T, Seybold V, Lutz-Meindl U (2012) Pb-induced ultrastructural alterations and subcellular localization of Pb in two species of Lespedeza by TEM-coupled electron energy loss spectroscopy. Environ Exp Bot 77:196–206
Zimmermann P, Zentgraf U (2005) The correlation between oxidative stress and leaf senescence during plant development. Cell Mol Biol Lett 10:515–534
Acknowledgments
The authors wish to acknowledge Dr. Douglas Rodrigues Miquita and Dra Elisabeth Ribeiro da Silva for analysis in transmission electron microscope FEI-TECNAI-G20 in the Electron Microscopy Center at Universidade Federal de Minas Gerais (UFMG). This research was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Alves, L.Q., de Jesus, R.M., de Almeida, AA.F. et al. Effects of lead on anatomy, ultrastructure and concentration of nutrients in plants Oxycaryum cubense (Poep. & Kunth) Palla: a species with phytoremediator potential in contaminated watersheds. Environ Sci Pollut Res 21, 6558–6570 (2014). https://doi.org/10.1007/s11356-014-2549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2549-9