Abstract
Recruitment of the European eel (Anguilla anguilla) has fallen steadily in recent decades, with current levels understood to be at around 5 % of those in the 1970s, and the species is now widely recognised as being endangered. Changes in ocean currents, climate shifts, habitat loss, overfishing, barriers to migration, increased predation, plastic litter and exposure to chemicals have all been postulated as potential causative factors. Several studies have shown a general decline in eel quality (lower lipid content and body condition) over time that may be linked to reduced reproductive success. In this study, data from an eel sampling campaign in 1987 are compared with recent data (2004–2008) for eels in Scotland to assess any temporal changes in eel quality indicators and also to assess any links between current levels of chemical exposure and eel quality. Mean lipid levels, as a percentage of wet muscle mass, were higher in 2004–2008 (37 ± 1.9 % SE) than in 1986 (21 ± 0.9 % SE). By contrast, mean body condition index (K) was slightly lower in the latter period. Considering the 2004–2008 samples, significant inter-site variation was observed for condition index K, while intra-site variation was observed for lipid content and physical parameters relative to age (i.e. mass/age, length/age and lipid/age ratios); however, the variations observed could not be linked to differences in chemical body burdens, indicating that no chemical impacts on the parameters assessed are discernible during the continental life stage of eels in Scotland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The population of the European eel (Anguilla anguilla) has undergone a severe and sustained decline over several decades (Belpaire et al. 2011; Dekker et al. 2007; ICES 2006), with an ongoing decline in recruitment estimated at 95 % from 1979 to the present throughout Europe (ICES 2011). The decline has led to the inclusion of the European eel on the International Union for Conservation of Nature (IUCN) list of critically endangered species (Freyhof and Kottelat 2008) and to the European Commission initiating a European Eel Recovery Plan which obliges member states to establish national eel management plans (EC 2007). Eels spawn in the vicinity of the Sargasso Sea, and their larvae cross the Atlantic on ocean currents to the continental shelf, where they metamorphose into unpigmented ‘glass eel’ and begin to recruit to the coasts and freshwaters of Europe. Their growth phase, when they are known as ‘yellow eel’, may take from 2 to 50 years, before they metamorphose again into their mature form, known as ‘silver eel’, and return to the Sargasso to breed. Eels may be impacted by pressures at all life stages, and changes in ocean conditions, increased predation, fishing exploitation, habitat loss, plastic litter, chemical contamination and introduced parasites and diseases have all been postulated as potential causative factors for their decline (Freyhof and Kottelat 2008; Geeraerts et al. 2011; ICES 2006; Knights 2003; Larsson et al. 1991).
Through direct and indirect toxicity, contaminant burdens could potentially lower the fitness of eels and so ultimately reduce breeding success (van Ginneken et al. 2009). Persistent organic pollutants (POPs) are of particular concern in this regard because eels have characteristics and lifestyle habits that render them susceptible to exposure to, and accumulation of, these contaminants, e.g. eels have high lipid contents, are long-lived in northern Europe, are benthic feeders and spawn only once during their lifetime (ICES 2011; Larsson et al. 1991). Research, monitoring and conservation programmes across the EU have contributed to a growing body of evidence on the contaminant levels in European eels (de Boer et al. 2010; de Boer and Hagel 1994; Macgregor et al. 2010; McHugh et al. 2010; Roose et al. 2003; Szlinder-Richert et al. 2010). Accumulation of contaminants may primarily impact upon eels during their long migration to the breeding grounds of the Sargasso Sea, when they metabolise lipid energy reserves and subsequently release stored contaminants into the bloodstream (Larsson et al. 1991). However, contaminants may also retard or impair the development of eels at an earlier stage of growth, e.g. by impeding lipid accumulation or other disruptive effects (see discussion in Belpaire et al. 2009).
