Abstract
Root exudates (REs) of Scirpus triqueter were extracted from the rhizosphere soil in this study. The components in the REs were identified by GC-MS. Many organic acids, such as hexadecanoic acid, pentadecanoic acid, vanillic acid, octadecanoic acid, citric acid, succinic acid, glutaric acid, and so on, were found. Batch simulated experiments were conducted to evaluate the impacts of different organic acids, such as citric acid, artificial root exudates (ARE), succinic acid, and glutaric acid in REs of S. triqueter on desorption of pyrene (PYR) and lead (Pb) in co-contaminated wetland soils. The desorption amount of PYR and Pb increased with the rise in concentrations of organic acids in the range of 0–50 g·L−1, within shaking time of 2–24 h. The desorption effects of PYR and Pb in soils with various organic acids treatments decreased in the following order: citric acid > ARE > succinic acid > glutaric acid. The desorption rate of PYR and Pb was higher in co-contaminated soil than in single pollution soil. The impacts of organic acids in REs of S. triqueter on bioavailability of PYR and Pb suggested that organic acids enhanced the bioavailability of PYR and Pb in wetland soil, and the bioavailability effects of organic acids generally followed the same order as that of desorption effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils always contain several contaminants that vary in concentration and composition, among all, organic chemicals such as polycyclic aromatic hydrocarbons (PAHs) and heavy metals are recognized as two major chemical families that cause water and soil pollution (Xiao et al. 2013; Hechmi et al. 2014). PAHs are by-products of the incomplete combustion or pyrolysis of organic materials, and they are priority organic pollutants in the environment (Gao and Zhu 2004). Several PAHs are carcinogenic and mutagenic, posing threats to human health (Gao and Zhu 2005). Soil contaminated with PAHs as well as other nonionic organic contaminants (NOCs) is of worldwide concerns (Patryk 2009). Heavy metals in soils are receiving increasing attention due to their toxicological importance in ecosystems (Huang et al. 2011). Heavy metals can be introduced into and accumulate in soil through agricultural application of sewage sludge and fertilizers, and through land disposal of metal-contaminated municipal and industrial wastes (Zhang and Dong 2008). The co-contamination of heavy metals and organic pollutants in water, air, and soil has become major environmental and human health concerns worldwide, due to rapid industrialization and urbanization (Pérez et al. 2010). Soil pollution by heavy metals and PAHs has been accelerated in China during the past decade (Wang et al. 2012a, b). Hence, it is critical to develop efficient and cost-effective approaches to simultaneously remove multiple contaminants from co-contaminated soils.
Plant roots can actively or passively release a variety of organic compounds, referred to as root exudates (REs), which include carbohydrates, organic acids, and amino acids (Petra et al. 2004). These REs may be an important resource for fast-growing microbes, subsequently alter the composition of species in the rhizosphere, and function in nutrient transformation, decomposition and the mineralization of organic substances, thus, these REs in the root zone or rhizosphere can induce the biodegradation of contaminants (Yang et al. 2001). Furthermore, some REs, such as citric acid, can increase the availability of weathered persistent organic pollutants (POPs) and PAHs in soils (Gao et al. 2010). The main effect of REs on the remediation of heavy metals can be best explained in the following two ways: (1) acidizing insoluble heavy metal fraction in soil and (2) chelating and reducing heavy metals in soil (Cunningham et al. 1996). Adsorption and desorption of heavy metals are important chemical processes occurring in the soil, the concentration of heavy metal ions in the soil is often under the control of adsorption equilibrium, adsorption, and desorption largely determines the bioavailability and distribution of heavy metals in solid and liquid phases (Chen et al. 1999). Adsorption-desorption characteristics of heavy metals in soil were affected by REs, fresh REs reduce the adsorption of heavy metals in the soil and improve its diffusion (Wu 1993). However, the desorption behavior was studied relatively less. Most desorption process was conducted immediately after the completion of adsorption process. This process is different from the desorption process alone.
