Abstract
Benthic diatom assemblages on the natural substrata were investigated at 21 sites of the Ganhe River watershed (China) once per season and in addition, early spring in 2013. A total of 487 diatom taxa from 36 genera were identified during five investigations. The assemblages were dominated by Achnanthidium minutissimum (Kützing) Czarnecki and Cocconeis placentula in the rural reach, whereas Navicula, Nitzschia, and Gomphonema species were characteristic of urbanized sites. Our results suggest that biodiversity was positively related to high nutrient levels and strongly negatively related to diatom-based indices. The periphyton biomass (expressed as chlorophyll a and ash-free dry mass) was not related to water quality. Canonical correspondence analysis (CCA) showed that the nutrient concentration gradient was the most important factor that affected the diatom assemblage composition and species distribution. The diatom-based indices (specific pollution sensitivity index (IPS), biological diatom index (IBD), and trophic diatom index (TDI)) were significantly positively correlated with water quality and are adequate for use in China. Slight changes in the biodiversity and diatom-based indices followed a temporal pattern. The species composition was less related to the season or hydrological characteristics of the river but more strongly related to differences in the trophic status. In this region, urbanization masked the impact of rural land use on benthic diatoms. The research will expand the understanding of using benthic diatom assemblages for water quality monitoring in urban streams and improve watershed-scale management and conservation efforts in the Ganhe River, China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms are a large and diverse group of unicellular algae, which are distributed throughout the world in most types of aquatic systems (Potapova and Charles 2002; Smucker and Vis 2011). They are very sensitive to environmental change, and their variation is expressed spatially and temporally (Urrea and Sabater 2009). Their composition and distribution may be influenced by a number of factors which includes geological, hydrological, climatic, physicochemical, and biological factors, such as nutrient concentration (Cardinale 2011), organic contamination (Kwandrans et al. 1998), electrical conductivity (Leira and Sabater 2005; Rimet 2009), pH (Charles 1985), etc. Water pollution caused by nutrients and organic contamination and subsequent environmental impacts are common problems in rivers throughout the world. Therefore, it is necessary to develop effective tools for monitoring rivers. Hambrook Berkman and Canova (2007) used algae biomass to assess water quality in lotic and lentic ecosystems. However, the response of algae biomass to water quality is not always predictable. Changes in biomass are not related to nutrients, which are not limiting factors for algae growth (Montuelle et al. 2010). Hill and Fanta (2008) showed that periphyton growth can be co-limited by phosphorus and irradiance in streams. Stephens et al. (2012) hypothesized that the biomass will increase as nutrients are added, unless it is limited by light. Like algae biomass, the use of algal biodiversity as an indicator of river pollution gave ambiguous results (Ndiritu et al. 2006; Blanco et al. 2012).
In contrast to algae biomass and biodiversity diatom, biomonitoring using diatom indices which use sensitivity and tolerance of diatoms to environmental degradation was useful and robust for pollution assessment (Rimet 2012). Diatoms are used widely as a biological monitoring tool which has been used to assess river pollution for 60 years (Rimet 2012). At least 18 diatom-based indices have been developed and used to assess aquatic environments (Blanco and Becares 2010). The specific pollution sensitivity index (IPS, Coste 1982) and biological diatom index (IBD, Lenoir and Coste 1996) are widely used in France, and IPS which incorporated the largest number of species among all diatom indices has usually been considered as the reference index to evaluate the applicability of other new diatom indices (Descy and Coste 1991). Trophic diatom index (TDI, Kelly and Whitton 1995) was developed in Great Britain for assessing the trophic status of river.
Aquatic ecosystems are faced with many pollution problems due to urbanization and rural development. Recently, studies have focused on the effects of human disturbance on diatom communities, particularly urbanization and rural land use (Newall and Walsh 2005; Urrea and Sabater 2009). However, very little is known about river systems in Central China where the Ganhe River flows through a rural basin and on to an urban area. In this study, we investigated the distribution of benthic diatom assemblages and their relationship with environmental factors based on spatial and temporal gradients and further explored three diatom-based indices to assess the water quality in the Ganhe River. Our major goals were (1) to analyze the relationships between diatom assemblages and environmental variables; (2) to identify suitable indicator species of river pollution; (3) to investigate whether the periphyton biomass and biodiversity correlated with the nutrient level or water pollution; and (4) to validate the applicability of diatom-base indices for water quality assessment in the Ganhe River of China.
