Abstract
Polychlorobiphenyl (PCB) biodegradation was followed for 1 year in microcosms containing marine sediments collected from Mar Piccolo (Taranto, Italy) chronically contaminated by this class of hazardous compounds. The microcosms were performed under strictly anaerobic conditions with or without the addition of Dehalococcoides mccartyi, the main microorganism known to degrade PCBs through the anaerobic reductive dechlorination process. Thirty PCB congeners were monitored during the experiments revealing that the biodegradation occurred in all microcosms with a decrease in hepta-, hexa-, and penta-chlorobiphenyls (CBs) and a parallel increase in low chlorinated PCBs (tri-CBs and tetra-CBs). The concentrations of the most representative congeners detected in the original sediment, such as 245-245-CB and 2345-245-CB, and of the mixture 2356-34-CB+234-245-CB, decreased by 32.5, 23.8, and 46.7 %, respectively, after only 70 days of anaerobic incubation without any bioaugmentation treatment. Additionally, the structure and population dynamics of the microbial key players involved in the biodegradative process and of the entire mixed microbial community were accurately defined by Catalyzed Reporter Deposition Fluorescence In Situ Hybridization (CARD-FISH) in both the original sediment and during the operation of the microcosm. The reductive dehalogenase genes of D. mccartyi, specifically involved in PCB dechlorination, were also quantified using real-time PCR (qPCR). Our results demonstrated that the autochthonous microbial community living in the marine sediment, including D. mccartyi (6.32E+06 16S rRNA gene copy numbers g−1 sediment), was able to efficiently sustain the biodegradation of PCBs when controlled anaerobic conditions were imposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) are persistent and ubiquitous organic pollutants widely used in industrial applications. Even if the industrial production of PCBs has been banned since the late 1970s, the chronic toxicity of these recalcitrant hazardous substances still remains, and the decontamination of lakes, rivers, harbors, and marine sediment continues to represent a challenge today (ATSDR 2000; Furukawa and Fujihara 2008; Robertson and Hansen 2015). However, contaminated matrices may involve naturally occurring biodegradation processes in company with the activity of specialized microbial communities able to survive in the presence of such toxic compounds. Anaerobic reductive dechlorination (RD) is the only process known to convert PCBs (chlorinated in para and meta positions) into less chlorinated congeners with a lower toxicity (e.g., ortho-chlorines) (Brown et al. 1987; Wu et al. 2002; Borja et al. 2005; Bedard 2008; Passatore et al. 2014; Kimbrough and Goyer 1985; Safe 1989; Quensen et al. 1990, 1998; Field and Sierra-Alvarez 2008). Eight distinct microbial dechlorination processes have been identified with the chlorine to be removed in various positions (processes M, Q, H’, H, P, N, LP, and T) (Wiegel and Wu 2000; Bedard 2003). Accurate identification of the PCB degradation pathways based on congener distribution can sometimes represent a challenge. Indeed, multiple pathways can dechlorinate most congeners, or vice versa, an individual congener may be dechlorinated by different dynamics, including cometabolic reactions (Bedard et al. 2005; Laroe et al. 2014).
In addition, the occurrence and the rate of dehalogenation are strongly dependent on the nature of the microbial population, the metabolic specificity of which depends on the number and position of chlorines on the phenyl rings and on the availability of electron donors (Wiegel and Wu 2000).
Several microbial enrichments have been obtained from PCB-contaminated sediments in which bacteria specialized in the degradation of halogenated organics were identified. They mainly belong to the strictly anaerobic Dehalococcoides (Dhc) genus able to derive energy from dehalorespiration processes in the presence of electron donors (Maymo-Gatell et al. 1997). Over the last few years, several Dhc strains with various dechlorinating capabilities have been isolated (i.e., Dhc strains 195, VS, GT, H10, BAV1, CBDB1, JNA) (Sung et al. 2006; Copeland et al. 2007a, b; He et al. 2005; Kube et al. 2005; Adrian et al. 2009; Loeffler et al. 2013; Laroe et al. 2014; Kube et al. 2005; Bedard et al. 2007; Pieper and Seeger 2008; Laroe et al. 2014; Fricker et al. 2014). Among these, the Dhc strain JNA has been found to be one of the most important PCB key degraders, as it is involved in 80 distinct PCB dechlorination reactions via various degradation pathways (Laroe et al. 2014). JNA is the only Dhc strain reported to be able to mediate the anaerobic degradation of hexa-CBs and hepta-CBs by process N into by-products, which can be aerobically degraded into di- and tri-CBs by processes LP or Q (Bedard 2003; Bedard and Quensen 1995; Pieper 2005; Field and Sierra-Alvarez 2008; Laroe et al. 2014). Moreover, PCB biodegradation has been also attributed to Dhc strains CDBD1 and 195, the activity of which has been reported in environmental matrices or microcosm studies (Brown et al. 1987; Bedard et al. 2005; Wiegel and Wu 2000). In particular, Dhc strain 195 is involved in the RD of hepta-CBs (from congener 170 to 193, IUPAC numbering) and octa-CBs (from 194 to 205, IUPAC numbering) and can also dechlorinate congeners with one-ring chlorination such as 23456-CB to 2346-CB, 2356-CB, and 246-CB (Fennell et al. 2004; Bedard 2008).