Gao et al. (2010) found that the addition of artificial root exudates (ARE) markedly influenced the desorption of phenanthrene and pyrene (PYR) in soils, and the effects depended on ARE concentration, aging time, and soil properties, implying that REs conditionally promotes the release of PAHs and REs, which in turn enhances the bioavailability of PAHs with the effects varying with REs concentration in soil and aging time. The positive effects of REs on the removal of heavy metals from soils have been studied by Kim et al. (2010). They found the accumulation of heavy metals in Echinochloa crus-galli that cultivated with the REs was two- to fourfold higher than that of the plants that were cultivated without the REs, and the bioconcentration factor (BF) and translocation factor (TF) values of lead (Pb) were greater when the REs were added. However, little is known about the effects of REs on desorption of PAHs and heavy metals in co-contaminated soils.
In this study, pyrene was used as a model of organic pollutant because it represents a class of organic compounds, PAHs, with carcinogenic potential that is present at many Superfund sites (Gao et al. 2010). Pb is a heavy metal of great concern in urban ecosystems because of its high toxicity to animals and human health, so it was used as a model of heavy metal pollutant. Scirpus triqueter is a dominant species in wetland of Huangpu-Yangtze estuary. The plants used in phytoremediation should be native to the area and should be tolerant to local soil conditions, so S. triqueter was chosen as a model plant. The main aims for the current investigation are (1) to extract and identify the REs of S. triqueter from soil, (2) to determine the effects of organic acids in REs of S. triqueter on desorption of PYR and Pb in soil, and (3) to evaluate the impacts of organic acids in REs of S. triqueter on bioavailability of PYR and Pb in soil. The findings of this study may prove to be useful in the assessment of PAHs and heavy metal-related risks to human health and the environment.
Experiment
REs extraction
S. triqueter plant samples were taken from the Huangpu-Yangtze estuary, and the rhizosphere soil of S. triqueter was collected by using the direct collection method. REs of S. triqueter were identified according to the method described by Yang et al. (2014) and Zhang et al. (2007) with minor modifications. First, S. triqueter plant samples were taken out of their growing land, the taken plants should be consistent in shape and height. Second, soils from 1 to 4 mm of the plant root surface were collected and placed in a stopper container. Finally, the collected rhizosphere soil was brought back to the laboratory to go further detection. The rhizosphere soil of S. triqueter was dried and sieved (0.4 mm) in the laboratory. Fifteen grams of rhizosphere soil samples were placed in a stoppered 100-mL flask. Methyl esterification continued for 12 h at 60 °C after adding 51 mL of anhydrous methanol-concentrated sulfuric acid solution (v:v = 10:7). After centrifugation (4000 rpm, 10 min), the esters were extracted with 1 mL of saturated sodium chloride solution, 20 mL of distilled water, and 5 mL of dichloromethane twice. The organic phase of the solution was extracted and rotary evaporated till dryness at 40 °C, and the residue was redissolved in 1 mL of dichloromethane. The solution was passed through a 0.45-μm filter for gas chromatography–mass spectrometry (GC-MS) analysis.
Soil treatment
The experiment soils, previously free of contaminants, were collected from the east campus of Shanghai University, China. They are typical soils in east China. Components and properties of the soil used in the experiment are as follows: texture-sand 32.20 %, silt 60.40 %, clay 7.40 %, organic matter 19.60 g·kg−1, and pH 8.30. Soil samples were air-dried and sieved (0.2 mm). Preparation of co-contaminated soil was carried out as follows: Firstly, the Pb-contaminated soil was prepared by completely mixing clean soils with Pb(CH3COO)2·3H2O solution, then the soils were air-dried at room temperature for a week. Secondly, PYR was initially dissolved in acetone, and then PYR solution was mixed with above Pb-contaminated soils, the mixture was then ventilated in a fume hood for 8 days to stabilize the contaminated soil. The contaminated soils have a final concentration of 50 and 250 mg·kg−1 of PYR and Pb in mixed contaminated soil, respectively. These concentrations were chosen according to the PAHs and heavy metals contamination levels generally found in soils around the world (Zhao et al. 2014). The soil water content was adjusted to 30 % of the soil water holding capacity.
