Abstract
Twenty-four surface sediment samples were collected from Liaohe River in June 2014 for the analysis of total concentrations of Cu, Pb, Zn, Cd, Ni, Fe, and Mn. The spatial distribution of heavy metals in Liaohe River was site specific, with Hun River as the most polluted river mainly affected by industrial and human activities. The contents of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEMs) in Liaohe River varied significantly, ranging from 0.03 to 19.4 μmol/g and 0.14 to 10.8 μmol/g, respectively. Sulfate-reducing bacteria (SRB) community size, organic matter and sulfate availability, and sediment redox status may be the main factors affecting the AVS distribution. Among all the acid-extracted metals, Zn was dominant in all samples, whereas much more toxic Cd contributed less than 1.0 % to the total SEMs. Sediment quality guidelines (SQGs) and AVS-SEM models were used to predict the sediment toxicity. Results revealed that only a small portion of sites exhibited potential metal toxicity to aquatic biota, while adverse effects should rarely occur in majority of sites. Comparison of the two assessment methods showed inconsistent results, indicating that each method had its own limitations. The combination of different methods will be more convincing as to the sediment quality assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been a great concern for decades about heavy metal contamination, because they are toxic, are resistant to biodegradation, and have potential to bioaccumulate and biomagnificate via food chains, thus posing risks to organisms and even human beings. Most metals entering into the aquatic environment are concentrated in the particle phase and finally deposited and immobilized in sediments. However, metals can be released to the interstitial or overlying water when environmental conditions change (pH, sediment redox potential, etc.) or by biological activities, increasing exposure and causing threats to organisms and humans (Van Den Berg et al. 1999). Evaluating the content, distribution, and potential ecological effect of heavy metals in sediments is therefore important and necessary.
To better assess metal pollution in sediments, a number of sediment quality guidelines (SQGs) have been developed for marine and freshwater ecosystems. These numerical SQGs have been proposed as an informal benchmark to aid in the interpretation of sediment chemistry data (Wenning and Ingersoll 2002), and to assess the potential adverse effects on aquatic biota based on the bulk metal contents (Long et al. 1995; Macdonald et al. 1996). However, increasing studies have shown that total concentrations do not reflect the bioavailability of metals in sediments and give an inaccurate estimate of the likely environmental impact (Vink 2002; Campana et al. 2013). In general, bioavailability and toxicity of metals in sediments are controlled by different metal-binding phases, e.g., organic matter, carbonate, iron and manganese oxides, clay, and acid-volatile sulfides (AVSs) (Chapman et al. 1999). As the major reactive phase for metals in anoxic sediments, AVS has received considerable attention in recent years (Garcia et al. 2011; Ribeiro et al. 2013; Li et al. 2014a). In the reaction with divalent metals, stable metal sulfide precipitates will be formed, causing a decreased level of free metal ions and therefore reducing metal bioavailability (De Jonge et al. 2009). Actually, AVS is operationally defined as the fraction of sulfides in sediments that are extracted by addition of 1.0 M HCl. The metals liberated during the extraction of AVS are called simultaneously extracted metals (SEMs). Accordingly, Di Toro et al. (1990) formulated the AVS-SEM model for evaluating sediment toxicology at the beginning of the 1990s. Since then, AVS-SEM models have been successfully used for predicting potential metal availability in laboratory and field studies (Di Toro et al. 1990; Berry et al. 1996) and are widely used in both marine and freshwater sediments (Burton et al. 2007; Nizoli and Luiz-Silva 2012). Up to date, both SQGs and AVS-SEM methods have been extensively applied to evaluate the sediment quality (Ribeiro et al. 2013; Mwanamoki et al. 2014; Li et al. 2014b). However, it is unclear whether consistent results can be obtained by the two different methods.