The ability of different Dhc strains to use various halogenated organics as growth substrates is mainly attributed to the presence of key enzymes coded by reductive dehalogenasegenes (RDase) characterized for their strain-specific function (e.g., tceA, bvcA, vcrA) (He et al. 2003, 2005). However, several RDase homologous genes not well characterized are present in the genome of diverse Dhc strains and may be involved in PCB degradation (Holscher et al. 2004; Bedard 2008; Fricker et al. 2014; Furukawa and Fujihara 2008). To date, the majority of PCB-dechlorinating bacteria were identified to be either Dhc species (Pinellas subgroup) or phylogenetically related but distinct Chloroflexi bacteria (Fagervold et al. 2005, 2007; May et al. 2006; Cutter et al. 2011; Wang and He 2013a, b). Nevertheless, it has been demonstrated that dechlorinating bacteria are present as minor populations in PCB-dechlorinating microcosms (e.g., Dhc in a JN culture represented 3.74 % of the total population) even if they are the main trigger for the PCB biodegradation (Bedard et al. 2007). In addition, other dechlorinating microorganisms (e.g., Dehalobacter, Desulfitobacterium, Sulfurospirillum, Sulfuromonas, SZ-type Geobacteraceae, Desulfuromonas, Anaeromixobacter) derive energy from the RD process and can supply nutrients and cofactors (i.e., cobalamins) to the mixed microbial community to assist the dechlorination activity of Dhc cells (He et al. 2007; Yan et al. 2012; He et al. 2007). Although they have been detected in matrices contaminated by PCBs, their role is not well documented (SrinivasaVaradhan et al. 2011).
In the present study, marine sediment from Mar Piccolo (Taranto) affected by long-term PCB contamination was examined. In order to evaluate the occurrence of PCB biodegradation and establish the role of autochthonous microbial populations, microcosms were constructed using the original marine sediment under strictly anaerobic conditions with and without the addition of a Dhc-enriched culture. More insights into the structure and distribution of the microbial community occurring in the presence of the chronic long-term PCB contamination were gained by using whole cell detection technique CAtalyzed Reporter Deposition Fluorescence In Situ Hybridization (CARD-FISH). Moreover, the presence and abundance of the Dhc 16S rRNA and RDase genes were ascertained by using real-time PCR.
The main objectives were as follows: (i) investigate the biodegradation potential of the marine sediment chronically contaminated by a long-term PCB presence,(ii) characterize the native microbial community involved in the PCB biodegradation processes, and (iii) evaluate the structure and population dynamics during the microcosm studies, paying particular attention to Dhc cells and RDase genes.
Material and methods
Microcosm preparation
Two sets of anaerobic microcosms were set up with the marine sediment collected from sampling station 11 (Cardellicchio et al. 2015, this issue) at Mar Piccolo (Taranto, Italy) affected by long-term PCB contamination. For each treatment, microcosms were prepared in duplicate in sterile 250-mL serum bottles. Marine sediment (90-g dry weight) was put in each bottle and amended with 70 and 55 mL of synthetic marine water in the T1 and T2 microcosms, respectively. In addition, the T2 microcosms were bioaugmented with 15 mL of Dhc-enriched culture containing 1.44 × 106 Dhc mccartyi cells mL−1 (Matturro et al. 2013a). No electron donors were added to the microcosms. After preparation, the bottles were sealed with Teflon-faced butyl rubber stoppers and fluxed for 10 min with a mixture of N2/CO2. All microcosms were incubated at 20 °C under rotation. A sterile control (T3) was also prepared with the autoclaved marine sediment, and no dechlorination was observed.
Sampling for chemical and biomolecular analysis
At fixed times (t = 0, 70, 320 days), samples were collected from the microcosms with sterile spatulas under an N2 flux. For the chemical analysis, 4 g of slurry from each microcosm were collected in 30-mLglass tubes and stored at −20 °C until further processing. For CARD-FISH analysis, 1 g of slurry was immediately fixed in formaldehyde (2 % v/v final concentration) and then processed to extract cells from sediment particles as elsewhere described (Barra Caracciolo et al. 2005). For DNA extraction and subsequent qPCR applications, 1 g of slurry was collected and immediately stored at −20 °C until DNA extraction, the latter performed with Power Soil (MoBio, Italy) by following the manufacturer’s instructions.
