Abstract
Currently, more than 10,000 ha of fens have been rewetted to re-establish their function as nutrient sinks in NE Germany. However, field investigations reveal that porewater concentrations of P, dissolved organic carbon (DOC) and ammonium in rewetted fens are orders of magnitude larger than under pristine conditions. Hence, the objective of this study was to investigate the reasons behind enhanced P, organic carbon (OC) and ammonium mobilisation due to rewetting by means of a long-term incubation experiment. Highly, moderately and slightly decomposed peat of a drained fen (polder Zarnekow) was incubated under waterlogged conditions. A time course of concentrations of P, DOC, ammonium, sulphate and other dissolved substances was investigated by means of permanently installed dialysis samplers during 54 weeks of incubation. Simultaneously, the concentrations of these dissolved substances were investigated after rewetting of the field site. Before, and at the end of the incubation study, the amounts of bicarbonate–dithionite (BD) and NaOH soluble P and OC of incubated peat samples were determined by a sequential extraction procedure. The highest mobilisation of P, OC and ammonium occurred in the highly decomposed peat. Final concentrations of P, DOC and ammonium reached about 143 μM, 46 and 1.9 mM, respectively. The initial sulphate concentrations in the rewetting experiment, as well as in the field investigations, were extremely high and ranged between 3 and 13 mM; however, a complete consumption of sulphate was only observed in highly decomposed peat. In conclusion, the reasons for enhanced P, OC and ammonium mobilisation are increased amounts of redox sensitive substances and enhanced availability of decomposable organic matter in the upper highly decomposed peat horizon. These results should be considered in future rewetting management strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Originally, about 10% of NE Germany were covered by minerotrophic peatlands (=fens). The drainage of these areas, mainly for low-intensity agricultural use and peat harvesting, began during the eighteenth century. Agricultural use was intensified at the end of the 1960s by complex dewatering systems and embankment. At present, more than 95% of fen areas are drained or have suffered from lowered ground water tables within their catchment. An obvious consequence has been the degradation and shrinkage of peat (Pfadenhauer and Klötzli 1996; Schindler et al. 2003). Furthermore, the change from anoxic to oxic conditions led to the decomposition of refractory organic substances due to the activation of oxidizing and hydrolytic enzymes (Freeman et al. 2001). Hence, elevated emissions of carbon dioxide, oxidation of iron sulphides to form Fe(III)-hydroxides and sulphates, and transformation of organic bound phosphorus, nitrogen and sulphur to inorganic substances have taken place (Lenz et al. 1992; Pfadenhauer and Klötzli 1996; Lamers et al. 1998; Schlichting et al. 2002; Litaor et al. 2004). Consequently, drained fens have lost some of their functions as nutrient sinks. In addition, due to seasonally induced and repeated drying/rewetting cycles, these fens act as sources of phosphorus, dissolved organic carbon (DOC) and nitrate which leach into adjacent surface waters (Lenz et al. 1992; Olde Venterink et al. 2002; Aldous et al. 2005; Worrall and Burt 2005).
Recently, more than 10,000 ha of drained fens in the state of Mecklenburg-Vorpommern (NE Germany) were rewetted to re-establish their ecological functions. Due to an extended drainage history, a re-establishment of their original state is not expected in the short-term. Thus, enhanced concentrations of P, DOC and ammonium can be found in the porewater of rewetted fens at a level of one to three orders of magnitude above pristine systems (Rupp et al. 2004; Zak et al. 2004; Tiemeyer et al. 2005). It can be assumed that the extent of peat mineralisation during drainage and varying geochemical conditions are decisive for the intensity of mobilisation processes after rewetting. Since upper peat horizons are most strongly affected by peat degradation, a characteristic vertical gradient from highly to slightly decomposed peat is formed in drained fens (Stegmann and Zeitz 2001; Schlichting et al. 2002; Schindler et al. 2003).
Past studies have pointed out that microbially induced redox reactions are important for the mobilisation of dissolved substances in rewetted fens (Lamers et al. 2002; Lucassen et al. 2004; Shenker et al. 2005). Therefore, we hypothesise that the upper highly decomposed peat layer is mostly responsible for the high mobilisation of P, DOC and ammonium due to enhanced supply of both oxidizing substances [Fe(III)-hydroxides, sulphate] and decomposable organic matter (OM) as electron donors.
To test this hypothesis, highly, moderately, and slightly decomposed peat samples of a drained fen were sampled and incubated under waterlogged conditions for the survey of P, OC and ammonium mobilisation. Results were compared with those from field measurements taken after the rewetting of the sampling site.