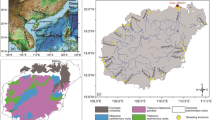
Liaohe River is the largest river in northeast China. It consists of Liao River, Taizi River, Hun River, and Daliao River (Fig. 1). The Taizi River and Hun River flow into Daliao River at their confluence, before finally entering the Bohai Sea. The mid-stream and downstream area of Liaohe River covers the largest industrial bases in northeast China, with metallurgy, machinery, petrochemical, electronic, and building material industries. In the past decade, along with the intensive industrial and human activities, plenty of industrial and domestic wastewater without effective treatment was directly discharged into water (Gao et al. 2012). Many studies have reported the pollution of metals and other potentially toxic substances in Liaohe River (Wu et al. 2011; Zhou et al. 2011; Lv et al. 2014). In terms of metal pollution, however, most studies were focused on the total metal concentrations on a tributary scale (Jiang et al. 2013; Wu et al. 2011, 2012). To our knowledge, no large-scale research on the bioavailability of metals and AVS and SEM pollution was conducted throughout Liaohe River. The aims of this study were to address these concerns, including (1) characterizing the spatial distribution of heavy metals, AVS, and SEM in sediments and exploring the possible relationship among variables; (2) assessing the potential bioavailability and toxicity of heavy metals based on SQGs and AVS-SEM models; and (3) elucidating environmental significance of predicting the environmental impacts of heavy metals by different evaluation methods.
Materials and methods
Sample collection
Duplicate sediment samples (0–10 cm) were collected from 24 sites in Liaohe River using a Van Veen grab sampler in June 2014 (Fig. 1). Among all sites, nine (L1–L9) were from Liao River, five (H1–H5) from Hun River, six (T1–T6) from Taizi River, and four (D1–D4) from Daliao River. The samples were placed into sealed polyethylene bags as quickly as possible to avoid oxidation, transferred to an ice box, and then immediately frozen and kept at −20 °C. Studies indicated that sediments stored refrigerated without a nitrogen atmosphere for up to 10 days exhibited no loss of AVS as compared to the original values (Boothman 1992). The samples for AVS determination were analyzed within 10 days of collection. Subsamples were freeze-dried, crushed, passed through a 2-mm sieve, and stored at 4 °C in dark until analysis.
Chemical analysis
Sediment characteristics analysis
Sediment pH was measured in a 0.01 M CaCl2 extract with a pH electrode. Water content was determined based on the weight loss dried at 105 °C for 24 h. The particle size distribution was analyzed by a Malvern Mastersizer 2000 laser diffractometer which allowed the measurement ranging from 0.02 to 2000 μm. According to DIN 4022 standard values, the size of particles in the sediment fraction was defined including clay (<2 μm), silt (2–63 μm), and sand (>63 μm) fractions. Potassium dichromate oxidation–ferrous sulfate titrimetry method was applied to analyze the total organic carbon (TOC) content (GAQS-IQ 2008).
Total heavy metal analysis
An amount of 0.10-g sample (<63 μm) was completely digested using the National Standard Method (GB/T17140) with HCl-HNO3-HF-HClO4 acids in a closed microwave digestion system. The obtained residue was diluted to 50.0 mL with 1.0 % HNO3 and transferred into polyethylene flasks for analysis. The concentrations of Cu, Pb, Zn, Cd, Ni, Fe, and Mn were analyzed by inductively coupled plasma mass spectroscopy (ICP-MS, Agilent Technologies 7500 Series, USA). Analytic accuracy was achieved by use of blanks and certified reference materials NIST-SRM 2704 (National Institute of Standards and Technology, riverine sediment). Replicate analysis of certified samples showed good accuracy with recoveries for all metals between 90.0 and 105.0 %.
AVS and SEM analysis
The determination of AVS and SEM was conducted according to the cold-acid purge and trap technique described by Allen et al. (1993). Briefly, the experimental setup consisted of a round-bottom reaction flask connected to a trapping vessel containing 10 mL of zinc acetate solution. After sparging for 10 min with N2, approximately 3.0 ± 0.01 g of damp sediment was added and sparged for another 10 min. Twenty milliliters of deoxygenated HCl (1.0 M) was then introduced into the reaction flask using a syringe, and the system was continuously bubbled with N2 at a flow rate of 40 mL/min for 45 min under constant magnetic stirring, with H2S produced being collected in the zinc acetate solution. The sulfide concentration was determined spectrophotometrically by the methylene blue method (Shimadzu UV1201 UV–vis spectrophotometer). A standard sodium sulfide was used to develop the calibration curve. The efficiency of the AVS extraction procedure was checked by using a sodium sulfide solution of known concentration in triplicates, and the average recovery was greater than 90.0 %. The remaining sediment suspension was separated by centrifugation and filtered through a 0.45-μm membrane, and the contents of SEM were detected by ICP-MS. The remaining sediments were dried and weighted. The results were expressed as μmol/g sediment (dry weight). Replicates were analyzed for 20.0 % of samples, and the relative standard deviations of AVS and SEM concentrations ranged from 10.0 to 15.0 %. Concentrations of SEM were calculated by summing five individual metals extracted (Cu, Pb, Zn, Cd, and Ni), denoted as ΣSEM.