Material and methods
Study area
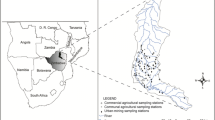
This research was conducted in the Ganhe River watershed, which goes through Xianning City, Hubei Province, which is located in Central China (113° 32′–114° 58′ E, 29° 02′–30° 19′ N) where the climate is subtropical. February, April, July, October, and December correspond to early spring, spring, summer, autumn, and winter, respectively. Low winter temperatures persist for almost three months. Rainfall is rare and the water transparency is the highest in winter. The annual average mean temperature and rainfall are approximately 17 °C and 1500 mm, respectively. Intense rainfall events and floods occur during the rainy season (April-July). The Ganhe River (Fig. 1) is 76.6 km long and the basin area occupies 854 km2. It is divided into two main catchment areas: five tributaries (Huangshui stream, Mingshui stream, Nanchuan stream, Bodun stream, and Longtan stream) are situated in the upstream region (S1–S15), which typically consists of hills (altitude from 88 to 211 m) and rural regions (agricultural area percentage is about 30 %), whereas the downstream region (S16–S21) is a flatland and urbanized area (altitude from 21 to 44 m), which is heavily industrialized and densely populated. Compared to the upstream region, flat terrain of the downstream region resulted in its slower water flow velocity in general. The riverbed matrix in the upstream was rocky whereas depositional habitats with rare rocks were found in the downstream.
Sample collection, preparation, and identification
Twenty-one sampling sites were selected for this study (Fig. 1). Periphyton samples were collected in triplicate from each site during February, April, July, October, and December 2013. To reduce the user error associated with potentially dissimilar estimates made by different people, because their subjectivity may affect their visual estimation of habitats (Smucker and Vis 2010), 3–5 pieces of rocks per sample were collected haphazardly along a zigzag pattern from each transect at the right, left, and center of the transect (Fore and Grafe 2002), thereby yielding a total of 9–15 pieces of rocks. We collected samples from the bottom sediment in these sites (S19 in Feb, S21 in Dec) because rocks were absent. Triplicate sediment samples were collected from areas of low flow by inverted petri dish along the riverbed to collect the surface material. To minimize the effects of light availability on benthic diatom (Hill et al. 1995), the selected sites had a canopy cover of 0–1.0 % and are saturated by light.
Each rock was fitted with a plastic O-ring. The area within the O-ring was scrubbed using a firm toothbrush and rinsed with stream water from near each rock. Small cores (surface area = 8 cm2) were taken from the surface of sediment samples to collect algae using modified 60 mL plastic syringes. Each of the three suspensions of epilithon and the three suspensions of epipelon from each sampling site was collected in a plastic bottle and transported to the laboratory inside a darkened ice box.
During sampling, the water temperature, pH (portable pH meter, PHB-4, China), dissolved oxygen (portable dissolved oxygen meter, JPB-607, China), and conductivity (portable conductivity meter, DDB-303A, China) were measured in the field, and water samples were collected from each location to measure the total nitrogen (TN), total phosphorus (TP), and chemical oxygen demand (COD) in the laboratory, according to APHA (1998).
Each suspension was divided into three portions in the laboratory: one was fixed in 4 % formaldehyde for diatom identification, the second was extracted in 90 % acetone for chlorophyll a analysis according to standard procedures (APHA 1998), while the third was processed to determine the ash-free dry mass based on APHA methods (1998).
One milliliter of captured diatoms were cleaned with a mixture of concentrated sulphuric and nitric acids and 40 μL of the cleaned diatom valves were embedded in synthetic resin as described previously by Poulı’cˇkova’ and Mann (2006). Diatoms were identified and enumerated using microscope fitted with a 100× oil immersion objective (numerical aperture, 1.25). A minimum of 500 valves per sample were counted unless the diatoms were sparse, and diatom identification was carried out according to Prygiel and Coste (2000), Krammer and Lange-Bertalot (1991–1997), Krammer (2002), and Zhu and Chen (2000). Samples with less than 200 valve counts were removed from the analysis. Thus, diatom count information of 12 samples (S6, S12 in Feb, S7, S10 in Apr, S2, S4, S10, S13, S19 in Jul, and S4, S14, S15 in Oct) was not included in the results.
Data analysis
To determine the relationship between the diatom assemblages and the environment gradient, a canonical correspondence analysis (CCA) was performed using CANOCO version 4.5 (Ter Braak and Smilauer 2002). Monte Carlo permutation tests (999 permutations, P < 0.01) were performed on each CCA. We selected the dominant species for the analysis if their relative abundance was >1.0 % in at least two sites. The environmental variables (except pH) and species data were log10 (x + 1) transformed to minimize the effects of extreme values as necessary.