Methods
Study site
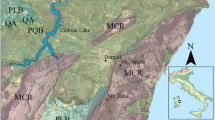
The study site “polder Zarnekow” (about 500 ha, latitude 53°52′N, longitude 12°53′E) is situated 8 km west of the town of Demmin in the valley of the River Peene in Mecklenburg-Vorpommern (NE Germany, Fig. 1). The climate is continentally influenced with a mean annual precipitation of 544 mm and a mean annual temperature of 8.1°C. The mean daily temperature is -0.8°C in January and 16.7°C in July (meteorological station Teterow, 24 km southwest of the sampling site). According to Joosten and Succow (2001), the studied fen can be classified as a river valley mire system consisting of spring mires at the valleys edge, wider percolation mires dominated by groundwater flow and a strip of flood mires along the River Peene. The fen is a carbonate buffered system and the peat is degraded to different degrees, which was determined by the “von Post-scale” (Puustjärvi 1970). The upper horizon is highly decomposed (0–0.3 m), followed by moderately decomposed peat until 1 m and a deep layer of slightly decomposed peat up to a maximum depth of 10 m. Drainage and low-intensive agricultural use began in the eighteen century (Lenschow et al. 2003) and land-use change to pastures and grassland was intensified by a complex dewatering system, embanking and a pump station to lower the groundwater tables in the mid-1970s.
Due to peat mineralisation and agricultural use, peat shrinkage of several decimetres took place. Therefore, after rewetting (October 2004), during the course of an EU-funded conservation project (European Agriculture Guidance and the Guarantee Fund) a larger area of the polder Zarnekow is now permanently flooded, with a water table depth of ∼0.1–0.5 m above peat surface.
Peat sampling and incubation study
Before the rewetting of the study site, altogether about 0.45 m³ of disturbed peat was extracted from three subsequent horizons of peat with differing degrees of peat decomposition (sampled horizons: 0.1–0.2 m with highly decomposed peat, 0.5–0.6 m with moderately decomposed peat, and 1–1.2 m with slightly decomposed peat). An overview of selected peat characteristics, including chemical data, is given in Table 1. Altogether, nine water-tight 96 L vessels (PVC-boxes: 0.6 × 0.4 × 0.4 m3) were filled with consistently about 40 kg of fresh peat (∼0.05 m3) from the above mentioned peat horizons (triplicates of highly, moderately and slightly decomposed peat samples). After water-logging the peat a water level of ∼5 cm above the peat surface was adjusted. This simulated rewetting was done with a sodium chloride solution (3.4 mM NaCl) to be in accordance with the average ionic strength of fen feeding groundwater and to prevent osmotic stress of micro-organisms in the rewetted peat. The water level of 5 cm was maintained by replenishment of evaporation loss with deionised water to guarantee waterlogged conditions in the peat throughout the experiment. The incubation experiment was carried out at water stagnant conditions and at a constant temperature of 20°C in a climate chamber. The porewater was taken in a passive way by permanently installed dialysis samplers according to Jacobs (2002). This sampling technique allows a non-disturbed monitoring of the course of dissolved substances in the porewater over several months. The applied one-chamber-sampler (about 0.05 L volume) was installed in the peat at a depth of 1–11 cm. For porewater sampling, the chamber water was extracted completely using pipettes (Multipette Eppendorf®) and then was directly renewed by oxygen free de-ionised water to avoid oxygen input into the anoxic porewater. Due to the small volume of the chamber water compared to the total peat porewater (0.05 L vs. ∼24 L) a dilution effect could be neglected. The sampling regime was biweekly for 8 weeks and monthly thereafter for a total of 54 weeks of incubation. Quick sampling and the fixation of samples with hydrochloric acids (2 M HCl) prevented the oxidation and subsequent precipitation of redox-sensitive substances like ferrous iron and the co-precipitation of DOC. Soluble reactive phosphorus (SRP) was directly fixed by the chemical agents for P analysis (see below) and measured within the same day. To deal with the strong supersaturation of the porewater with respect to CO2, air tight vessels were filled completely with non-diluted samples for the determination of dissolved inorganic carbon (DIC) immediately after collection of porewater.
Field measurements
After 2 weeks of the rewetting of the polder Zarnekow the in-situ monitoring of dissolved substances in the porewater was started. Contrary to the laboratory study, non-rechargeable dialysis samplers with 14 spaced chambers were used according to Hesslein (1976). Always three dialysis samplers were inserted into the peat close to the sampling point of the peat used for the incubation study. New samplers were introduced in the same sampling slits to neglect effects of peat heterogeneity. The sampling interval was monthly and the sampling depth was restricted to 0.7 m (length of the dialysis sampler). Sampling in accordance to the incubation study, after 10, 14 and 54 weeks after rewetting, was not possible due to ice cover during winter. The sampling procedure is described in detail by Zak et al. (2004). Accordingly, a homogenised composite sample of the 14 chambers (over the whole sampling horizon) was obtained for further analysis. In one case study only, a 56 chambers sampler (1 cm spaced chambers) was taken to yield the vertical profiles of SRP, DOC and ammonium after 42 weeks of rewetting.