Statistical analysis
Normality and homogeneity of data were evaluated with Kolomogorov-Simirnov test and Levene test. The spatial variation in total metal, AVS, and SEM concentrations was tested by a one-way ANOVA, while Krustal-Wallis nonparametric test was applied since the variables were not normally distributed. If significant differences were found, least significant difference (LSD) multiple comparison test or nonparametric Mann–Whitney U test was conducted for pairwise comparison. A spearman correlation analysis was used to determine the potential correlations between different variables. The level of significance was set at 0.05. All statistical analyses were performed with SPSS 19.0.
Results and discussion
Sediment physicochemical characterization
An overview of sediment characteristics at different sites is provided in Table 1. The water content of sediments varied greatly from 17.6 to 85.2 %. Sediment pH ranged from 7.0 to 8.5. Obvious differences in the organic carbon contents occurred in Liaohe River, ranging from 1.4 to 105.1 mg/g, with the highest value recorded at site H3, which received a large amount of sewage from wastewater treatment plant in the upstream. Fine-grained sediments were predominant in Liaohe River. The ternary diagram in Fig. 2 categorizes all the sediments revealing that sediments in Liaohe River were predominantly composed of silty sand and sandy silt.
Spatial distribution of total metals
Total concentrations of Ni, Cu, Zn, Cd, and Pb in sediments of Liaohe River are listed in Table 2. Among these metals, Zn had the highest values (34.3–398.7 mg/kg), followed by Cu (5.2–67.1 mg/kg), Ni (7.4–16.2 mg/kg), Pb (5.2–13.7 mg/kg), and Cd (0.2–1.1 mg/kg). Compared with the mean levels of heavy metals in other areas (Table 3), sediments in Liaohe River were slightly polluted. The maximum levels of metals detected were all observed at site H2 located in Fushun City, which could be related to the contribution from industrial sources and residential activities. The sites adjacent to big cities (e.g., sites L9, H3, H4, T3, and D2) and mining areas (e.g., sites T2 and T4) were also highly contaminated. For the entire basin, the levels of heavy metals exhibited significant spatial variations (p < 0.05) with the exception of Pb. In general, the mean concentrations followed an increasing order of Liao River < Daliao River < Taizi River < Hun River. This spatial heterogeneity was site specific, and the serious pollution resulted from the rapid industrial development and urbanization of Liaoning province.
The relationship between heavy metals and sediment characteristics was revealed by a Spearman correlation analysis. Positive correlations (p < 0.05) between metals and TOC except for Cd (r s = 0.51 to 0.73) were observed, suggesting that organic matter influenced the distribution of these metals. The organic matter may provide sorption or reaction sites, retaining metals in sediments or forming the more toxic organo-metallic complexes driving their distributions in the aquatic environment (Ribeiro et al. 2013). This positive correlation also indicated a possible unique source and transport pathway of these metals (Mwanamoki et al. 2014). In addition, the close relationships between Fe, Mn, and metals were found with correlation coefficients of 0.42 (Fe-Pb, p < 0.05) to 0.86 (Fe-Ni, p < 0.01) and 0.50 (Mn-Pb, p < 0.05) to 0.90 (Mn-Ni, p < 0.01), respectively. These findings revealed that iron oxides or hydroxides, manganese oxides, or magnesium hydroxides had great affinity for these metals.