Desorption of PYR and Pb in soil
Batch experiments were conducted to measure PYR and Pb released from soils. Citric acid, succinic acid, glutaric acid, and ARE solutions (10 mL, 0–50 g·L−1) were mixed with 1 g of contaminated soil (PYR-contaminated soil, Pb-contaminated soil, and PYR and Pb co-contaminated soil) in 25 mL centrifuge tubes. The tubes were shaken in the dark at 200 rpm on a gyratory shaker to reach equilibrium (36 h). After centrifugation (4000 rpm, 10 min), 3 mL aliquots of solution were taken out and mixed with 7 mL of methanol (HPLC grade). The mixture was filtered through a 0.22-μm filter, and the concentrations of PYR were determined by HPLC. Another 2 mL aliquots of solution were taken out and diluted to 25 mL with 0.5 M nitrate to analyze Pb concentrations. The concentrations of Pb were measured by ICP-AAS. Losses such as photochemical decomposition, volatilization, and sorption onto tubes were negligible.
Bioavailability of PYR and Pb in wetland soil
PYR and Pb co-contaminated soil (20 g) was filled into amber glass microcosms similar to those reported previously (Macleod and Semple 2003). Organic acid solutions (citric acid, succinic acid, glutaric acid, and ARE) with concentrations of 0, 1, 5, 10, 20, 40, and 50 g·kg−1 were added, respectively. Three replicates were prepared for each treatment. Soil water content was adjusted to 30 % of the soil water holding capacity. Amber glass microcosms were sealed and incubated at 25 °C for 30 days. Soil was then sampled and PYR was extracted. An n-butanol extraction technique for the measure of the bioavailability of PYR was tested (Liste and Alexander 2002). Briefly, PYR and Pb co-contaminated soil from each microcosm was freeze-dried and sieved. Two grams of soil for each treatment were placed in a 30-mL glass centrifuge tube, 10 mL of 100 % n-butanol were added, and the tube cap was screwed on tightly. Next, the samples were incubated in an ultrasonic bath for 60 min. After centrifugation (4000 rpm, 10 min), the solvent (5 mL) was collected and concentrated to near dryness in a rotary evaporator at 70 °C, and the extract was then resuspended in methanol to a final volume of 2 mL. After filtered through a 0.22-μm filter, the concentration of PYR was measured by high-performance liquid chromatography (HPLC).
Acid-extractable fraction of Pb (bioavailability fraction) was extracted from soil in the following three steps: First, add 40 mL 0.11 mol·L−1 acetic acid to 1 g of PYR and Pb co-contaminated soil. Second, shake the mixed solution for 16 h at 20 ± 2 °C. Third, centrifugate the mixed solution at 4000 rpm for 20 min. Finally, decante the supernatant liquid into a polyethylene container and analyze it by ICP-AAS (Zhu et al. 2010).
Chemical analysis
Analysis of the REs of S. triqueter was performed on Agilent 7890GC-5975MS, which was fitted with a manual injector. Compounds were separated on HP-5 (30 m × 0.25 mm × 0.25 μm) silica capillary column. Samples (2 μL) were injected in the splitless mode. The temperature at injector was 280 °C, the temperature at ion-source was 200 °C, and the temperature at detector was 250 °C. Helium was used as carrier gas at a velocity of 1 mL·min−1. The temperature program used was as follows: started at 40 °C, held for 2 min; increased to 100 °C at 5 °C·min−1, held for 1 min; then rose to 280 °C at 15 °C·min−1, held for 8 min; finally dropped to 240 °C at 2.5 °C·min−1. The scan mode was full at m/z 29–400.
The desorption and bioavailability amount of PYR were analyzed by HPLC (LC-20A), the HPLC system was fitted with a UV detector and a 4.6 × 250 mm reverse phase C18 column, using methanol as the mobile phase, at a flow rate of 1 mL·min−1 (40 °C). The wavelength of the UV detector was 245 nm for PYR. The sample solution (20 μL) was injected into the HPLC system by an autosampler. The concentration of Pb was measured by ICP-AAS.
Statistical analysis
All data were processed using Microsoft Excel and SPSS 17.0. PYR and Pb concentrations of each fraction were tested for differences using p < 0.05 unless otherwise indicated. One-way analysis of variance followed by Duncan’s multiple range test is used to compare the mean value.