Chemical analysis
For the determination of total P, C, N, Fe, and Ca contents (Pt, Ct, Fet, Nt, and Cat) of the incubated highly, moderately and slightly decomposed peat, always three samples (∼20 g) were dried to a constant weight at 105°C and homogenised in a stainless steel mill. Pt content of peat was determined as SRP after acid digestion in a solution of 2 ml 10 M H2SO4, 2 ml 30% H2O2 and 20 ml deionised water at 160°C for 6 h. Ct and Nt contents of peat were measured with a CN-Analyser (Vario EL, Elementar, Mt Laurel, NJ, USA). The Fet, Alt and Cat contents were analysed after digestion with “aqua regia” in a high pressure microwave oven (MLS GmbH) by flame atomic absorption spectrometry. Dry weight (dw) of peat was determined by drying three trials of each sample at 105°C until constant weight. The content of OM of the dry samples was measured as loss on ignition (550°C, 4 h).
Before, and at the end of the incubation study, the amount of bicarbonate–dithionite (BD) and NaOH soluble P and OC of the highly, moderately and slightly decomposed peat was determined using a sequential extraction procedure slightly modified from Psenner et al. (1984). Contrary to the “Psenner method”, the HCl step was done prior to the NaOH extraction to release P and OC bound to Fe or Al oxides, or calcium bound P, similar to Ivanoff et al. (1998). Accordingly, the BD fractions consist of reductant soluble P and OC mainly bound to Fe(III)-hydroxides (Lijklema 1980; Buffle 1988), and NaOH release labile, semi-labile and refractory P and OC substances such as phosphormonoester, poly-P, fulvic and humic acids (Hupfer et al. 1995; Ivanoff et al. 1998).
P concentrations in the BD and NaOH extracts were measured after above mentioned acid digestion to exclude analytical interferences with humic acids. Hence, from now on the inorganic and organic forms of P will not be distinguished between.
Soluble reactive phosphorus in the digested extracts and in the porewater samples was measured photometrically by the molybdenum blue method (Varian, Cary 1E) according to Murphy and Riley (1962) and ammonium in the porewater by the indophenol method (Flow Solution III, Perstop). DOC concentrations in the BD and NaOH extracts and in the porewater samples and DIC porewater concentrations were analysed with a C-Analyser (TOC 5000, Shimadzu, Kyoto, Japan). Iron and calcium concentrations in the porewater samples and iron concentrations in the BD extracts were determined by flame atomic absorption spectrometry (Perkin-Elmer, 3300), sulphate and chloride porewater concentrations by ion chromatography (Shimadzu). Porewater pH was measured by means of probes (WTW®) as well as redox potential (E h) using a Pt electrode with Ag/AgCl reference electrode. Hence, measured values were adjusted to standard hydrogen potential and pH = 7 (E 7 in V) according to Galster (2000).
Saturation indices of mineral phases were computed from the major dissolved species: Ca (2+), Fe (2+, 3+), N (3-), Cl (1-), S (6-), P (5+), C (4+) in porewater of rewetted peat according to the pH and E h using PHREEQC, Version 2 software (Parkhurst and Apello 1999).
The computer program SPSS 9 for Windows was used for statistical analysis (Repeated Measures Analysis of Variance and Post Hoc-Scheffé-test).
Results
BD and NaOH fractions of peat
An overview of the amount of BD and NaOH soluble substances before and after the incubation experiment (time: 54 weeks) is given in Table 2. The (initial) amount of redox-sensitive Fe(III)-hydroxides (BD–Fe) was significantly enhanced with increasing degree of peat decomposition. Likewise, the highest portion of BD–P was found in the highly decomposed peat; on average fivefold higher than in moderately decomposed peat and about 30-fold higher than in slightly decomposed peat. The portions of BD–OC were similar in highly and moderately decomposed peat, but fivefold higher than in slightly decomposed peat. Similar to the BD fractions, the amount of NaOH soluble P and OC was related to the degree of peat decomposition (see Table 2).