Pearson’s correlation analysis was used to test the relationships between diatom-based indices and environmental factors. Sites were compared using a repeated measure analysis of variance (ANOVA) model to account for differences in the spatial and temporal gradients. A log transformation was applied to the diatom indices when necessary to ensure normality and the homogeneity of variances. All of the statistical analyses were performed using SPSS v.17.0 (SPSS Inc. 2008). The relationship between the concentration of chlorophyll a and total phosphorus was assessed using a simple linear regression. Pielou evenness (Pielou 1966), species richness (Sample contains the total number of species), and Shannon diversity (Magurran 2004) were calculated based on species composition. Diatom-based indices, i.e., the trophic diatom index (TDI: Kelly and Whitton 1995), the specific polluosensitivity index (IPS: Coste 1982), and the biological diatom index (IBD: Lenoir and Coste 1996), were calculated using OMNIDIA 5.3 (Lecointe et al. 1993). To facilitate comparisons, they were all transformed so they varied from 0 to 20.
Results
Aquatic environmental factors
The annual mean values for the aquatic environmental factors are shown in Table 1. The sampling sites were in the Ganhe River along seasonal and rural/urbanized gradients. The annual mean values of the water temperature (WT), electrical conductivity (EC), COD, TP, and TN at the urbanized sites (S16 to S21) were higher than those at the rural sites (S1 to S15), whereas the water pH and dissolved oxygen (DO) were lower at the urbanized sites. In general, the water was slightly alkaline and the pH varied from 7.3 to 9.4. The water temperature, electrical conductivity, and dissolved oxygen varied from 8.7 to 25.7 °C, 154 to 467 μs/cm, and from 6.5 to 19.8 mg/L, respectively. The mean EC and DO were the lowest in summer, whereas the COD and TP were the lowest in winter, indicating seasonal variation. The concentration of AFDM (0.75–88.79 g/m2) was lowest in summer, and it ranged from 0.75 to 12.28 g/m2 (median = 5.24; 25th, 75th percentile = 3.69, 7.49). The annual TP content ranged from 0.002 to 0.253 mg/L and was lower in winter compared with other seasons. The concentration of TN (0.147–5.104 mg/L) was higher in spring (median = 1.171; 25th, 75th percentile = 0.874, 1.613) and the lowest during October (median = 0.604; 25th, 75th percentile = 0.491, 0.822).
Diatom assemblages in the Ganhe River basin
A total of 487 diatom taxa (including variations) from 36 genera were identified in Ganhe River. The diatom assemblage distribution and composition differed between months (seasons) in rural/urbanized regions (Fig. 2). We divided sampling time into the following: early spring (February), spring (April), summer (July), autumn (October), and winter (December). On the spatial scale, the species such as Achnanthidium minutissimum, Cocconeis placentula, and Cymbella affinis were persistently dominant species at rural sites (S1–S15). However, Eolimna minima (Grunow) Lange-Bertalot, species such as from the genera Navicula and Nitzschia predominated at urbanized sites (S16–S21). The most common species was C. placentula, which was present at all sites in each season. C. affinis was also a common taxon from S1–S15, and it was dominant at S1, S3, and S13. There were clear differences between rural and urban areas, i.e., A. minutissimum was the most abundant species at many rural sites whereas it was rare or absent at urbanized sites throughout the year. Its maximum relative abundance reached 85.5 % at S8 during early spring. On a temporal scale, there were few changes in the dominant species at rural sites. In winter, C. placentula had the highest mean relative abundances whereas A. minutissimum had the lowest mean value. However, the major seasonal variations in diatom assemblages were most apparent at urban sites (S16–S21), i.e., Navicula trivialis and Nitzschia palea were dominant during the early spring decreasing shortly after; Luticola goeppertiana (Bleisch in Rabenhorst) D. G. Mann, Gomphonema olivaceum, and Gomphonema parvulum increased in spring. Eolimna minima was the predominant species in most seasons (except spring). Nitzschia frustulum and G. parvulum were the second most abundant species at these sites in autumn and winter, respectively.