Results and discussion
Identification of the REs from Scirpus triqueter
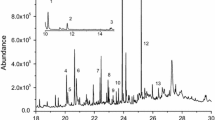
GC-MS results showed that the REs mainly contained 27 kinds of compounds (Fig. 1 and Table 1). The majority were organic acids including hexadecanoic acid, pentadecanoic acid, vanillic acid, octadecanoic acid, citric acid, o-phthalic acid, 2-ketoglutaric acid, p-phthalic acid, myristic acid, oleic acid, succinic acid, decanoic acid, crotonic acid, glutaric acid, propanedioic acid, benzoic acid, 2-methyl butyric acid, and octanoic acid. Zou et al. (2013) verified that the existence of hexadecanoic acid in rhizosphere soil of S. triqueter has an effect on the composition of the microbial community of diesel-spiked simulated wetland.
As a complexing agent and a chelating agent, citric acid forms soluble complexes with the metals in the soil, constitutes an easily accessible carbon source for soil microorganisms, and further reduces the toxic effect of metals on plant (Arwidsson et al. 2010). Stearic acid and myristic acid are mainly used as raw materials for production of surfactants.
REs of different plants may contain many different kinds of compounds. Several compounds in rice REs named 5-hydroxy-2-indolecarboxylic acid, 5-hydroxyindole-3-acetic acid, mercaptoacetic acid, 4-hydroxyphenylacetic acid, and 4-vinylphenol were identified by Seal et al. (2004). Twenty-nine kinds of compounds from the roots of Flaveria bidentis were identified by Yang et al. (2014), including esters, hydrocarbons, ketones, thiazole, amines, etc. Zhang et al. (2007) found that the REs of apple seedlings mainly contained organic acids, glycol, esters, and phenol derivatives, peach REs contained phenolic acids and phenol derivatives in addition to two unidentified compounds, and REs of jujube did not contain any phenolic acids. Several organic acids in the REs of watermelon such as oxalic acid, malic acid, and citric acid were identified by Ling et al. (2011). Citric acid is also contained in REs of S. triqueter in this study. The REs of S. triqueter also contained hydrocarbons, amino acids, and some unidentified compounds. Among the organic acids in REs of S. triqueter, only several of them were soluble in water, mainly including citric acid, succinic acid, and glutaric acid. So, in this study, the three organic acids and ARE, made by 65 % citric acid, 25 % succinic acid, and 10 % glutaric acid according to the rough proportion in Table 1, were chosen to be studied further.
Effects of organic acids on the desorption of PYR and Pb
Organic acids and concentrations
In this experiment, the desorption amount of PYR and Pb in soils by ARE and organic acids was compared. The desorption amounts of PYR and Pb from each soil type in the presence of citric acid, succinic acid, glutaric acid, and ARE, at concentrations of 0–50 g·L−1 with shaking for 36 h, are shown in Fig. 2.
Comparison of the impacts of different concentration of organic acids and ARE on the desorption amount of pyrene and Pb from test soils shaking 36 h. a Desorption amount of pyrene in pyrene single pollution soil. b Desorption amount of Pb in Pb single pollution soil. c Desorption amount of pyrene in pyrene and Pb co-contaminated soil. d Desorption amount of Pb in pyrene and Pb combined pollution soil. Error bars are standard deviations (SD)
Although PYR and Pb desorption amount increased in all soils with increased concentrations of these substances, the increment of PYR and Pb desorption was always higher with the addition of citric acid than ARE, succinic acid, and glutaric acid of the same concentration. For example, in PYR and Pb co-contaminated soil, the desorption amounts of PYR and Pb were 25.09 and 215.80 mg·kg−1 with citric acid at 50 g·L−1. At the same concentration, the desorption amounts of PYR and Pb by ARE, succinic acid, and glutaric acid were 23.74 and 152.30 mg·kg−1, 22.32 and 80.28 mg·kg−1, and 20.94 and 60.07 mg·kg−1, respectively, which is 5.38 and 29.40 %, 11.00 and 62.80 %, and 16.60 and 72.20 % lower, respectively, than that of citric acid. The same trend was found in single pollution soil. The enhancement of the desorption amounts of PYR and Pb in soil with various organic acids treatments decreased in the following order: citric acid > ARE > succinic acid > glutaric acid. Citric acid enhanced PAHs and heavy metals desorption to a more significant extent than other acids, which might be a result of their chemical structures; citric acid is a ternary organic acid, and it has a stronger desorption effect than other acids (Gerhardt et al. 2009).