Spatial distribution of AVS
The AVS contents in sediments of Liaohe River ranged from 0.03 to 19.4 μmol/g, with a mean value of 2.4 μmol/g (Fig. 3). This value was at a moderate level compared with the mean AVS contents of other areas (Table 3). The highest mean concentration was observed in Taizi River, followed by Hun River, Daliao River, and Liao River. The AVS level in sediments is the result of the equilibrium between the generation via the reduction of sulfate to sulfide by SRB and loss by oxidation or diffusion (Lasorsa and Casas 1996). Factors affecting the supply of organic matter and SO4 2−, the rate of SO4 2− reduction, and redox condition of sediments could cause the AVS variation. The highest AVS level occurred at site H3, where the sediment had the greatest TOC content. The organic matter could supply bacteria with carbon for their metabolism, increasing their activity hence favoring the reduction reaction and consequently AVS production (Oenema 1990). Relatively high levels were found in sediments at sites H5, T2, T4, T6, and D4, corresponding to the regions with more frequent human activities. These regions were impacted by terrigenous inputs such as waste discharges which were rich in organic matter, potentially contributing to the formation of hypoxia status and inhibition of AVS oxidation (Garcia et al. 2011). Therefore, the spatial distribution of AVS content was mainly affected by factors including SRB community size, organic matter and sulfate availability, and sediment redox status (Prica et al. 2008). In addition, flood, storm, wind, bioturbation, and dredging activities may also be the contributing factors (Audry et al. 2004).
The correlation matrix for AVS and sediment characteristics was summarized in Table 4. The AVS was highly correlated with water content and TOC and moderately correlated with the silt content. Lower water content allowed more penetration of O2 into sediments and hence increased the loss of AVS by oxidation (Van Griethuysen et al. 2006). Organic matter was the source of energy for SRB. In the bacteria living, AVS was the by-product of organic matter degradation which led to the production of fine-grained organic material simultaneously. So AVS contents were positively related with silt fraction. Besides, sediments with high TOC content and small particle size could contribute to the formation of anoxic conditions caused by TOC oxidation and low oxygen renewal and provide an ideal condition for SRB (Machado et al. 2008).
Spatial distribution of SEM
Concentrations of ΣSEM in sediments of Liaohe River were in the range of 0.14–10.8 μmol/g, with an average value of 1.9 μmol/g (Fig. 3). As revealed by comparison with the mean ΣSEM levels of other regions in Table 3, this concentration was low. The highest level of ΣSEM was observed at site H2, consistent with the result of total metals. Relatively high levels were observed in Hun River, Taizi River, and Daliao River, which was also in accordance with the distribution of total metals, indicating the impact of human activities. Sediment characteristics, including pH, redox potential, cationic exchange capacity, carbonates, and organic matter contents may regulate the dynamics of metal precipitation or solubilization, partly explaining the variation of ΣSEM contents (Nizoli and Luiz-Silva 2012).
Among all the extracted metals, Zn was dominant in all samples (Fig. 4). This element accounted for approximately 41.5–97.7 % of ΣSEM, whereas much more toxic Cd contributed less than 1 % to ΣSEM. The average concentrations of the extracted metals were ranked in the following order of [SEM]Cd < [SEM]Pb < [SEM]Ni < [SEM]Cu < [SEM]Zn. The different solubility of metal sulfides was an important factor affecting the proportion of different metals in ΣSEM. In natural sediments, AVS existed primarily as iron monosulfide complexes commonly referred to as mackinawite and greigite (Leonard et al. 1993). The Ksp of metal sulfides increased in the order of PbS < CdS < NiS < ZnS < CuS < FeS (Cooper and Morse 1998a, b). Cationic metals (Cu, Zn, Ni, Cd, and Pb) with lower solubility than FeS can displace Fe from their mono-sulfides to form highly insoluble metal sulfides, which were extracted together with AVS. Therefore, Zn can dissolve more easily than Pb and Cd in sediments during the extraction procedure. The different proportions of metals also depended on the reaction characters of metals with HCl. Cooper and Morse 1998a, b pointed that nickel and copper sulfides (NiS, NiS2, Ni3S2, CuS, and Cu2S) were poorly extracted in cold acid (1.0–31.0 % after 1.0 h in 6.0 M HCl); therefore, these metals were not easily extracted by acids.