The molar ratios of Fe and P and OC of the BD and NaOH fractions are summarised in Table 2. The (initial) Fe : P ratios (BD-fractions) were lowest in the highly decomposed peat (∼15) compared to moderately and slightly decomposed peat (44–94). The lowest OC : P ratios (NaOH-fractions) were found in the highly decomposed peat (734–831); approximately twice as much in moderately decomposed peat (1,336–1,754) and three times as much in slightly decomposed peat (1,845–2,674).
Overall, only slight decreases, or in some cases also minor increases, of Fe, P and OC of the BD and NaOH fractions and were found during incubation regardless of the degree of peat decomposition (Table 2). For example, for highly decomposed peat, after 54 weeks of incubation, BD–P and NaOH–P had diminished by 7 and 4%, respectively. However, all changes were not significant (Repeated Measures Analysis of Variance, p > 0.05).
Changes of redox potential and pH in the porewater
The waterlogged conditions after rewetting led to a continuously lowering of the redox potential (E 7) in the porewater of incubated peat layers, as well as under in-situ conditions within the first 20 weeks of rewetting (Fig. 2a). Lowest values of about 0.1 V were reached in highly decomposed peat, somewhat higher values in moderately decomposed peat as well as in the field and highest values of about 0.3 V in slightly decomposed peat. The initial pH of 5.5–6.0, with lowest values in slightly decomposed peat, increased to final values of 6.5–6.8 in porewater of all incubated peat layers (Fig. 2b). Contrary to the incubation study, an increase of pH was not observed in the field study. After rewetting, pH shifted to the bicarbonate buffering range so all values became 6.6–7.1.
a–f The changes in the redox potential (E7) and the pH and the trends of sulphate (SO 2-4 ), iron (Fe), calcium (Ca) and dissolved inorganic carbon (DIC) concentrations in porewater of incubated peat layers with different degrees of peat decomposition (highly, moderately and slightly) according to von Post-scale (Puustjärvi 1970) and of in-situ measurements (mean ± SD, n = 3). No in-situ porewater samples could be obtained after 10, 14 and 54 weeks of rewetting because of ice cover during winter
Trends of porewater sulphate and iron concentrations
The beginning of rewetting was characterised by extremely high sulphate concentrations, both in all incubated peat layers and in-situ, in the range of 3–13 mM (Fig. 2c). However, a rapid decrease of sulphate concentrations was only observed in the incubated highly decomposed peat layer. After 22 weeks of rewetting, sulphate disappeared almost completely, whereas only slight decreases were found in moderately decomposed peat and continued to increase slowly for the slightly decomposed peat during the whole incubation period. A strong decrease of sulphate concentrations was also observed under field conditions after 26 weeks of rewetting, but the concentrations remained on a high level of about 3 mM until the end of the investigation.
Iron concentrations were below 0.01 mM in all incubated peat layers at the beginning and about 0.1 mM under in-situ conditions after 4 weeks of rewetting (Fig. 2d). Highest concentrations were found in the porewater of highly decomposed peat and under in-situ conditions after 22 weeks of rewetting (2.5 and 1.7 mM, respectively). Subsequently, a distinct decrease of iron concentrations occurred in highly decomposed peat until 30 weeks of incubation and until 34 weeks under in-situ conditions. The iron concentrations started to increase again in the porewater of highly decomposed peat, but not in-situ (Fig. 2d). Somewhat lower increases of iron concentrations were found in the porewater of moderately decomposed peat until a mean value of 1.2 mM was reached after 34 weeks of rewetting. Only a minor increase of iron concentrations was measured in slightly decomposed peat, resulting in a final mean concentration of about 0.1 mM.
Trends of porewater calcium and DIC concentrations
The highest increases of calcium and DIC concentrations were found in the porewater of incubated highly decomposed peat and under in-situ conditions (Fig. 2e, f). Again, somewhat lower increases were measured in moderately decomposed peat and zero or only minor increases in the porewater of slightly decomposed peat. According to computed saturation indices, the precipitation of siderite (FeCO3), aragonite (CaCO3) and other Ca and Fe containing minerals took place after 22 weeks of rewetting in porewater of highly decomposed peat as well as under field conditions.