In PYR and Pb single pollution soil, the desorption amounts were 16.56–23.75 mg·kg−1 for PYR and 9.18–180.20 mg·kg−1 for Pb, and desorption rates of PYR and Pb were 33.10–47.50 % and 3.67–72.10 %, respectively. However, in PYR and Pb co-contaminated soil, the desorption amounts were 17.02–25.09 mg·kg−1 for PYR and 3.99–215.80 mg·kg−1 for Pb, and desorption rates of PYR and Pb were 34.00–50.20 % and 1.60–86.30 %, respectively. The desorption rate is defined as the desorption amount of pollutant divided by the original contaminant amount in soil. Wang et al. (2012a, b) revealed that removal rate of PYR decreased at the elevated Cd level (6.38 mg·kg−1) in the soil planted with hyperaccumulator plant Sedum alfredii. In this study, the desorption amount of PYR slightly increased under high level of Pb (250 mg·kg−1), and the desorption amount of Pb was also increased under low level of PYR (50 mg·kg−1). Therefore, an interactive effect of heavy metals and organic pollutants on contaminants removal from co-contaminated soil can either cause a negative or positive effect depending on the type and concentration of both PAHs and metals (Zhang et al. 2012). Cachada et al. (2012) confirmed PAHs and polychlorinated biphenyls (PCBs) were associated with anthropogenic toxic elements such as Cu, Pb, Zn, and Hg, especially between PAHs and Pb, with correlations higher than 0.767 (p < 0.01), and this may explain why Pb and PYR could make each other desorbed from soils and utilized by plants more easily.
Shaking time
Effects of shaking time on PYR and Pb desorption in the presence of organic acids or ARE at concentration of 50 g·L−1 are shown in Fig. 3. In this study, shaking time ranges from 2 to 36 h enhanced the desorption amount of PYR and Pb, and a shaking time of 36 h had the greatest desorption amount for PYR and Pb. For example, in PYR and Pb single pollution soil, only 15.22 and 0.58 mg·kg−1 of PYR and Pb were desorbed by citric acid (50 g·L−1) at a shaking time of 0 h, whereas 23.75 and 180.20 mg·kg−1 were desorbed by citric acid (50 g·L−1) at a shaking time of 36 h, respectively. The desorption rates of PYR and Pb in single pollution soil were 30.10–31.20 % for PYR and 0.21–0.27 % for Pb at a shaking time of 0 h, compared to 40.00–47.50 % for PYR and 24.20–72.10 % for Pb at a shaking time of 36 h. In single pollution soil, the desorption rates of PYR and Pb increased 9.22–17.10 % and 24.00–71.90 %, respectively. The desorption rates of PYR and Pb in co-contaminated soil were 32.10–33.20 % for PYR and 0.16–0.22 % for Pb at a shaking time of 0 h, compared to 36.60–45.90 % and 24.70–85.20 %, respectively, at a shaking time of 36 h. In co-contaminated soil, the desorption rates of PYR and Pb increased by 3.75–13.50 % and 24.50–85.00 %, respectively, compared with the single pollution soil. In single pollution soil, PYR and Pb desorption rates peaked at 47.50 and 72.10 %, while in co-contaminated soil, PYR and Pb desorption rates peaked at 45.90 and 85.20 %. Thus, shaking time had a strong effect on PAHs and heavy metals desorption, the desorption effect of organic acids and ARE increased with increasing shaking time.