The water content, TOC, and silt fraction of sediments turned out to be significant factors explaining the spatial variations in ΣSEM content (Table 4). Due to the adsorption and flocculation effect of fine particle fractions and organic matter, smaller silt and organic-rich sediments may have an elevated capacity for binding metals, increasing the accumulation of metals in sediments (Machado et al. 2004). Significantly positive correlations between ΣSEM and AVS (p < 0.01) confirmed that AVS was one of the major carriers for heavy metals. It has been reported that AVS had the priority to bind with metals, although organic matter, Fe-Mn oxyhydroxide, and carbonate may be the important binding phases (Yu et al. 2001). Even a small amount of AVS can sequester a significant quantity of metals and should be considered in determining the potential toxicity of metals (Di Toro et al. 1992).
Toxicity assessment of heavy metals in sediment
Two empirical SQGs were used to evaluate the possible toxicity of total metals to aquatic biota, including effect range low (ERL) and effect range mean (ERM) (USA), and threshold effect level (TEL) and probable effect level (PEL) (Australia and New Zealand). These SQGs delineate concentration ranges where adverse biological effects are expected rarely (below low-range value), occasionally (between low and mid-range values), and frequently (above mid-range value). The comparison results in Table 2 demonstrated that for Zn, 20.9 % of samples were above PEL, while 50.0 and 58.3 % of samples exceeded ERL and TEL, respectively, indicating that sediments in Liaohe River were severely polluted with Zn and corresponding toxicity may occur. As for other metals, the concentrations were all below ERM and PEL, whereas some values exceeded ERL or TEL, suggesting that occasional adverse effects might occur at these sites. However, the frequency, nature, and severity of adverse effects were difficult to predict, and further investigation was needed (CCME 2002). It was notable that sediments at H2 and H3 may pose serious hazard to aquatic biota, due to the levels of three or four metals exceeding ERL or TEL.
AVS-SEM models are based on the difference between the AVS and ΣSEM molar concentrations to predict the bioavailability and toxicity of heavy metals. If there is sufficient AVS, a large amount of metals is retained in the form of thermodynamically stable metal sulfide precipitates, which results in a decreased content of free metal ions and therefore reduced metal bioavailability. Based upon the threshold ratio of SEM to AVS in Table 5 (Burton et al. 2005), sediments at six sites in Liaohe River were expected to be potentially toxic to aquatic life, whereas sediments at other sites may have no risk of adverse effects. USEPA also proposed the criteria on the basis of AVS-SEM (USEPA 2004, 2005). In these criteria, three tiers were set as revealed in Table 6, i.e., associated adverse effects on aquatic life were probable (tier 1), possible (tier 2), or no indication of associated adverse effects (tier 3). According to the criterion of 2004, eight samples were classified as tier 3 with no adverse effect while two at sites L7 and H2 were classified as tier 1 with probable hazard to aquatic organisms, and adverse effects were uncertain for other sites. Owing to the fact that there existed many other metal-binding phases in sediments such as TOC, not all sediments with ΣSEM > AVS can cause toxicity (Di Toro et al. 2005). The complementary evaluation method taking into consideration of TOC content was proposed (USEPA 2005). Based on this criterion, the number of the sites with no adverse effects increased, because the binding phase of organic matter mediated bioavailability of metals. It was noted that adverse effects were expected at site L7, while 20 sites were not likely to be toxic and toxic effects were uncertain for the rest 3 sites. Given the uncertainty, a comparison of the three models perhaps provided a stronger indication of the potential metal bioavailability. One site (L7) exceeded all the three AVS models, one site (H2) exceeded two models, and four sites (L2, L9, H1, and D1) exceeded one model only. These results indicated that L7 was in great potential for hazard effect, while H2 was potentially at risk and other four sites (L2, L9, H1, and D1) were possibly having metal toxicity.