The mean relative abundance of the predominant species in rural/urbanized regions. The relative abundance of the predominant species was >5 % at two sites or >10 % at one site. The species codes and their corresponding full names are given in the Appendix
Environmental factors and diatom assemblages
The predominant species (relative abundance >5 % at two sites or >10 % at one site) were used in the CCA analysis (Fig. 3). During early spring, the first two CCA axes combined explained 78.8 % of the species data, and the species-environmental correlations were 0.986 and 0.914, respectively. The first CCA axis was positively correlated with TP (r = 0.98), TN (r = 0.96), and EC (r = 0.82), while the second axis was correlated with pH (r = 0.50) and DO (r = 0.39). Similarly, the first CCA axis was strongly correlated with TP (r = 0.93, 0.77, 0.84, 0.93), TN (r = 0.94, 0.39, 0.69, 0.89), and EC (r = 0.87, 0.08, 0.79, 0.73) in all seasons. pH (r = 0.60, 0.71) and DO (r = 0.45, 0.64) were correlated with the second axis in spring and winter, but they were usually negatively correlated with the first axis in summer and October. Water temperature was positively correlated with the first axis in all CCA analyses. In general, two main gradients appeared in all CCAs: the first axis represented a nutrient or urbanization gradient and the second axis represented an alkalinity gradient. E. minima and Navicula, Nitzschia, and Gomphonema species were often abundant at sites where the urbanized areas had higher nutrient levels. On the other gradient, Achnanthidium, Coccoenis, and Cymbella were associated with sites in the rural basin.
The relationships between benthic diatom communities and environmental factors in the CCA ordination diagrams. The codes of the diatoms and their corresponding full names are given in the Appendix
Richness, evenness, and diversity
The benthic diatom species richness and Pielou evenness ranged from 18 to 83 and from 0.24 to 0.8, respectively. Shannon diversity index (log base = e) varied from 0.75 to 3.37 (Fig. 4). The maximum values were recorded at site S16. The results indicated a noticeable difference in the spatial gradient (P < 0.01), but there was no significant difference in the temporal pattern (P = 0.436). The biodiversity increased in the urbanized areas with higher nutrient levels (TP and TN).
Diatom-based indices and water quality assessment
The IBD, IPS, and TDI indices were clearly different in the urbanized sites and the rural sites, and they decreased from 19.9 to 4.3 (median = 16.3), 18.7 to 3.3 (median = 16.6), and from 18.8 to 4.8 (median = 14.3), respectively (Fig. 5). These diatom-based indices exhibited a similar pattern of seasonal variation. They had significant correlations with some environmental factors (Table 2). The IBD, IPS, and TDI values decreased with higher electrical conductivity (r = −0.612, −0.660, and −0.572, respectively; P < 0.001), total phosphorus (r = −0.831, −0.826, and −0.635, respectively; P < 0.001), and total nitrogen (r = −0.720, −0.756 and, −0.651, respectively; P < 0.001), whereas they increased with greater pH (r = 0.302, 0.340, and 0.270, respectively; P < 0.05) and dissolved oxygen (r = 0.218, 0.259 and 0.284, respectively; P < 0.05). The mean IBD values were the lowest (mean = 14.0) during early spring and the highest (mean = 15.9) in winter. By contrast, the mean TDI index maximum value occurred in early spring (mean = 14.7) whereas its minimum (mean = 11.0) was in winter. The IBD (P = 0.612) and IPS (P = 0.985) indices were not significantly different among seasons, whereas the TDI index differed significantly (P < 0.05) according to the ANOVA.
Discussion
Factors that affect the composition and distribution of benthic diatoms are complex which includes geological, hydrological, climatic, physicochemical, and biological factors. The upstream region (S1–S15) of the Ganhe basin typically consists of hills with high altitude (altitude from 88 to 211 m) which results in faster drop and higher flow velocity, whereas the downstream region (S16–S21) of the Ganhe basin is a flatland (altitude from 21 to 44 m) which results in its lower current velocity in general. Eutrophication of water bodies must have three conditions, including nutrients, slow current velocity, and suitable temperature, illumination conditions (Lei et al. 2010). Therefore, the downstream of the river eutrophication are the comprehensive results of multiple factors, and nutrition input is one of the important conditions. The specie Achnanthidium, which dominated in rural upstream areas with higher flow velocity, characterized the good water quality (Kwandrans et al. 1998; Fore and Grafe 2002). By contrast, the species (e.g., N. palea, E. minima) that dominated in urbanized downstream with lower flow velocity areas were indicators of bad water quality (Duong et al. 2006; Bere and Tundisi 2011; Raven 1998).