PCB extraction and quantification
The PCBs were extracted from the original marine sediment and from the slurry collected in each microcosm and then quantified. The slurry was mixed with Silica Gel 60 (Merck, Darmstadt, Germany) (1:1 w/w). Before extraction, five PCB congeners (IUPAC nos. 29, 55, 122, 166, and 186) were added to the samples as surrogate standards. The PCBs were extracted with Accelerated Solvent Extractor (ASE 200, Dionex Corp., Sunnyvale, CA, USA) by using a mixture of acetone/hexane 1:1at 100 °C and 1200 psi. The extracts were shaken with distilled water to remove acetone, and the organic phase was then treated with tetrabutylammonium sulfite for sulfur removal as elsewhere described (Jensen et al. 1977). The PCBs were eluted with hexane on a multilayer column containing silica gel impregnated with H2SO4 and aluminum oxide 90 (Merck, Darmstadt, Germany) partially deactivated with 10 % water. The hexane was then concentrated to 0.5 mL using a rotary evaporator. Analyses were performed by gas chromatography (GC) with amass spectrometric detector on a TraceGC equipped with a 30-m × 0.25-mm × 0.25-μm HP5-MS capillary column (Agilent technologies, Palo alto, CA, USA), connected to a TraceMS (Thermo, Austin, TX, USA) operated in the electron impact ionization (EI) mode. Two ions were monitored for each PCB homologous group. The injector and transfer line temperatures were 250 and 300 °C, respectively. The oven temperature was held at 60 °C for 1 min, then increased to 160 °C at a rate of 20 °C/min, further increased to 300 °C at a rate of 6 °C/min, and held for 2 min. Helium was employed as the carrier gas with a constant flow of 1 mL/min. For quantitative analysis, a WHO/ISS calibration mixture was used (UltraScientific, North Kingstown, RI, USA) containing 32 PCB congeners. Quantification was based on a three-point calibration curve, and data were reported as ng of PCBs per g of dry sediment (hereafter cited as ng g−1).
CARD-FISH
Cells extracted from sediment particles were filtered through a 0.2-μm polycarbonate membrane (Millipore, 25-mm diameter) by gentle vacuum (<0.2 bar) and were analyzed by CARD-FISH as reported in Matturro et al. (2012). CARD-FISH probes targeting 16S rRNA or 23S rRNA were used to detect total Bacteria (Eub338I, Eub338II, Eub338III), Archaea (Arch915), α-Proteobacteria (Alf968), β-Proteobacteria (Bet42a + Gam42a competitor), γ-Proteobacteria (Gam42a + Bet42a competitor), δ-Proteobacteria (Delta495a, Delta495b, Delta495c), Firmicutes (LGCa, LGCb, LGCc), Chloroflexi (GNSB941), Desulfitobacterium (Dsf440 + Dsf475 competitor), and Dehalococcoides mccartyi (Dhc1259c, Dhc1259t). Probe details and conditions are reported in probeBase (http://www.microbial-ecology.net/probebase/). 4′,6-Diamidino-2-phenylindole (DAPI) fluorescent staining was performed to determine total cell numbers, from which the relative abundance of each targeted bacterial population was calculated. A total cell count was performed at the end of the hybridization assay using a Vectashield Mounting Medium with DAPI (Vector Labs, Italy). For each sample, at least 20 randomly selected microscopic fields were analyzed to count the cells through microscopic analysis. Cell abundances were expressed in terms of cells per dry weight of the marine sediment from which they were extracted (hereafter cited as cells g−1). Means and standard deviations were calculated with Microsoft Excel®.
qPCR
qPCR absolute quantification was performed on 1:100 dilutions of DNA extracted from sediment. qPCR targeting Dhc 16S rRNA and RDase genes tceA, bvcA, and vcrA was performed in triplicate. Reactions and standard curves were performed as already described in Matturro et al. (2013b). Quantitative data were expressed as gene copy numbers g−1 sediment, and error bars were estimated with Microsoft Excel® on triplicate reactions for each sample.