Trends of porewater P, DOC and ammonium concentrations
The porewater concentrations of P, DOC and ammonium were low at the beginning of the rewetting both in the incubation and in the field study (Fig. 3a–c). Generally, the highest increases of these dissolved substances were measured in the porewater of highly decomposed peat. Increases in P concentrations took place until 50 weeks of rewetting, resulting in a final P concentration of 143 μM. In comparison, the in-situ P concentrations were about twofold lower during the whole rewetting period. Almost no increases in P concentrations could be found in porewater of moderately and slightly decomposed peat (Fig. 3a). Likewise, the highest in-situ P concentrations were found in the upper highly decomposed peat layer and significantly lower concentrations in the underlain moderately decomposed peat layer (Fig. 3d). However, the in-situ P concentrations in the porewater of the highly decomposed peat layer were two times smaller, and of moderately decomposed peat a hundred times larger, when compared to the incubation study.
a–c The trends of soluble reactive phosphorus (SRP), DOC and ammonium (NH +4 ) concentrations in porewater of incubated peat layers with different degrees of peat decomposition (highly, moderately and slightly) according to von Post-scale (Puustjärvi 1970) and of in-situ measurements (mean ± SD, n = 3). No in-situ porewater samples could be obtained after 10, 14 and 54 weeks of rewetting because of ice cap during winter. d–f The vertical profiles (1 cm resolution) of in-situ porewater concentrations of SRP, DOC and NH +4 in polder Zarnekow fen after 42 weeks of rewetting. The sampled horizon of (0–60 cm) consisted of two peat layers with different degrees of peat decomposition: highly decomposed peat (H 7–10), and moderately decomposed peat (H 5–6)
The highest increases in DOC concentrations were found in the highly decomposed peat up to 14 weeks of rewetting (Fig. 3b). Subsequently, concentrations varied between 38 and 46 mM until the end of the investigation. DOC concentrations in moderately decomposed peat and in-situ increased until 30 weeks of rewetting and reached values about two to three times lower than in highly decomposed peat. The initial porewater DOC concentrations of slightly decomposed peat declined from about 1.5 mM to a final mean value of 1.1 mM. The vertical in-situ measurement of the DOC concentrations after 42 weeks of rewetting showed a slight gradient of minor increasing concentrations up to 20 mM until a depth of 15 cm (Fig. 3e). Thereafter, the porewater DOC concentrations were about two times lower in highly decomposed peat and similar in moderately decomposed compared to the incubation study (Fig. 3b).
The ammonium concentrations at the beginning of rewetting were below 0.2 mM both in the incubation and the field study. Highest increases up to final mean concentrations of 1.9 mM were measured in porewater of incubated highly decomposed peat compared to 0.3 and 0.03 mM in moderately decomposed peat and slightly decomposed peat, respectively (Fig. 3c). Likewise high increases were found in the field study until 30 weeks of rewetting. Subsequently mean concentrations ranged between 0.7 and 0.8 mM. The vertical in-situ measurement of the ammonium concentrations after 42 weeks of rewetting showed a distinct gradient of increasing concentrations up to 0.5 mM until a depth of 10 cm (Fig. 3e). Below, the concentrations ranged between 0.4 and 0.6 mM. Thereafter, the porewater ammonium concentrations were about three times lower in highly decomposed peat and slightly higher in moderately decomposed compared to the incubation study (Fig. 3c).
Discussion
Rewetting of long-term drained peatlands has been shown to increase porewater concentrations of P, DOC and ammonium (van Dijk et al. 2004; Zak et al. 2004; Tiemeyer et al. 2005; Worrall and Burt 2005), which was also recorded for the site under investigation in this study (polder Zarnekow). Porewater is also characterised by high sulphate concentrations in the initial stage of rewetting (Fig. 2c) and is due to sulphide oxidation during the desiccation period, as demonstrated for other fens in northern Central Europe (Lamers et al. 1998; Gelbrecht et al. 2002; Lucassen et al. 2004). Specifically, sulphate can be stored as gypsum (CaSO4) in the calcareous peat of the investigated fen, which will be dissolved after rewetting. Surprisingly the initial sulphate porewater concentrations of incubated slightly decomposed peat were also found to be high, probably caused by sulphate leaching from upper degraded peat horizons and/or seepage of sulphate rich groundwater.
Our hypothesis that the upper highly decomposed peat layer is mostly responsible for the high mobilisation of P, OC and ammonium after rewetting of fens was confirmed by the results. Highest concentrations of P, DOC and ammonium were found in porewater of highly decomposed peat (Fig. 3a–c). Following processes must be taken into account to explain the high increases of P, DOC and ammonium concentrations:
-
ion-exchange reactions between solid and aqueous phase (Olila et al. 1997; Beltman et al. 2000; Kalbitz et al. 2000; Olde Venterink et al. 2002)
-
cleavage of particulate OM by hydrolytic and fermentation processes (Robinson et al. 1998; Turner et al. 2003)
-
microbial catalysed redox reactions in dependence of the availability of reducible substances (electron acceptors) and OM as electron donors (Lamers et al. 2002; Lucassen et al. 2004; Aldous et al. 2005; Shenker et al. 2005)
-
abiotic redox reactions such as reduction of Fe(III)-hydroxides by hydrosulphides (Lamers et al. 2002; Lucassen et al. 2004) or by phenolic substances (Deiana et al. 1995; Pracht et al. 2001)
The continuously lowering of the redox potential, coupled with high increases in iron and ammonium concentrations and rapid decreases in sulphate concentrations during the incubation study imply that the best conditions for microbial decomposition processes exist in highly decomposed peat (Figs. 2a, c, d, and 3c). Microbial decomposition of OM leads furthermore to a release of CO2 and large concentrations can be dissolved in the porewater, up to supersaturation levels and with the potential for dissolving existing carbonates (e.g. St Louis et al. 2003). Both processes can explain the high increases in DIC and calcium concentrations of highly decomposed peat.