Comparison of the impacts of different shaking time on the desorption amount of pyrene and Pb from test soils with organic acids concentration of 50 g·L−1. a Desorption amount of pyrene in pyrene single pollution soil. b Desorption amount of Pb in Pb single pollution soil. c Desorption amount of pyrene in pyrene and Pb co-contaminated soil. d Desorption amount of Pb in pyrene and Pb combined pollution soil. Error bars are standard deviations (SD)
Bioavailability of PYR and Pb
Figure 4 shows the concentrations of bioavailable fraction of PYR and Pb as a function of amounts of organic acids added in soil after 30 days of incubation. The addition of organic acids enhanced the bioavailability of PYR and Pb in soil irrespective of the different organic acids, and the concentrations of bioavailable PYR and Pb in soil with organic acids were significantly higher than those in control soil (without organic acids). Additionally, the bioavailable amount of PYR and Pb increased with increasing organic acids concentrations in soils. For instance, the bioavailable concentrations of PYR and Pb in control soil after 30 days were 14.46 and 12.62 mg·kg−1, respectively. By contrast, the bioavailable concentrations of PYR in soil increased to 15.91, 17.91, 21.07, 22.71, 23.39, and 23.44 mg·kg−1 with increasing amounts of citric acid (1, 5, 10, 20, 40, and 50 g·kg−1, respectively), and the bioavailable concentrations in soil of Pb increased to 34.23, 51.89, 61.04, 66.50, 71.93, and 73.15 mg·kg−1 with increasing amounts of citric acid (1, 5, 10, 20, 40, and 50 g·kg−1, respectively). This suggests that organic acids enhanced the bioavailability of PYR and Pb in soil, and that addition of organic acids resulted in enhancement of bioavailability of PYR and Pb.
As seen in Fig. 4., the improvement of the bioavailability of PYR and Pb in soil by adding organic acids generally decreased in the following order: citric acid > ARE > succinic acid > glutaric acid, which is similar to the results reported by Ling et al. (2009). They found that the bioavailability of phenanthrene and PYR in soil increased with increasing amount of citric and oxalic acid, and the addition of citric acid enhanced the bioavailability of phenanthrene and PYR greater than oxalic acid. The studies on the correlations between the bioavailable fraction of Pb and organic acids (citric acid, ARE, succinic acid, and glutaric acid) indicated that the contents of low molecular weight organic acids (LMWOAs) were positively correlated to the bioavailable fraction of heavy metals, which showed LMWOAs addition could improve the bioavailability of heavy metal (Zhu et al. 2010). The effect of REs on the dissipation of organic chemicals is not just a general increase in the mass, group, and activity of microorganisms, but rather improves their bioavailabilities in soil environment (Sun et al. 2012). Unfortunately, current studies just focus on the effects of REs from common species or ARE on desorption of contaminants, not really related to a certain kind of plant species.
Conclusions
In this study, the REs of S. triqueter were extracted, isolated, and identified from soil. Results presented here show that the REs of S. triqueter included many organic acids and other kinds of compounds, including 18 kinds of organic acids, several kinds of hydrocarbons, amino acids, and unidentified compounds. Typical water-soluble organic acids were citric acid, succinic acid, and glutaric acid.
The desorption amount of PYR and Pb increased with the increase of organic acids and ARE concentration (0–50 g·L−1). With the extension of shaking time from 0 to 36 h, PYR and Pb desorption amount increased. PYR and Pb desorption rates were higher in PYR and Pb co-contaminated soil than PYR and Pb single pollution soil. Organic acids enhanced the bioavailability of PYR and Pb in soil, and larger amounts of organic acids resulted in further enhancement of PYR and Pb bioavailability. The desorption and bioavailability effects of PYR and Pb in soils with various organic acids treatments decreased in the following order: citric acid > ARE > succinic acid > glutaric acid.
In this study, the clearly positive effects of organic acid, such as citric acid, ARE, succinic acid, and glutaric acid on availabilities of PAHs and heavy metals, provide a new method to enhance the remediation efficiency of co-contaminated wetland soil by LMWOAs addition.