Results
PCB biodegradation
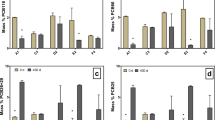
Chemical analyses were conducted on the original marine sediment before treatment (t = 0 day) and on samples collected from the strictly anaerobic microcosms at different times (t = 70, 320 days). Thirty PCB congeners were analyzed, and a total of 4244.7 ng g−1 were detected (Table S1). Among all of them, the most abundant congeners were hexa-CBs, penta-CBs, and hepta-CBs. In the original marine sediment, the congener 245-245-CB was the most abundant, followed by the mixture of 2356-34-CB+234-245-CB and 2345-245-CB congeners, which, respectively, represented 15.5, 14, and 9.3 % of the total PCBs analyzed at t = 0. Moreover, the congeners 2356-245-CB, 236-245-CB, 2345-234-CB, 236-24-CB, 245-24-CB, 245-25-CB, 2345-234-CB, and 2346-245-CB represented >2.5 % each of all the congeners analyzed with concentrations >100 ng g−1 (Table S1). Interestingly, the presence of some tri-CBs and tetra-CBs (e.g., 25-2-CB, 24-4-CB, 25-4-CB, 24-24-CB, 23-25-CB, 25-25-CB, 34-34-CB) was also observed in the original sediment and, respectively, represented 0.4, 1.5, 1.5, 0.9, 3.6, and <0.1 % of the total PCBs analyzed. The chemical analysis, conducted after 70 and 320 days of incubation, revealed a significant decrease in highly chlorinated PCBs and an increase in PCBs with four or three chlorines on their rings (Fig. 1). Hepta-CBs (e.g., 2345-245-CB, 2345-234-CB, 2356-245P-CB, 2346-245-CB) were reduced to hexa-CBs (e.g., 245-245-CB, 234-245-CB, 2356-34-CB, 236-245-CB, 2356-25-CB, 234-234-CB) and consequently to penta-CBs (e.g., 245-24), tetra-CBs (e.g., 24-24-CB, 25-25-CB, 23-25-CB), and tri-CBs (e.g., 25-2-CB, 24-4-CB, 25-4-CB). In particular, the concentration of the congeners 2356-34-CB + 234-245-CBs, 2345-234-CB, 245-245-CB, and 2345-245-CB significantly decreased, by 46.7, 34.6, 32.5, and 23.8 % after only 70 days in the T1 microcosms without any bioaugmentation treatment (Fig. 1a, c; Table S1). Interestingly, under these conditions, an increase in by-products or final products of the RD process, such as 245-24-CB (+12.6 %), 23-25-CB (23.3 %), 25-25-CB (+18 %), 24-24-CB (+47 %), 25-4-CB+24-4-CB (+72.2 %), was observed (Fig. 1a, c; Table S1). Comparing the increase/decrease trends, no differences were observed in the decrease in hepta-CBs, hexa-CBs, and penta-CBs between the microcosm without (T1) and with (T2) bioaugmentation after either 70 or 320 days (Fig. 1, Table S1). It is worth noting that the total concentration of the congeners analyzed decreased while the microcosm was being performed, from 4244.7 ng g−1 estimated before treatment to 3111.7 and 3401.9 ng g−1 in T1 and T2, respectively, after 320 days of incubation. This finding may suggest that, in addition to the total of 30PCBs analyzed, other low chlorinated compounds, such as tetra-CB and/or tri-CB congeners, not analyzed here, were probably obtained as by-products of the biodegradation process.
PCB congener distribution in microcosms T1 (a) and T2 (b) during the microcosm operations. Decreasing/increasing patterns of each congener were evaluated by calculating the difference of congener concentration (ng g−1 dry sediment) estimated in the original marine sediment (t = 0) and at the end of the treatments in microcosms T1 (c) and T2 (d)
Microbial community distribution
CARD-FISH was applied for the characterization of the microbial community structure in the original contaminated marine sediment and during the anaerobic treatments. Total cell numbers, estimated in the original sediment by DAPI-staining, accounted for 2.19E+07+1.96E+06 cells g−1 (Fig. 2). After 70 days of incubation, total biomass was higher in T2 (9.33E+07 cells g−1) than in T1 (2.7E+07 cells g−1) while comparable abundances were observed after 320 days (6.67E+07 and 6.97E+07 total cells g−1in T1 and T2, respectively). Bacteria and Archaea were detected in the sediment before the treatments with relative abundances of >60 and >20 % of total cells, respectively (Fig. 2). Both Archaea and Bacteria increased during the anaerobic incubation period, and Archaea notably rose to 51.5 and 35.4 % of total cells in T1 and T2, respectively (Fig. 2).
Quantitative analyses were also performed at the phylum level to gain more insights into the bacterial community structure and distribution in both the original sediment and during the microcosm experiments (Fig. 3). More than 80 % of the total bacterial community was identified, and cell abundances of each phylum were accurately quantified. In detail, in the original sediment, δ-Proteobacteria represented 23.4 % of total Bacteria followed by Chloroflexi (16 %), γ-Proteobacteria (15 %), α-Proteobacteria (13.5 %), β-Proteobacteria (6.5 %), and Firmicutes (4.7 %) (Fig. 3). Sixty-six percent of Chloroflexi were not affiliated to Dhc. The microbial community structure changed during the incubation period, and very similar distributional patterns were observed both in T1 and T2 (Fig. 3a–d). In particular, cell abundances of the Chloroflexi phylum showed a significant increase after 70 days of incubation, representing 45and 22 % of total Bacteria in T1 and T2, respectively (Fig. 3). Dhc cells represented 34 % of total Chloroflexi in the original sediment, and the Dhc/Chloroflexi ratio reached 24.7 and 43.4 % in T1 and T2, respectively, after 320 days of incubation (Fig. 4a, b). The higher increase in the Dhc/Chloroflexi ratio in the T2microcosm was probably due to the bioaugmentation of Dhc. Nevertheless, a significant portion of non-Dhc-Chloroflexi was observed and represented >50 % of total Chloroflexi under all tested conditions (Fig. 4). Moreover, Firmicutes also represented a significant portion of the entire bacterial community in the original sediment (47.3 % of total Bacteria). However, Firmicutes cell abundances strongly decreased during the experiments, representing ≤4 % of total Bacteria after 320 days of incubation. Within this phylum, Desulfitobacterium species were observed in the original sediment, representing 26 % of total Firmicutes. Even though Desulfitobacterium cells decreased from 1.47E+06 cells g−1, as estimated in the original sediment, to 1.5E+04 (in T1) and 1.22E+04 (in T2) cells g−1, members of this genus represented>35 % of total Firmicutes after the treatments. Interestingly, the distribution of Proteobacteria also changed during the microcosm experiment and substantially increased, from 59 % of total Bacteria in the original sediment to >80 % after 320 days both in T1 and T2 (Fig. 3). Among these, the major portion was represented by α-Proteobacteria, which substantially increased during the treatments, representing >70 % of total Bacteria both in T1 and T2 at the end of the incubation period.