Environmental significance of adopting different evaluation methods
Sites in Liaohe River predicted to be toxic were different based on ERL-ERM, TEL-PEL, and AVS-SEM models, respectively. The inconsistent results were primarily due to the limitations of each method. The empirical SQGs generally developed for a specific region were greatly influenced by geography, environment, and derivation factors (Macdonald et al. 2000; Ingersoll et al. 2001). These SQGs have not been validated effectively for other areas with possible differences in sediment geochemistry or biological diversity. In addition, the grain size, pH, specific species, exposure time, or population stress, which affected the bioavailability, toxicity, and susceptibility of SQGs were not fully considered (Hübner et al. 2009). AVS-SEM models have shown their value to estimate toxic effects on aquatic organisms in laboratory and field studies (Van Griethuysena et al. 2003). However, even if there is much higher levels of ΣSEM than of AVS, metals in sediments may not cause toxicity to biota (Allen et al. 1993). Other geochemical processes could remove metals from porewater and reduce their bioavailability, including metal ion adsorption, complexation with organic matter, precipitation, and dissolution with clays, carbonates, and/or oxyhydroxide minerals (Leonard et al. 1996). Furthermore, AVS was an operationally defined parameter, affected by a variety of dynamic biochemical processes, such as deposition, oxidation or bioturbation, resulting in the AVS variation temporally and spatially (Burton et al. 2007). Therefore AVS-SEM models could not lead to a definitive conclusion about the sediment toxicity. In conclusion, using only one single approach for sediment toxicity prediction may not be sufficient. The supplement of AVS-SEM criteria to sediment quality assessment will be necessary due to its simple operation and high efficiency for ecotoxicological assessment (De Lange et al. 2008). For example, the AVS-SEM results can help identify the areas of major environmental concern, and thus priority sites can be established for further detailed studies (Campana et al. 2009). The combination of sediment quality guidelines and AVS-SEM models will be more convincing as to the final decision of sediment quality and environmental management strategies. In addition to chemical analysis, the complement of biological effect assessments, such as sediment toxicity tests or benthic community surveys, can provide a powerful weight of evidence for future ecological risk assessment.
Conclusions
The concentrations of total heavy metals, AVS, and SEM in sediments of Liaohe River were investigated to determine their pollution status and evaluate the potential toxic risks. The spatial distribution of these pollutants indicated that Hun River, Taizi River, and Daliao River were heavily polluted than Liao River, due to the anthropogenic inputs from industry and frequent human activities. Although the pollution of heavy metals and AVS in Liaohe River was identified, the potential toxicity to aquatic life was unexpected in most sites examined by empirical SQGs and AVS-SEM models. The comparison of the results based on the two methods indicated that a single approach to toxicity assessment may be insufficient. The combination of different methods will be more convincing. In addition to chemical analysis, biological effect assessments are required as well in the future study.
References
Allen HE, Fu G, Deng B (1993) Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ Toxicol Chem 12:1441–1453
Audry S, Schäfer J, Blanc G et al (2004) Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot river reservoirs (France). Environ Pollut 132:413–426
Berry WJ, Hansen DJ, Boothman WS et al (1996) Predicting the toxicity of metal-spiked laboratory sediments using acid-volatile sulfide and interstitial water normalizations. Environ Toxicol Chem 15:2067–2079
Boothman WS (1992) Vertical and seasonal variability of acid volatile sulfides in marine sediments. In 13th Annual Meeting Society of Environmental Toxicology and Chemistry- Abstracts
Burton GA, Nguyen LTH, Janssen C et al (2005) Field validation of sediment zinc toxicity. Environ Toxicol Chem 24:541–553
Burton GA, Green A, Baudo R et al (2007) Characterizing sediment acid volatile sulfide concentrations in European streams. Environ Toxicol Chem 26:1–12
Campana O, Rodríguez A, Blasco J (2009) Identification of a potential toxic hot spot associated with AVS spatial and seasonal variation. Arch Environ Contam Toxicol 56:416–425
Campana O, Blasco J, Simpson SL (2013) Demonstrating the appropriateness of developing sediment quality guidelines based on sediment geochemical properties. Environ Sci Technol 47:7483–7489
CCME, Canadian Council of Ministers of the Environment (2002) Canadian sediment quality guidelines for the protection of aquatic life: Summary tables. Updated. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg.