All of the CCAs showed that the TP, TN, and EC arrows pointed in the same direction along the first ordination axis. The conductivity mainly indicated the concentrations of ions, which is consistent with nutrient enrichment (Leira and Sabater 2005). This suggests that the first axis corresponded to a nutrient gradient, particularly TP, which was the principal nutrient gradient. The best indicator species for high nutrient levels or pollution were as follows: N. palea, N. trivialis, and L goeppertiana in spring; E. minima and Gomphonema angustum in summer; E. minima and N. frustulum in autumn; and E. minima in winter. These results confirmed that N. palea is cosmopolitan and a pollution-tolerant species, and it was dominant in polluted rivers in Spain (Urrea and Sabater 2009), Kenya (Ndiritu et al. 2006), Vietnam (Duong et al. 2006), and Brazil (Bere and Tundisi 2011). E. minima, a small cell-sized diatom, can adapt to different conditions (Raven 1998), and it was the dominant taxon at polluted sites after the summer due to harsh conditions such as flooding and excess light. Another major gradient emerged in the CCAs where the second axis represented the pH and DO gradients. The pH has a major effect on diatom assemblage composition (Verb and Vis 2000; Smucker and Vis 2011). C. placentula and C. affinis are alkaliphilous taxa and they prefer alkaline water (Urrea and Sabater 2009), which may explain this effect. The pH is regulated by alkaline metals, and it may be related to local agricultural activities. Some studies have shown that agriculturally impacted rivers have a high pH due to animal waste, liming, erosion, and fertilizers (Johnson et al. 1997; Carpenter and Waite 2000). However, this gradient was disrupted by anthropogenic nutrient stressors when the stream flowed into the urban area.
Algal growth and biomass is widely used to evaluate the trophic status of aquatic ecosystems. A high algae biomass can indicate eutrophication, but it can also accumulate in less productive habitats after long periods of stable flow (Stevenson and Bahls 1999). We found that total phosphorus (TP) explained only 5.07 % of the variability and total nitrogen (TN) only 4.62 % of the variability in benthic algae Chl a (Fig. 6). High or nuisance levels (Chl a >10 μg/cm2, Biggs 1996) of periphyton biomass were also present at sites with low nutrient levels in the Ganhe River. Nutrients, particularly phosphate, have important effects on algal growth (Pan et al. 1999; Camargo et al. 2005), but they are unlikely to be a factor that limits algal growth (Kelly and Wilson 2004; Wu et al. 2007). Our results showed that the concentration of periphyton Chl a was not related to the nutrient levels (TP and TN). There is good evidence that nutrient concentrations are poor predictors of Chl a (Figueroa-Nieves et al. 2006), which has been shown by other researchers (Munn et al. 1989). However, others have shown the opposite effect (LaPerriere et al. 1989). O’Brien and Wehr (2009) observed that the periphyton biomass was significantly greater in urbanized locations with a higher phosphate level. The effects of light availability on algal growth throughout the water column were not monitored, and we support the view that multiple factors may control the biomass of algae. Thus, the interaction between hydrology and light probably controls the periphyton biomass in nutrient-rich, agricultural streams (Figueroa-Nieves et al. 2006). Therefore, we suggest that using the biomass of periphyton as a parameter to assess the water quality in streams is not suitable because it can produce ambiguous results. The biomass was the lowest in July, and there were almost no filaments in a visual observation. This low biomass may have been the result of a recent storm event followed by spate. It may be related to the hydrological characteristics of the Ganhe River, which experiences a wet season in summer (Yang et al. 2009).
Biodiversity indices (e.g., diversity, species richness, and evenness) are not always predictable in response to environmental conditions (Ndiritu et al. 2006). We found that the diversity was higher in the urbanized area with an intensity of anthropogenic disturbance, with lower values in the upper reaches of the lower trophic level. The community composition exhibited a distinct change of species with the environmental gradients in the Ganhe River. The communities with a relatively low biodiversity in good water quality were dominated by only a few species of such as C. placentula and A. minutissimum.
C. placentula was the predominant species in weak alkaline streams in Italy (Bona et al. 2007) and Spain (Urrea and Sabater 2009). Similarly, it was present at all sites of the Ganhe River that were characterized by alkaline water in all seasons. A. minutissimum is known for its rapid colonization of new habitats because of its short generation time and high immigration rate, and it was prevalent and abundant on substrates due to its short mucilaginous stalks (Round 1990; Jöbgen et al. 2004). A. minutissimum is highly sensitive to organic pollution (Kwandrans et al. 1998), and it is regarded as a good indicator of disturbances (Fore and Grafe 2002). It dominated in the rural sites, as it does in similar areas elsewhere (Zampella et al. 2007; Urrea and Sabater 2009; Pei and Liu 2011).