Estimation of RDase genes
RDase (tceA, bvcA, and vcrA) and Dhc 16S rRNA gene copies were quantified by qPCR (Fig. 5). As expected from the CARD-FISH results, the presence of Dhc in the original marine sediment chronically contaminated by PCBs was further confirmed by qPCR (Fig. 5a). In the T1 microcosms, an increase in the Dhc 16S rRNA genes of 29 and 53 % was observed after 70 and 320 days, respectively. In the bioaugmented T2 microcosms, a significant increase of 83.62 % in Dhc 16S rRNA gene copies was found at 70 days. However, after 320 days, a decrease in 33 % of Dhc 16S rRNA gene copies was detected in T2, probably owing to the consumption of the electron donors available in the original sediment. Of all RDase genes screened, tceA and vcrA genes were detected, and no significant increase in these RDase genes was observed during the treatments, except for the tceA gene in the T2 microcosms (Fig. 5b, c). Furthermore, the sum of tceA and vcrA genes represented only 0.15 % of the Dhc 16S rRNA gene copies estimated in the marine sediment before the treatments. This percentage decreased in T1 at the end of the experiments (0.03 %) while in the T2 microcosms, in line with the bioaugmentation, the tceA gene represented ~5 % of the total Dhc 16S rRNA gene copies.
Discussion
In this study, the biodegradation potential of the marine sediment from Mar Piccolo (Taranto, Italy), affected by chronically long-term PCB contamination, was evaluated. The chemical analysis conducted on the original marine sediment demonstrated the presence of at least 30PCB congeners. Among these, PCBs with a high degree of chlorination (mainly hepta-CBs, hexa-CBs, and penta-CBs) were detected in addition to a substantial presence of tri-CBs or tetra-CBs (such as 25-2-CB, 24-4-CB, 25-4-CB, 23-25-CB, 24-24-CB, 25-25-CB, and 34-34-CB). The presence of congeners with a low degree of chlorination indicates that some RD processes are likely to have occurred at the site. As a matter of fact, the congener 24-24-CB, one of the most common final products obtained by dechlorination of highly chlorinated CBs (e.g., 2345-245-CB, 2346-245-CB, 2345-234-CB, 245-24-CB, 234-245-CB, 245-245-CB) and often associated with the metabolic activity of specialized microorganisms (i.e., Dhc), was detected in the original sediment. These indications were also supported by the detection of the Dhc 16S rRNA gene in the original sediment (8.32E+06 16S rRNA gene copies g−1), suggesting a possible involvement of this dechlorinating microorganism in attenuation of the PCBs occurring in the sediments. However, not all Dhc strains present in the sediments were identified. Indeed, the analysis of RDase genes demonstrated that strains carrying tceA or vcrA genes represented only 0.15 % of total Dhc 16S rRNA, suggesting that “other” Dhc strains, carrying other RDase genes probably involved in the PCB biodegradation, were present. It is well known that at least 35 related, but distinct, RDase homologous genes are present in the genome of dechlorinating microorganisms even if the functions are not yet defined (Holscher et al. 2004). Moreover, no PCB dechlorinase responsible for mediating the RD process has been fully characterized, except for a chlorobenzene RDase gene, found in the Dhc strain CBDB1 (Adrian et al. 2007), and for three PCB RDase genes (pcbA1, pcbA4, pcbA5) recently identified in three new Dhc strains CG1, CG4, CG5 (Wang et al. 2014).
Nevertheless, the RDase encoded by this gene might also not be the only one able to dechlorinate PCBs, and more potential candidate genes for PCB dechlorinases are likely to exist, probably belonging to Chloroflexi strains (Bedard 2008). Indeed, as shown by CARD-FISH analysis, Dhc cells in the original sediment represented ~40 % of the total cells affiliated to the Chloroflexi phylum, suggesting that other dechlorinating non-Dhc-Chloroflexi able to grow in the presence of PCBs (e.g., o-17/DF-1-like Bacteria) may play a role in the RD process.