Since Fe(III)-hydroxides are known to have high binding affinities to both P and OC substances (Lijklema 1980; Buffle 1988; Kalbitz et al. 2000), the reduction of Fe(III)-hydroxides can explain the strong increases of P and DOC in porewater of highly decomposed peat. Hence, we expected a significant decrease of Fe(III)-hydroxides and of the binding partners P and OC in highly decomposed peat, which was not confirmed by the results (Table 2). According to a simplified calculation (upward diffusion or microbial uptake not considered) about 8% of BD–P must be dissolved to reach the final P concentrations of 143 μM (water content of peat: 80%, amount of BD-P: 4.6 μmol g-1 dw), which is similar to the measured, but not significant decrease, of BD–P (7%, Table 2). However, NaOH–P may also be responsible for P release, since hydrolytic cleavage of particulate OM is supposed to be an important process for P mobilisation in peat soils too (Robinson et al. 1998; Turner et al. 2003). The comparatively low OC : P ratios of the NaOH-fractions suggest an enhanced microbial availability of OM in highly decomposed peat, supporting P mineralisation and/or mobilisation (Chapin et al. 2003). The minor or non-significant changes of P and OC fractions reveal that microbial processes may have significant effects on the porewater concentrations of dissolved substances, but only little or even no effect on the composition of the solid phase during the incubation period of 54 weeks. A release of Fe(III)-hydroxide bound substances due to increasing pH up to maximum values of 7.2 (Fig. 2b) can be neglected, since the zero point charge of Fe(III)-hydroxides occurs at pH 7.9–8.1 (Buffle 1988).
Strong increases of iron and DOC concentrations were found in moderately decomposed peat too (Figs. 2d, 3 b). The surprising lack of an increase of P concentrations in porewater of moderately decomposed peat can only be explained by the high molar Fe : P ratios in the BD extracts of moderately decomposed peat (∼73, Table 2), whereas the ratio was much lower in highly decomposed peat (∼15). Presumably, iron reduction did not result in a net P release in the porewater of moderately decomposed peat because P can be re-adsorbed at free sorption sites on the remaining excess of Fe(III)-hydroxides (Lijklema 1980; Jensen et al. 1999).
The lack of increases of P, DOC and ammonium porewater concentrations reveal that P, C and N mobilisation processes are of minor importance in slightly decomposed peat (Fig. 3a–c). The significantly lower amount of redox-sensitive bound P and OC, as well as of NaOH soluble P and OC, may be an explanation for these findings. In the slightly decomposed peat, the consistently very low-DIC concentrations over the incubation period, the lack of a decrease of sulphate concentrations and the high-OC : P ratios of NaOH extractable organic substances reveal that microbial decomposition processes are inhibited. An explanation might be the presence of phenolic compounds as potent inhibitors of microbial activity (Freeman et al. 2001).
Conclusions
-
1.
The rewetting and incubation of different peat layers clearly show the dependency of P, OC and ammonium mobilisation processes on the degree of peat decomposition. The significantly increased supply of oxidising substances [Fe(III)-hydroxides and sulphate] and the greater availability of OM as electron donors in highly decomposed peat, are responsible for the high P, OC and ammonium mobilisation after fen rewetting. And, strongly enhanced in-situ P, DOC and ammonium concentrations in underlain less decomposed peat layers are caused by downward diffusion or leaching processes.
-
2.
The drainage of the studied area led to significantly increased quantities of redox-sensitive bound P and OC and also of NaOH soluble P and OC substances in highly decomposed peat layers. The minor decrease of these solid pools implies that the enhanced P and OC mobilisation could last for a longer period of time in rewetted fens. The absence of an increase of P concentrations in moderately decomposed peat, despite high iron mobilisation, reveals the importance of the Fe : P ratio of the redox-sensitive solid phases.
-
3.
The findings of this incubation study are important for the management of fen rewetting. According to the results, a removal of the highly decomposed peat layer is recommended to reduce the high mobilisation of water polluting substances after rewetting and to support the re-establishment of the important ecological functions of fens.