These diatoms appeared to have many advantages that helped them to live in the environmental conditions present in the upstream sites of the Ganhe River. Their interspecific competitive advantages may be reflected in oligotrophic water bodies. Interspecies competition is not common in nutrient-rich water bodies so more species can coexist in the same environment. This may explain why the biodiversity was higher in the urbanized section of the Ganhe River. However, the river had an appreciable self-purification capacity (Mbuligwe and Kaseva 2005). Biodiversity may help to buffer natural ecosystems against the ecological impacts of nutrient pollution (Cardinale 2011). A higher biodiversity may be a manifestation of river self-purification and a result of long-term interactions between species and environmental factors in polluted rivers.
The diatom indices (IPS, IBD, and TDI) show different water quality classes when comparing upstream and downstream, and the 3 diatom indices tested showed similar results for a given site/time. The diatom indices (IPS, IBD, and TDI) indices were all relatively low in the urban sites, which reflected poor to bad (or meso-eutrophy to eutrophy) water quality. In contrast, the diatom indices from the sites of the rural area revealed high values which represent excellent, good to moderate (or oligotrophy, oligo-mesotrophy to mesotrophy) water quality. The diatom indices indicated differences in the water quality based on the relative abundances of indicators of trophic or saprobic stages and different types of diatom communities (Kwandrans et al. 1998). These indices had similar responses to environmental factors and were significantly negative correlated with the trophic level (TP and TN concentrations). These significant correlations indicated that the water pollution was mainly caused by nutrients. The IPS, IBD, and TDI were developed to assess the nutrient concentrations or eutrophication (Kelly and Whitton 1995), the pollution level (Coste 1982), and the general water quality (Lenoir and Coste 1996), respectively. The dominant diatoms at urban sites were nutrient-tolerant and pollution-tolerant taxa (e.g., N. palea, E. minima) in the Ganhe River basin (Duong et al. 2006; Bere and Tundisi 2011; Raven 1998). The diatom (Achnanthidium minutissimum) that dominated at the rural region was less polluted in the Ganhe River (Kwandrans et al. 1998; Fore and Grafe 2002). However, the most important factors that affected the distribution of diatom assemblages were the nutrient concentrations according to the CCA analysis of the Ganhe River. These diatom indices were also strongly negatively correlated with biodiversity (Table 2). Thus, we suggested that the pollution was due to excessive nutrient input into the Ganhe River.
Our investigations suggested that the diatom indices (IPS, IBD, and TDI) should be able to differentiate sites of various degrees of contamination and therefore seem adequate to use in China. This suggested that the diatom indices, especially IPS and IBD, should be suitable for monitoring rivers. The TDI index detected differences that may be attributed to A. minutissmum, which is considered to be a good water quality indicator in TDI (Rimet et al. 2005), although it was only distributed at urban sites and it had a relatively low abundance in winter. There were 96 % of taxa using for the calculation of the indices in our study. Xiang Tan et al. (2013) demonstrated the applicability of diatom indices for water quality and ecohealth assessment in subtropical rivers. These diatom indices produced good results in the water quality evaluation based on the spatial and temporal patterns, but this survey design only studied one river system for 1 year. Thus, further long-term research is necessary to determine whether these trends are seasonal cycles and whether the diatom assemblages exhibit seasonal species succession.
References
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Organisation, Washington
Bere T, Tundisi J (2011) Influence of land-use patterns on benthic diatom communities and water quality in the tropical Monjolinho hydrological basin, São Carlos-SP, Brazil. Water SA 37(1):93–102
Biggs B (1996) Patterns in benthic algae of streams. In: Stevenson JR, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 31–56
Blanco S, Becares E (2010) Are biotic indices sensitive to river toxicants? A comparison of metrics based on diatoms and macro-invertebrates. Chemosphere 79(1):18–25