The chemical and biological characterization conducted on the original sediment provided preliminary indications on the biodegradation potential of the site, which can be enhanced by imposing more favorable conditions. Indeed, results from microcosm studies showed that the biodegradation of PCBs occurred rapidly under strictly anaerobic conditions. Interestingly, after 70 days of incubation, both T1 and T2 microcosms showed a strong decrease in the highly chlorinated and most representative congeners (245-245-CB, 2356-34-CB+234-245-CB, 2345-235-CB, 2345-245-CB) and a significant increase in less chlorinated congeners 245-24-CB, 23-25-CB, 24-24-CB, 25-25-CB, 25-4-CB, and 24-4-CB. In particular, the congeners 2345-245-CB, 245-245-CB, and the mixture 2356-34-CB+234-245-CB, which were present in very high concentrations (respectively, 395, 658, 594.4 ng g−1), decreased after 70 days by 23, 32, and 46.7 %, respectively, of their initial concentrations (Fig. 1, Table S1).
In addition, other hepta-CBs, hexa-CBs, and penta-CBs decreased after 70 days of incubation with falls in the ranges of 20–40, 10–50, and 11–52 %, respectively, of the concentrations observed in the original sediment (Table S1). At the same time, significant increases in tri-CBs and tetra-CBs and some intermediates and/or final products of processes N and H (e.g., 245-24-CB, 24-24-CB, 25-25-CB) were observed. The mixture of the congeners 24-4-CB+25-4-CB increased by 72 % of the original concentration, followed by the congener 24-24-CB (+47 %), known to be the final product of the biodegradation process mediated by dechlorinating microorganisms. It is interesting to note that some biodegradation pathways, mainly related to process N and led by Dhc (JNA strain) were previously identified and found to be involved in the transformation of 2345-245-CB → 245-245-CB → 245-24-CB→ 24-24-CB or alternatively 2345-245-CB → 235-245-CB →235-24 → 25-24 (Laroe et al. 2014). In the microcosms carried out in this study, the congeners 2345-245 and 245-245-CB decreased by 23 and 32.5 %, and the congeners 245-24-CB and 24-24-CB increased by 12.6 and 47 %, respectively, after only 70 days of anaerobic treatment without bioaugmentation (Fig. 1, Table S1). These observations supported the involvement of Dhc cells, already detected in the original sediment, in the PCB biodegradation process under strictly anaerobic conditions. It is possible that Dhc strain 195, the presence of which was ascertained by the detection of the tceA gene, is involved in the biodegradation of some hepta-CBs and octa-CBs (particularly, from 170 to 193 and from 194 to 205—IUPAC numbering) both in the original sediment and consequently in the microcosm enrichments. Indeed, congeners 2345-234-CB, 2356-234-CB, 2346-245-CB, 2356-245-CB, 2345-345-CB decreased overtime during the microcosm experiments. The anaerobic fermentation of the organic carbon in the sediment was able to support the PCB dechlorination and probably determined the bacterial community structure. Similarly, the increase in Archaea observed at the end of the anaerobic treatment is also linked to the availability of fermentation products and consistent with the observations that PCB dechlorination can be accompanied by the production of methane (Nies and Vogel 1990; Field and Sierra-Alvarez 2008; Loyd et al. 2013). Moreover, even though the only metabolisms known for cultured Archaea are methane production and methane consumption, recent isotopic evidence has shown that sedimentary Archaea can be heterotrophic, but the potential carbon substrates remain unknown.
Surprisingly, comparable PCB biodegradation patterns were observed in the microcosms with and without bioaugmentation, suggesting that the addition of the Dhc-enriched consortium did not improve the biodegradation kinetics. This finding clearly shows the biodegradation potential of an autochthonous microbial community adapted to a chronically long-term contamination, whereas the impact of inoculated specialized dechlorinators was negligible as they were not acclimatized to the stringent conditions (i.e., high PCB concentrations) present in the contaminated sediment.
To sum up, this study reports for the first time experimental evidence of PCB biodegradation in marine sediment from Mar Piccolo (Taranto, Italy). The microcosm study confirmed the involvement of the autochthonous microbial community, including PCB-degrading bacteria, the decrease in highly chlorinated PCBs, and the formation of by-products and/or final products shortly after the anaerobic incubation. Moreover, these findings indicate the importance of biological data estimation (e.g., biomarkers of contamination, structure of the microbial community) for the predictive evaluation of the bioremediation potential of PCB-contaminated sediments.