References
Arwidsson Z, Johansson E, Von Kronhelm T, Allard B, Van Hees P (2010) Remediation of metal contaminated soil by organic metabolites from fungi-production of organic acids. Water Air Soil Pollut 205:215–226. doi:10.1007/s11270-009-0222-6
Cachada A, Pato P, Rocha-Santos T, Ferreira da Silva E, Duarte AC (2012) Levels, sources and potential human health risks of organic pollutants in urban soils. Sci Total Environ 430:184–192. doi:10.1016/j.scitotenv.2014.07.097
Chen Z, Tang C, Xu J (1999) Non-suppressed conductivity and indirect UV detection of carboxylic acids in environmental samples by ion exclusion chromatography using 2, 6-pyridinedicarboxylic acidic eluent. Chromatogr 859:173–181. doi:10.1016/S0021-9673(99)00885-7
Cunningham SD, Anderson TA, Schwab AP, Hsu FC (1996) Phytoremediation of soils contaminated with organic pollutants. Adv Agron 56:55–114. doi:10.1016/S0065-2113(08)60179-0
Gao YZ, Ren LL, Ling WT, Gong SS, Sun BQ, Zhang Y (2010) Desorption of phenanthrene and pyrene in soils by root exudates. Bioresour Technol 101:1159–1165. doi:10.1016/j.biortech.2009.09.062
Gao YZ, Zhu LZ (2004) Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere 55:1169–1178. doi:10.1016/j.chemosphere.2004.01.037
Gao YZ, Zhu LZ (2005) Phytoremediation for phenanthrene and pyrene contaminated soils. J Environ Sci 16:12–18
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants potential and challenges. Plant Sci 176:20–30. doi:10.1016/j.plantsci.2008.09.014
Hechmi N, Aissa NB, Abdenaceur H, Jedidi N (2014) Evaluating the phytoremediation potential of Phragmites australis grown in pentachlorophenol and cadmium co-contaminated soils. Environ Sci Pollut Res 21:1304–1313
Huang H, Yu N, Wang L, Gupta DK, He Z, Wang K, Zhu Z, Yan X, Li T, Yang X (2011) The phytoremediation potential of bioenergy crop Ricinus communis for DDTs and cadmium co-contaminated soil. Bioresour Technol 102:11034–11038. doi:10.1016/j.biortech.2011.09.067
Kim S, Lim H, Lee I (2010) Enhanced heavy metal phytoextraction by Echinochloa crus-galli using root exudates. J Biosci Bioeng 109:47–50. doi:10.1016/j.jbiosc.2009.06.018
Ling N, Raza W, Ma JH, Huang QW, Shen QR (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47:374–379. doi:10.1016/j.ejsobi.2011.08.009
Ling WT, Ren LL, Gao YZ, Zhu XZ, Sun BQ (2009) Impact of low-molecular-weight organic acids on the availability of phenanthrene and pyrene in soil. Soil Biol Biochem 41:2187–2195. doi:10.1016/j.soilbio.2009.08.003
Liste HH, Alexander M (2002) Butanol extraction to predict bioavailability of PAHs in soil. Chemosphere 46:1011–1017. doi:10.1016/S0045-6535(01)00165-5
Macleod CJA, Semple KT (2003) Sequential extraction of low concentrations of pyrene and formation of non-extractable residues in sterile and non-sterile soils. Soil Biol Biochem 35:1443–1450. doi:10.1016/S0038-0717(03)00238-4
Patryk O (2009) Application of three methods used for the evaluation of polycyclic aromatic hydrocarbons (PAHs) bioaccessibility for sewage sludge composting. Bioresour Technol 100:413–420. doi:10.1016/j.biortech.2008.05.039
Pérez RM, Cabrera G, Gómez JM, Ábalos A, Cantero D (2010) Combined strategy for the precipitation of heavy metals and biodegradation of petroleum in industrial wastewaters. J Hazard Mater 182:896–902. doi:10.1016/j.jhazmat.2010.07.003
Petra M, David C, Ching HY (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261:199–208. doi:10.1023/B:PLSO.0000035569.80747.c5
Seal AN, Pratley JE, Haig T, An M (2004) Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J Chem Ecol 30:1647–1662. doi:10.1023/B:JOEC.0000042074.96036.14