Chapman PM, Wang F, Adams WJ et al (1999) Appropriate applications of sediment quality values for metals and metalloids. Environ Sci Technol 33:3937–3941
Cooper DC, Morse JW (1998a) Biogeochemical controls on trace metal cycling in anoxic marine sediments. Environ Sci Technol 32:327–330
Cooper DC, Morse JW (1998b) Extractability of metal sulfide minerals in acidic solutions: application to environmental studies of trace metal contamination within anoxic sediments. Environ Sci Technol 32:1076–1078
De Jonge M, Dreesen F, De Paepe J et al (2009) Do acid volatile sulfides (AVS) influence the accumulation of sediment-bound metals to benthic invertebrates under natural field conditions? Environ Sci Technol 43:4510–4516
De Lange HJ, Van Griethuysen C, Koelmans AA (2008) Sampling method, storage and pretreatment of sediment affect AVS concentrations with consequences for bioassay responses. Environ Pollut 151:243–251
Di Toro DM, Mahony JD, Hansen DJ et al (1990) Toxicity of cadmium in sediments: the role of acid volatile sulfide. Environ Toxicol Chem 9:1487–1502
Di Toro DM, Mahony JD, Hansen DJ et al (1992) Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments. Environ Sci Technol 26:96–101
Di Toro DM, McGrath JA, Hansen DJ et al (2005) Predicting sediment metal toxicity using a sediment biotic ligand model: methodology and initial application. Environ Toxicol Chem 24:2410–2427
Gao Y, Zhang H, Su F et al (2012) Environmental occurrence and distribution of short chain chlorinated paraffins in sediments and soils from the Liaohe River Basin, PR China. Environ Sci Technol 46:3771–3778
GAQS-IQ (General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China) (2008) The speciation for marine monitoring - part 5: sediment analysis. Beijing, pp. 50–52 (in Chinese)
Garcia CAB, de Andrade PE, Alves JPH (2011) Assessment of trace metals pollution in estuarine sediments using SEM-AVS and ERM-ERL predictions. Environ Monit Assess 181:385–397
Hübner R, Astin KB, Herbert RJH (2009) Comparison of sediment quality guidelines (SQGs) for the assessment of metal contamination in marine and estuarine environments. J Environ Monit 11:713–722
Ingersoll CG, MacDonald DD, Wang N et al (2001) Predictions of sediment toxicity using consensus-based freshwater sediment quality guidelines. Arch Environ Contam Toxicol 41:8–21
Jiang J, Wang J, Liu S et al (2013) Background, baseline, normalization, and contamination of heavy metals in the Liao River Watershed sediments of China. J Asian Earth Sci 73:87–94
Lasorsa B, Casas A (1996) A comparison of sample handling and analytical methods for determination of acid volatile sulfides in sediment. Mar Chem 52:211–220
Leonard EN, Mattson VR, Benoit DA et al (1993) Seasonal variation of acid volatile sulfide concentration in sediment cores from three northeastern Minnesota lakes. Hydrobiologia 271:87–95
Leonard EN, Ankley GT, Hoke RA (1996) Evaluation of metals in marine and freshwater surficial sediments from the environmental monitoring and assessment program relative to proposed sediment quality criteria for metals. Environ Toxicol Chem 15:2221–2232
Li F, Wen YM, Zhu PT (2008) Bioavailability and toxicity of heavy metals in a heavily polluted river, in PRD, China. Bull Environ Contam Toxicol 81:90–94
Li F, Lin J, Liang Y et al (2014a) Coastal surface sediment quality assessment in Leizhou Peninsula (South China Sea) based on SEM–AVS analysis. Mar Pollut Bull 84:424–436
Li L, Wang X, Zhu A et al (2014b) Assessing metal toxicity in sediments of Yellow River wetland and its surrounding coastal areas, China. Estuar Coast Shelf Sci 151:302–309
Long ER, MacDonald DD, Smith SL et al (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Lv J, Zhang Y, Zhao X et al (2014) Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in sediments of Liaohe River: levels, spatial and temporal distribution, possible sources, and inventory. Environ Sci Pollut Res. doi:10.1007/s11356-014-3666-1
Macdonald DD, Carr RS, Calder FD et al (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5:253–278
Macdonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Machado W, Carvalho MF, Santelli RE et al (2004) Reactive sulfides relationship with metals in sediments from an eutrophicated estuary in Southeast Brazil. Mar Pollut Bull 49:89–92