Blanco S, Cejudo-Figueiras C, Tudesque L, Bécares E, Hoffmann L, Ector L (2012) Are diatom diversity indices reliable monitoring metrics? Hydrobiologia 695(1):199–206
Bona F, Falasco E, Fassina S, Griselli B, Badino G (2007) Characterization of diatom assemblages in mid-altitude streams of NW Italy. Hydrobiologia 583(1):265–274
Camargo JA, Alonso A, Puente M (2005) Eutrophication downstream from small reservoirs in mountain rivers of Central Spain. Water Res 39(14):3376–3384
Cardinale BJ (2011) Biodiversity improves water quality through niche partitioning. Nature 472(7341):86–89
Carpenter KD, Waite IR (2000) Relations of habitat-specific algal assemblages to land use and water chemistry in the Willamette Basin Oregon. Environ Monit Assess 64(1):247–257
Charles DF (1985) Relationships between surface sediment diatom assemblages and lakewater characteristics in Adirondack lakes. Ecology 66(3):994–1011
Coste M (1982) Étude des méthodes biologiques d’appréciation quantitative de la qualité des eaux. Rapport QE Lyon AF Bassin Rhône-Méditéranée-Corse CEMAGREF
Descy JP, Coste M (1991) A test of methods for assessing water quality based on diatoms. Verh Int Ver Limnol 24:2112–2116
Duong TT, Coste M, Feurtet-Mazel A et al (2006) Impact of urban pollution from the hanoi area on benthic diatom communities collected from the Red Nhue and Tolich Rivers (Vietnam). Hydrobiologia 563(1):201–216
Figueroa-Nieves D, Royer TV, David MB (2006) Controls on chlorophyll-a in nutrient-rich agricultural streams in Illinois USA. Hydrobiologia 568(1):287–298
Fore LS, Grafe C (2002) Using diatoms to assess the biological condition of large rivers in Idaho (U.S.A.). Freshw Biol 47(10):2015–2037
Hambrook Berkman JA, Canova MG (2007) Algae biomass for indicators (ver. 1.0). U.S. Geological Survey Techniques of Water-Resources Investigations. http://water.usgs.gov/owq/FieldManual /Chapter7/7.4. Accessed August 2007
Hill WR, Fanta SE (2008) Phosphorus and light colimit periphyton growth at subsaturating irradiances. Freshw Biol 53(2):215–225
Hill WR, Ryon MG, Schilling EM (1995) Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76:1297–1309
Jöbgen A, Palm A, Melkonian M (2004) Phosphorus removal from eutrophic lakes using periphyton on submerged artificial substrata. Hydrobiologia 528(1):123–142
Johnson L, Richards C, Host G, Arthur J (1997) Landscape influences on water chemistry in Midwestern stream ecosystems. Freshw Biol 37(1):193–208
Kelly M, Whitton B (1995) The trophic diatom index: a new index for monitoring eutrophication in rivers. J Appl Phycol 7(4):433–444
Kelly MG, Wilson S (2004) Effect of phosphorus stripping on water chemistry and diatom ecology in an eastern lowland river. Water Res 38(6):1559–1567
Krammer, Lange-Bertalot (1991–1997) Bacillariophyceae 1.Teil, Naviculaceae; 2. Teil, Bacillariaceae Epthimiaceae Surirellaceae; 3.Teil, Centrales Fragilariaceae Eunotiaceae; 4.Teil, Achnanthaceae Achnanthes and Gomphonema. Spektrum Akademischer Verlag Heidelberg, Berlin
Krammer K (2002) Cymbella. In: Lange-Bertalot H (ed) Diatoms of Europe. Gantner, Ruggell
Kwandrans J, Eloranta P, Kawecka B, Wojtan K (1998) Use of benthic diatom communities to evaluate water quality in rivers of southern Poland. J Appl Phycol 10(2):193–201
LaPerriere JD, Nieuwenhuyse EE, Anderson PR (1989) Benthic algal biomass and productivity in high subarctic streams Alaska. Hydrobiologia 172(1):63–75
Lecointe C, Coste M, Prygiel J (1993) “Omnidia”: software for taxonomy calculation of diatom indices and inventories management. Hydrobiologia 269(1):509–513
Lei H, Liang YQ, Zhu AM (2010) Phytoplankton and its relationship with water quality in Tongzhuang river of the three Gorges reservoir. J Lake Sci 2010(002):195–200 (in Chinese)
Leira M, Sabater S (2005) Diatom assemblages distribution in catalan rivers NE Spain in relation to chemical and physiographical factors. Water Res 39(1):73–82
Lenoir A, Coste M (1996) Development of a practical diatom index of overall water quality applicable to the French National Water Board Network. Use of algae for monitoring rivers II: 29–43
Magurra AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Mbuligwe SE, Kaseva ME (2005) Pollution and self-cleansing of an urban river in a developing country: a case study in Dar es Salaam Tanzania. Environ Manag 36(2):328–342
Montuelle B, Dorigo U, Bérard A et al (2010) The periphyton as a multimetric bioindicator for assessing the impact of land use on rivers: an overview of the Ardières-Morcille experimental watershed (France). Hydrobiologia 657(1):123–141
Munn M, Osborne L, Wiley M (1989) Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia 174(2):89–97