References
Adrian L, Rahnenfhrer J, Gobom J, Holscher T (2007) Identification of a chlorobenzene reductive dehalogenase in Dehalococcoidessp. Strain CBDB1. Appl Environ Microbiol 73:7717–7724
Adrian L, Dudková V, Demnerová K, Bedard DL (2009) Dehalococcoides strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl (PCB) mixture Aroclor 1260. Appl Environ Microbiol 75:4516–4524
Agency for Toxic Substances and Disease Registry, ATSDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs). U.S. Department of Health and Human Services, Public Health Service, Atlanta
Barra Caracciolo A, Grenni P, Cupo C, Rossetti S (2005) In situ analysis of native microbial communities in complex samples with high particulate loads. FEMS Microbiol Lett 253:55–58
Bedard DL (2003) Polychlorinated biphenyls in aquatic sediments: environmental fate and outlook for biological treatment. In Dehalogenation: microbial processes and environmental applications, ed. MM Haggblom, I Bossert, pp. 443-65
Bedard DL (2008) A case study for microbial degradation: anaerobic bacterial reductive dechlorination of polychlorinated biphenyls—from sediment to defined medium. Annu Rev Microbiol 62:253–270
Bedard DL, Quensen JF III (1995) Microbial reductive dechlorination of polychlorinated biphenyls. In Microbial transformation and degradation of toxic organic chemicals. Edited by LY Young, CE Cerniglia, New York Wiley-Liss: 127-216
Bedard DL, Pohl EA, Bailey JJ, Murphy A (2005) Characterization of the PCB substrate range of microbial dechlorination process LP. Environ Sci Technol 39:6831–6839
Bedard DL, Ritalahti KM, Löffler FE (2007) The Dehaloccoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol 73:2513–2521
Borja J, Taleon DM, Auresenia J, Gallardo S (2005) Polychlorinated biphenyls and their biodegradation. Process Biochem 40:999–2013
Brown JF Jr, Wagner RE, Feng H, Bedard DL, Brennan MJ et al (1987) Environmental dechlorination of PCBs. Environ Toxicol Chem 6:579–593
Cardellicchio N, Annicchiarico C, Di Leo A, Giandomenico S, Spada L (2015) The Mar Piccolo of Taranto: an interesting marine ecosystem for the environmental problems studies. Accepted in this issue
Copeland A, Lucas S, Lapidus A, Barry K, Detter JC et al (2007a) Complete sequence of Dehalococcoides sp. BAV1. U.S. Dep. Energy Joint Genome Inst. http://www.jgi.doe.gov/
Copeland A, Lucas S, Lapidus A, Barry K, Glavina del Rio T et al (2007b) Sequencing of the draft genome and assembly of Dehalococcoides sp. VS. U.S. Dep. Energy Joint Genome Inst. http://www.jgi.doe.gov/
Cutter LA, Watts JE, Sowers KR, May HD (2011) Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ Microbiol 3(11):699–709
Fagervold SK, Watts JEM, May HD, Sowers KR (2005) Sequential reductive dechlorination of metachlorinated polychlorinated biphenyl congeners in sediment microcosms by two different Chloroflexiphylotypes. Appl Environ Microbiol 71:8085–8090
Fagervold SK, May HD, Sowers KR (2007) Microbial reductive dechlorination of Aroclor 1260 in Baltimore Harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Appl Environ Microbiol 73:3009–3018
Fennell DE, Nijenhuis I, Wilson SF, Zinder SH, Hӓggblom MM (2004) Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ Sci Technol 38:2075–2081
Field JA, Sierra-Alvarez R (2008) Microbial transformation and degradation of polychlorinated biphenyls. Environ Pollut 155(1):1–12
Fricker AD, LaRoe SL, Shea ME, Bedard DL (2014) Dehalococcoides mccartyi strain JNA dechlorinates multiple chlorinated phenols including pentachlorophenol and harbors at least 19 reductive dehalogenase homologous genes. Environ Sci Technol 48(24):14300–14308
Furukawa K, Fujihara H (2008) Microbial degradation of polychlorinated biphenyls: biochemical and molecular features. J Biosci Bioeng 105(5):433–449
He JZ, Ritalahti KM, Yang KL, Koenigsberg SS, Löffler FE (2003) Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62–65
He J, Sung Y, Krajmalnik-Brown R, Ritalahti KM, Loffler FE (2005) Isolation and characterization of Dehalococcoidessp. strain FL2, a trichloroethene (TCE)- and 1,2-dichloroethene-respiring anaerobe. Environ Microbiol 7:1442–1450
He J, Holmes VF, Lee PK, Alvarez-Cohen L (2007) Influence of vitamin B12 and cocultures on the growth of Dehalococcoides isolates in defined medium. Appl Environ Microbiol 73(9):2847–2853
Holscher T, Krajmalnik-Brown R, Ritalahti KM, vonWintzingerode F, Gorisch H et al (2004) Multiple nonidentical reductive-dehalogenase-homologous genes are common in Dehalococcoides. Appl Environ Microbiol 70:5290–5297
Jensen S, Renberg L, Reutergardh L (1977) Residue analysis of sediment and sewage sludge for organochlorines in the presence of elemental sulfur. Anal Chem 49:316–319
Kimbrough RD, Goyer RA (1985) Toxicity of chlorinated biphenyls, dibenzofurans, dibenzodioxins and related-compounds. In: Japan United States Joint Seminar. Environ Health Perspect 59:3–3
Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L (2005) Genome sequence of the chlorinated compound respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol 23:1269–1273