Machado W, Santelli RE, Carvalho MF et al (2008) Relation of reactive sulfides with organic carbon, iron, and manganese in anaerobic mangrove sediments: implications for sediment suitability to trap trace metals. J Coast Res 24:25–32
Mwanamoki PM, Devarajan N, Thevenon F et al (2014) Trace metals and persistent organic pollutants in sediments from river-reservoir systems in democratic republic of Congo (DRC): spatial distribution and potential ecotoxicological effects. Chemosphere 111:485–492
Nizoli EC, Luiz-Silva W (2012) Seasonal AVS–SEM relationship in sediments and potential bioavailability of metals in industrialized estuary, southeastern Brazil. Environ Geochem Health 34:263–272
Oenema O (1990) Pyrite accumulation in salt marshes in the Eastern Scheldt, southwest Netherlands. Biogeochemistry 9:75–98
Prica M, Dalmacija B, Rončević S et al (2008) A comparison of sediment quality results with acid volatile sulfide (AVS) and simultaneously extracted metals (SEM) ratio in Vojvodina (Serbia) sediments. Sci Total Environ 389:235–244
Ribeiro AP, Figueiredo AMG, Santos JO et al (2013) Combined SEM/AVS and attenuation of concentration models for the assessment of bioavailability and mobility of metals in sediments of Sepetiba Bay (SE Brazil). Mar Pollut Bull 68:55–63
USEPA, United States Environmental Protection Agency (2004) The Incidence and Severity of Sediment Contamination in Surface Waters of the United States. National Sediment Quality Survey. EPA-823-R-04-007, second ed., Washington, DC: United States Environmental Protection Agency, Office of Science and Technology
USEPA, United States Environmental Protection Agency (2005) Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Metal Mixtures (Cadmium, Copper, Lead, Nickel, Silver, and Zinc). EPA-600-R-02-011, Washington, DC: United States Environmental Protection Agency, Office of Research and Development
Van Den Berg GA, Loch JPG, Van Der Heijdt LM et al (1999) Mobilisation of heavy metals in contaminated sediments in the river Meuse, The Netherlands. Water Air Soil Pollut 116:567–586
Van Griethuysen C, De Lange HJ, Van den Heuij M et al (2006) Temporal dynamics of AVS and SEM in sediment of shallow freshwater floodplain lakes. Appl Geochem 21:632–642
Van Griethuysena C, Meijboom EW, Koelmans AA (2003) Spatial variation of metals and acid volatile sulfide in floodplain lake sediment. Environ Toxicol Chem 22:457–465
Vink JPM (2002) Measurement of heavy metal speciation over redox gradients in natural water-sediment interfaces and implications for uptake by benthic organisms. Environ Sci Technol 36:5130–5138
Wenning RJ, Ingersoll CG (2002) Executive summary of the SETAC Pellston workshop on use of sediment quality guidelines and related tools for the assessment of contaminated sediments. Society of Environmental Toxicology and Chemistry (SETAC), Pensacola, FL, USA
Wu Z, He M, Lin C et al (2011) Distribution and speciation of four heavy metals (Cd, Cr, Mn and Ni) in the surficial sediments from estuary in daliao river and yingkou bay. Environ Earth Sci 63:163–175
Wu Z, He M, Lin C (2012) Environmental impacts of heavy metals (Co, Cu, Pb, Zn) in surficial sediments of estuary in Daliao River and Yingkou Bay (northeast China): concentration level and chemical fraction. Environ Earth Sci 66:2417–2430
Yin H, Deng J, Shao S et al (2011) Distribution characteristics and toxicity assessment of heavy metals in the sediments of Lake Chaohu, China. Environ Monit Assess 179:431–442
Yu KC, Tsai LJ, Chen SH et al (2001) Chemical binding of heavy metals in anoxic river sediments. Water Res 35:4086–4094
Zhou LJ, Ying GG, Zhao JL et al (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Acknowledgments
This work was financially supported by Major Science and Technology Program for Water Pollution Control and Treatment (2012ZX07501-001) and National Science Foundation of China (51178438).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
About this article
Cite this article
He, Y., Meng, W., Xu, J. et al. Spatial distribution and toxicity assessment of heavy metals in sediments of Liaohe River, northeast China. Environ Sci Pollut Res 22, 14960–14970 (2015). https://doi.org/10.1007/s11356-015-4632-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4632-2