Ndiritu GG, Gichuki NN, Triest L (2006) Distribution of epilithic diatoms in response to environmental conditions in an urban tropical stream Central Kenya. Biodivers Conserv 15(10):3267–3293
Newall P, Walsh CJ (2005) Response of epilithic diatom assemblages to urbanization influences. Hydrobiologia 532(1–3):53–67
O’Brien PJ, Wehr JD (2009) Periphyton biomass and ecological stoichiometry in streams within an urban to rural land-use gradient. Hydrobiologia 657(1):89–105
Pan Y, Stevenson RJ, Hill BH, Kaufmann PR, Herlihy AT (1999) Spatial patterns and ecological determinants of benthic algal assemblages in mid-atlantic streams, USA. J Phycol 35(3):460–468
Pei G, Liu G (2011) Distribution patterns of benthic diatoms during summer in the Niyang River Tibet China. Chin J Oceanol Limnol 29(6):1192–1198
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Potapova MG, Charles DF (2002) Benthic diatoms in USA rivers distributions along spatial and environmental gradients. J Biogeogr 29(2):167–187
Poulı’cˇkova’ A, Mann DG (2006) Sexual reproduction in Navicula cryptocephala (Bacillariophyceae). J Phycol 42:872–886
Prygiel J, Coste M (2000) Guide méthodologique pour la mise enoeuvre de l’Indice Biologique Diatomées NF T 90–354. Agences de l’Eau-Cemagref-Groupement de Bordeaux. Agences de l’Eau, Mars 2000
Raven J (1998) The twelfth Tansley Lecture. Small is beautiful: the picophytoplankton. Funct Ecol 12(4):503–513
Rimet F (2009) Benthic diatom assemblages and their correspondence with ecoregional classifications: case study of rivers in north-eastern France. Hydrobiologia 636(1):137–151
Rimet F (2012) Recent views on river pollution and diatoms. Hydrobiologia 683(1):1–24
Rimet F, Cauchie HM, Hoffmann L, Ector L (2005) Response of diatom indices to simulated water quality improvements in a river. J Appl Phycol 17(2):119–128
Round F (1990) The effect of liming on the benthic diatom populations in three upland Welsh lakes. Diatom Res 5(1):129–140
Smucker NJ, Vis ML (2010) Using diatoms to assess human impacts on streams benefits from multiple-habitat sampling. Hydrobiologia 654(1):93–109
Smucker NJ, Vis ML (2011) Spatial factors contribute to benthic diatom structure in streams across spatial scales: considerations for biomonitoring. Ecol Indic 11(5):1191–1203
SPSS Inc (2008) SPSS for windows (vers.17.0). SPSS Inc, New York
Stephens SH, Brasher AMD, Smith CM (2012) Response of an algal assemblage to nutrient enrichment and shading in a Hawaiian stream. Hydrobiologia 683(1):135–150
Stevenson RJ, Bahls LL (1999) Periphyton benthic macroinvertebrates and fish. In: Barbour MT, Gerritsen J, Snyder BD, Stribling JB (eds) Periphyton protocols. rapid bioassessment protocols for use in streams and Wadeable Rivers, 2nd edn.EPA 841-B-99-002. Office of Water US Environmental Protection Agency, Washington DC
Tan X, Ma P, Xia X, Zhang Q (2013) Spatial pattern of benthic diatoms and water quality assessment using diatom indices in a subtropical river, China. Clean Soil Air Water. doi:10.1002/clen.201200152
Ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for windows user’s guide: software for canonical community ordination (vers. 4.5.). Microcomputer Power, Ithaca
Urrea G, Sabater S (2009) Epilithic diatom assemblages and their relationship to environmental characteristics in an agricultural watershed (Guadiana River SW Spain). Ecol Indic 9(4):693–703
Verb RG, Vis ML (2000) Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and nonimpacted sites. J N Am Benthol Soc 19(2):274–288
Wu N, Tang T, Qu X, Cai Q (2007) Spatial distribution of benthic algae in the Gangqu River Shangrila China. Aquat Ecol 43(1):37–49
Yang GY, Tang T, Dudgeon D (2009) Spatial and seasonal variations in benthic algal assemblages in streams in monsoonal Hong Kong. Hydrobiologia 632(1):189–200
Zampella RA, Laidig KJ, Lowe RL (2007) Distribution of diatoms in relation to land use and pH in blackwater coastal plain streams. Environ Manag 39(3):369–384
Zhu H, Chen J (2000) Bacillariophyta of the Xizang Plateau. Science Press, Beijing (in Chinese)
Acknowledgments
This research was supported by the National Science Foundation of China (Grant No 30970550) and Hubei Province (2015CFB416).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Yang, Y., Cao, JX., Pei, GF. et al. Using benthic diatom assemblages to assess human impacts on streams across a rural to urban gradient. Environ Sci Pollut Res 22, 18093–18106 (2015). https://doi.org/10.1007/s11356-015-5026-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5026-1