References
Aldous A, McCormick P, Ferguson C, Graham S, Craft C (2005) Hydrologic Regime controls soil phosphorus fluxes in restoration and undisturbed wetlands. Restor Ecol 13(2):341–347
Beltman B, Rouwenhorst TG, Van Kerkhoven MB, Van der Krift T, Verhoeven JTA (2000) Internal eutrophication in peat soils through competition between chloride and sulphate with phosphate for binding sites. Biogeochemistry 50:183–194
Buffle J (1988) Complexation properties of homologous comlexants and choice of measuring methods. In: Chalmers RA, Masson M (eds) Complexation reactions in aquatic systems: an analytical approach. Ellis Horwood series in analytical chemistry. Chichester, UK, pp 304–383, 692 pp
Chapin CT, Bridgham SD, Pastor J, Updegraff K (2003) Nitrogen, Phosphorus, and Carbon mineralisation in response to nutrient and lime additions in peatlands. Soil Sci 168(6):409–420
Deiana S, Gessa C, Manunza B, Rausa R, Solinas V (1995) Iron(III) reduction by natural humic acids: a potentiometric and spectroscopic study. Eur J Soil Sci 46:103–108
Freeman C, Ostle N, Kang H (2001) An enzymatic latch on a global carbon store. Nature 409:149
Galster H (2000) Technique of measurement, electrode processes and electrode treatment. In: Schüring J, Schulz HD, Fischer WR, Böttcher WHM (eds) Redox fundamentals, processes and applications. Springer, Berlin, pp 13–23, 248 pp
Gelbrecht J, Exner H-J, Conradt S, Rehfeld-Klein M, Sensel F (2002) Water Chemistry. In: Köhler J, Gelbrecht J, Pusch M (eds) Die Spree—Zustand, Probleme und Entwicklungsmöglichkeiten. Limnologie aktuell, Bd. 10, Schweizerbarth, Stuttgart, pp 74–85, 384 pp
Hesslein RH (1976) An in situ sampler for close interval porewater studies. Limnol Oceanogr 21:912–914
Hupfer M, Gächter R, Giovanoli R (1995) Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci 57:305–324
Ivanoff DB, Reddy KR, Robinson S (1998) Chemical fractionation of organic phosphorus in selected histosols. Soil Sci 163:36–45
Jacobs PH (2002) A new rechargeable dialysis pore water sampler for monitoring sub-aqueous in-situ sediment caps. Wat Res 36:3121–3129
Jensen MB, Hansen HCB, Nielsen NE, Magid J (1999) Phosphate leaching from intact soil column in response to reducing conditions. Water Air Soil Pollut 113:411–423
Joosten H, Succow M (2001) Hydrogenetische Moortypen. In: Succow M, Joosten H (eds) Landschaftsökologische Moorkunde. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, pp 234–240, 622 pp
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Lamers LPM, van Roozendaal SME, Roelofs JGM (1998).Acidification of freshwater wetlands: Combined effects of non-airborne sulfur pollution and desiccation. Water Air Soil Pollut 105:95–106
Lamers LPM, Falla SJ, Samborska EM, Dulken IAR, Hengstum G, Roelofs JGM (2002) Factors controlling the extent of eutrophication and toxicity in sulphate-polluted freshwater wetlands. Limnol Oceanogr 47:585–593
Lenschow U, Jeschke L, Zscheile KH, Ziese B (2003) Geologie und Landschaftsgeschichte Mecklenburg-Vorpommerns. In: Department of Environment of Mecklenburg-Vorpommern (ed) Die Naturschutzgebiete in Mecklenburg-Vorpommern. Demmler Verlag GmbH, pp 8–18, 713 pp
Lenz A, Kleyn KP, Geller G (1992) Leaching of nitrogen and carbon by draining peat soils. Wasser + Boden 2:61–62
Lijklema L (1980) Interaction of orthophosphate with iron (III) and aluminium hydroxides. Environ Sci Technol 14:537–541
Litaor MI, Reichmann O, Auerswald K, Haim A, Shenker M (2004) The geochemistry of phosphorus in peat soils of a semiarid altered wetland. Soil Sci Soc Am J 68:2078–2085
Lucassen ECHET, Smolders AJP, Van de Crommenacker J, Roelofs JGM (2004) Effects of stagnating sulphate-rich groundwater on the mobility of phosphate in freshwater wetlands: a field experiment. Arch Hydrobiol 160:117–131
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Olde Venterink H, Davidsson TE, Kiehl K, Leonardson L (2002) Impact of drying and rewetting on N, P and K dynamics in a wetland soil. Plant Soil 243:119–130
Olila OG, Reddy KR, Stites DL (1997) Influence of draining on soil phosphorus forms and distribution in a constructed wetland. Ecol Eng 9:157–169