Sun BQ, Gao YZ, Liu J, Sun YD (2012) The impact of different root exudate components on phenanthrene availability in Soil. Soil Sci Soc Am J 76:2041–2050. doi:10.2136/sssaj2011.0417
Wang K, Zhang J, Zhu ZQ, Huang HG, Li TQ, He ZL, Yang XE, Alva A (2012a) Pig manure vermicompost (PMVC) can improve phytoremediation of Cd and PAHs co-contaminated soil by Sedum alfredii. J Soil Sediment 12:1089–1099. doi:10.1007/s11368-012-0539-4
Wang K, Zhu ZQ, Huang HG, Li TQ, He ZL, Yang XE, Alva A (2012b) Interactive effects of Cd and PAHs on contaminants removal from co-contaminated soil planted with hyperaccumulator plant Sedum alfredii. J Soil Sediment 12:556–564. doi:10.1007/s11368-012-0471-7
Wu QT (1993) Effect of exudates on Cd bioefficiency. Soils 25:227–259
Xiao WD, Wang H, Li TQ, Zhu ZQ, Zhang J, He ZL, Yang XE (2013) Bioremediation of Cd and carbendazim co-contaminated soil by Cd-hyperaccumulator Sedum alfredii associated with carbendazim-degrading bacterial strains. Environ Sci Pollut Res 20:380–389
Yang X, Zhang LH, Shi CP, Shang Y, Zhang JL, Han JM, Dong JG (2014) The extraction, isolation and identification of exudates from the roots of Flaveria bidentis. J Integr Agr 13:105–114. doi:10.1016/S2095-3119(13)60495-5
Yang Y, Ratte D, Smets B, Pignatello JJ, Grasso D (2001) Mobilization of soil organic matter by complexing agents and implications for polycyclic aromatic hydrocarbon desorption. Chemosphere 43:1013–1021. doi:10.1016/S0045-6535(00)00498-7
Zhang JH, Mao ZQ, Wang LQ, Shu HR (2007) Bioassay and identification of root exudates of three fruit tree species. J Integr Plant Biol 49:257–261. doi:10.1111/j.1672-9072.2006.00307.x
Zhang JQ, Dong YH (2008) Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J Hazard Mater 151:833–839. doi:10.1016/j.jhazmat.2007.11.046
Zhang Z, Rengel Z, Chang H, Meney K, Pantelic L, Tomanovic R (2012) Phytoremediation potential of Juncus subsecundus in soils contaminated with cadmium and polynuclear aromatic hydrocarbons (PAHs). Geoderma 175–176:1–8. doi:10.1016/j.geoderma.2012.01.020
Zhao L, Hou H, Shangguan YX, Cheng B, Xu YF, Zhao RF, Zhang YG, Hua XZ, Huo XL, Zhao XF (2014) Occurrence, sources, and potential human health risks of polycyclic aromatic hydrocarbons in agricultural soils of the coal production area surrounding Xinzhou, China. Ecotoxicol Environ Saf 108:120–128. doi:10.1016/j.ecoenv.2014.05.034
Zhu MH, Fang BX, Pang YH, Chen J, Huang ST, Yan XJ, Ding DW (2010) Effects of low molecular weight organic acids of root exudates on heavy metal bioavailability around Scirpus mariqueter rhizosphere sediments. Oceanol Limnol Sin 41:583–589
Zou JC, Liu XY, He CQ, Zhang XY, Zhong CL, Wang CH, Wei J (2013) Effect of Scripus triqueter of its rhizosphere and root exudates on microbial community structure of simulated diesel-spiked wetland. Int Biodeterior Biodegrad 82:110–116. doi:10.1016/j.ibiod.2013.03.006
Acknowledgments
The work was funded by the National Natural Science Foundation of China (Nos. 41373097, 41101230, 41203051), Program for Innovative Research Team in University (No. IRT13078).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hou, Y., Liu, X., Zhang, X. et al. Identification of Scirpus triqueter root exudates and the effects of organic acids on desorption and bioavailability of pyrene and lead in co-contaminated wetland soils. Environ Sci Pollut Res 22, 17780–17788 (2015). https://doi.org/10.1007/s11356-015-4995-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4995-4