LaRoe SL, Fricker AD, Bedard DL (2014) Dehalococcoides mccartyi strain JNA in pure culture extensively dechlorinates Aroclor 1260 according to polychlorinated biphenyl (PCB) dechlorination Process N. Environ Sci Technol 48(16):9187–9196
Lloyd KJ, Schreiber L, Petersen DG, Kjeldsen KU, Lever MA, Steen AD, Stepanauskas R, Richter M, Kleindienst S, Lenk S, Schramm A, Jørgensen BB (2013) Predominant archaea in marine sediments degrade detrital proteins. Nature 496:215–218
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Müller JA, Fullerton H, Zinder SH, Spormann AM (2013) Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia class is nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol 63(Pt 2):625–635
Matturro B, Aulenta F, Majone M, Papini MP, Tandoi V, Rossetti S (2012) Field distribution and activity of chlorinated solvents degrading bacteria by combining CARD-FISH and real time PCR. Nat Biotechnol 30(1):23–32
Matturro B, Tandoi V, Rossetti S (2013a) Different activity levels of Dehalococcoides mccartyi revealed by FISH and CARD-FISH under non-steady and pseudo-steady state conditions. Nat Biotechnol 30(6):756–762
Matturro B, Heavner GL, Richardson RE, Rossetti S (2013b) Quantitative estimation of Dehalococcoides mccartyi at laboratory and field scale: comparative study between CARD-FISH and real time PCR. J Microbiol Methods 93(2):127–133
May HD, Cutter LA, Miller GS, Milliken CE, Watts JEM, Sowers KR (2006) Stimulatory and inhibitory effects of organohalides on the dehalogenating activities of PCB-dechlorinating bacterium o-17. Environ Sci Technol 40:5704–5709
Maymó-Gatell X, Chien Y, Gossett JM, Zinder SH (1997) Isolation of a bacterium that reductively dechlorinate stetrachloroethene to ethene. Science 276(5318):1568–1571
Nies L, Vogel TM (1990) Effects of organic substrates on dechlorination of Aroclor 1242 in anaerobic sediments. Appl Environ Microbiol 56(9):2612–2617
Passatore L, Rossetti S, Juwarkar AA, Massacci A (2014) Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): state of knowledge and research perspectives. J Hazard Mater 278:185–202
Pieper DH (2005) Aerobic degradation of polychlorinated biphenyls. Appl Microbiol Biotechnol 67(2):170–191
Pieper DH, Seeger M (2008) Bacterial metabolism of polychlorinated biphenyls. J Mol Microbiol Biotechnol 15(2-3):121–138
Quensen JF, Boyd SA, Tiedje JM (1990) Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microbiol 56(8):2360–2369
Quensen JF, Mueller SA, Jain MK, Tiedje JM (1998) Reductive dechlorination of DDE to DDMU in marine sediment microcosms. Science 280(5364):722–724
Robertson LW, Hansen LG (2015) PCBs: recent advances in environmental toxicology and health effects. University Press of Kentucky, Lexington
Safe S (1989) Polychlorinated biphenyls PCBs mutagenicity and carcinogenicity. Mutat Res 220:31–47
SrinivasaVaradhan A, Khodadoust AP, Brenner RC (2011) Effect of biostimulation on the microbial community in PCB-contaminated sediments through periodic amendment of sediment with iron. J Ind Microbiol Biotechnol 38(10):1691–1707
Sung Y, Ritalahti KM, Apkarian RP, Löffler FE (2006) Quantitative PCR confirms purity of strain GT, a novel trichloroethene-to-ethene-respiring Dehalococcoides isolate. Appl Environ Microbiol 72:1980–1987
Wang S, He J (2013a) Phylogenetically distinct bacteria involve extensive dechlorination of Aroclor 1260 in sediment-free cultures. PLoS One 8(3):e59178
Wang S, He J (2013b) Dechlorination of commercial PCBs and other multiple halogenated compounds by a sediment-free culture containing Dehalococcoides and Dehalobacter. Environ Sci Technol 47(18):10526–10534
Wang S, Chng KR, Wilm A, Zhao S, Yang KL, Nagarajan N, He J (2014) Genomic characterization of three unique Dehalococcoides that respire on persistent polychlorinated biphenyls. PNAS 111(33):12103–12108
Wiegel J, Wu QZ (2000) Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microbiol Ecol 32:1–15
Wu QZ, Watts JEM, Sowers KR, May HD (2002) Identification of a bacterium that specifically catalyzes the reductive dechlorination of polychlorinated biphenyls with doubly flanked chlorines. Appl Environ Microbiol 68:807–812
Yan J, Ritalahti KM, Wagner DD, Löffler FE (2012) Unexpected specificity of interspecies cobamide transfer from Geobacter spp. to organohalide-respiring Dehalococcoides mccartyi strains. Appl Environ Microbiol 78(18):6630–6636
Acknowledgments
The activities described in this publication were funded by the “Project Bandiera RITMARE-La Ricerca Italiana per il Mare” coordinated by the National Research Council and funded by the Ministry for Education, University and Research within the National Research Program 2011–2013. The authors thank Dr. Nicola Cardellicchio and Dr. Tamara Cibicfor their support and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Matturro, B., Ubaldi, C., Grenni, P. et al. Polychlorinated biphenyl (PCB) anaerobic degradation in marine sediments: microcosm study and role of autochthonous microbial communities. Environ Sci Pollut Res 23, 12613–12623 (2016). https://doi.org/10.1007/s11356-015-4960-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4960-2