Parkhurst DL, Apello CAJ (1999) User’s guide to PHREEQC (Version 2)—a computer programm for speciation, batch reaction, one dimensional transport, and inverse agrochemicals calculations. Water-Resources Investigations Report 4259, US Department of the Interior, Denver, Colorado, 312 pp
Pfadenhauer J, Klötzli F (1996) Restoration experiments in middle European wet terrestrial ecosystems: an overview. Vegetatio 126:101–115
Psenner R, Pucsko R, Sager M (1984) Fractionation of organic and inorganic phosphorus compounds in lake sediments. An attempt to characterize ecologically important fractions. Arch Hydrobiol/Suppl 70:111–155
Pracht J, Boenigk J, Isenbeck-Schröter M, Keppler F, Schöler HF (2001) Abiotic Fe(III) induced mineralisation of phenolic substances. Chemosphere 44:613–619
Puustjärvi V (1970) Degree of humification. Peat Plant News 3:48–52
Robinson JS, Johnston CT, Reddy KR (1998) Combined chemical and 31P-NMR spectroscopic analysis of phosphorus in wetland organic soils. Soil Sci 163(9):705–713
Rupp H, Meissner R, Leinweber P (2004) Effects of extensive land use and rewetting on diffuse phosphorus pollution in fen areas—results from a case study in the Drömling catchment, Germany. J Plant Nutr Soil Sci 167:408–416
Shenker M, Seitelbach S, Brand S, Haim A, Litaor MI (2005) Redox reactions and phosphorus release in re-flooded soils of an altered wetland. Eur J Soil Sci 56:515–525
Scheffer B, Blankenburg J (1993) The determination of the bulk density of peat soils. Agribiol Res 46(1):46–53
Schindler U, Behrendt A, Müller L (2003) Change of soil hydrological properties of fens as a result of soil development. J Plant Nutr Soil Sci 166:357–363
Schlichting A, Leinweber P, Meissner R, Altermann M (2002) Sequentially extracted phosphorus fractions in peat-derived soils. J Plant Nutr Soil Sci 165:290–298
Stegmann H, Zeitz J (2001) Bodenbildende Prozesse entwässerter Moore. In: Succow M, Joosten H (eds) Landschaftsökologische Moorkunde. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, pp 47–57, 622 pp
St Louis VL, Partridge AD, Kelly CA, Rudd JWM (2003) Mineralization rates of peat from eroding peat islands in reservoirs. Biogeochemistry 64(1):97–110
Tiemeyer B, Lennartz B, Schlichting A, Vegelin K (2005) Risk assessment of the phosphorus export from a rewetted peatland. Phys Chem Earth 30:550–560
Turner BL, Chudek JA, Whitton BA, Baxter R (2003) Phosphorus composition of upland soils polluted by long-term atmospheric nitrogen deposition. Biogeochemistry 65:259–274
Van Dijk J, Stroetenga M, Bos L, van Bodegom PM, Verhoef HA, Aerts R (2004) Restoring natural seepage conditions on former agricultural grasslands does not lead to reduction of organic matter decomposition and soil nutrient dynamics. Biogeochemistry 71:317–337
Worrall F, Burt T (2005) Predicting the future DOC flux from upland peatland catchments. J Hydrol 300:126–139
Zak D, Gelbrecht J, Steinberg CEW (2004) Phosphorus retention at the redox interface of peatlands adjacent to surface waters in northeast Germany. Biogeochemistry 70:357–368
Acknowledgements
Timothy Jones (Bangor, UK) is gratefully acknowledged for editing the manuscript and three anonymous reviewers for the helpful comments to a previous version of the manuscript. Many thanks to all colleagues of the Central Chemical Laboratory of IGB who supported the comprehensive chemical analyses: Hans-J. Exner for the assistance of the AAS analyses, Elke Zwirnmann and Thomas Rossoll for the carbon and the sulphate measurements. Special thanks to Titus Rohde for the support at the incubation study. We offer thanks to Bernd Schütze and Antje Lüder for the technical assistance during field work and Sabine Jordan (Humboldt-Universität zu Berlin) for the peat characterisation and determination of the degree of peat decomposition. The study was supported by the Department of Environment of Mecklenburg-Vorpommern as well as by the European Agriculture Guidance and Guarantee Fund (EAGGF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zak, D., Gelbrecht, J. The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 85, 141–151 (2007). https://doi.org/10.1007/s10533-007-9122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-007-9122-2