Decreases in the mean lipid content of yellow eels (from ~21 to ~13 % of wet muscle mass) over a 30-year period have been recorded in the Netherlands (de Boer et al. 2010), while very similar decreases (from 20 to 12 %) have also been reported in Belgium between 1994 and 2006 (Belpaire et al. 2009). Such reductions in lipid content may indicate a decrease in the general fitness of eels which could have a bearing on their reproductive success. Indeed, a lipid content of 20 % or greater has been estimated as the fat level threshold needed for eels to have sufficient energy reserves to undergo normal migration and reproduction (Belpaire et al. 2009; Boetius and Boetius 1980). It is currently unclear whether the fat level declines observed independently in the Netherlands and Belgium reflect a Europe-wide trend; thus, there is a pressing need for data from other countries. More studies examining inter-relationships between POPs in eels and the factors that influence or indicate their health status (i.e. lipid content, growth rates and condition index) are also needed, particularly those that investigate multiple catchments across regions and/or countries. Such needs have been formally recognised and have prompted the recent foundation of the European eel quality database (Belpaire et al. 2011; ICES 2011).
In this study, we use historical data from an eel survey in 1986 together with data generated from eels sampled during the period 2004–2008 to examine trends in lipid content and body condition index (K) in eels in Scotland. Moreover, relationships between pollutant concentrations (polychlorinated biphenyls (PCBs), brominated diphenyl ethers (BDEs) and organochlorine pesticides) in muscle tissues and eel quality indicators are investigated. This represents an important step forward in understanding the status of eels in this part of Europe and the factors that may influence it.
Methods
Sites and sample collection
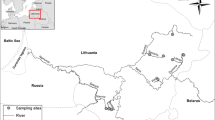
European eels (Anguilla anguilla) in the yellow life stage were collected from various sites across Scotland via electrofishing (Fig. 1). Data from eels captured at 33 sites in 1986 (Wells et al. 1987) were used alongside data from eels captured at 31 sites between 2004 and 2008 (see Macgregor et al. 2010). Eels of length >25 cm were targeted in both time periods and 19 of the sampling sites featured in both campaigns. Details of the sample collection and handling methods employed, as well as a list of sites investigated, have been given previously (Macgregor et al. 2010; Wells et al. 1987; see also Fig. 1, Table 1 and Supplementary Information Table 1).
Map of Scotland (mainland and near-shore islands, with inset of Great Britain) showing eel collection sites from the 1986 and 2004–2008 surveys. Sites from the 2004–2008 survey where eel ages were determined are also indicated, and their site numbers are shown (see Table 1 for location names)
Sample processing and chemical analysis
In the laboratory, following measurement of eel length and weight, muscle tissue samples (taken from posterior to the anus after skin removal) were prepared for lipid and chemical analyses. The analytical and quality control procedures used for the 2004–2008 samples and, where relevant, their minor differences to those employed in 1986 have been described and discussed previously (Macgregor et al. 2010), but in brief, a 1–2-g aliquot of freeze-dried tissue from each eel was extracted by accelerated solvent extraction (ASE). The ASE extracts underwent cleanup using an acid silica column or gel permeation chromatography (GPC), followed by an alumina column. Sample extracts were analysed for hexachlorocyclohexane (γ-HCH) and dichlorodiphenyldichloroethylene (DDE; the long-lived derivative of DDT, used here together with γ-HCH as representatives of organochlorine pesticides), PCBs (including the seven ICES indicator congeners) and BDEs (congeners 28, 47, 66, 71, 75, 85, 99, 100, 138, 153, 154, 183) using gas chromatography (GC) with electron capture detector (Hewlett-Packard 5890, GC-ECD) and GC-tandem mass spectrometry (Varian 1200 L triple quadrupole, GC-MS/MS) with rapid MS capabilities. Certified standards, used for calibration and precision checks, purchased from Ultra Scientific included 31 PCB congeners, HCH (α, β, γ), p,p′-DDE, p,p′-DDD and p,p′-DDT. Standards for BDE congeners were purchased from Cambridge Isotope Laboratory and Greyhound. Extractions were conducted in small batches (five samples), with each batch also including a process blank, eel matrix blank, spiked eel matrix and a certified reference material. For analysis, the samples were batched with independent standards and also with calibration drift standards to assess concentration drift throughout the run. Calibration curves used for quantification were all better than r 2 > 0.995. Percentage recoveries (100 × measured/certified values) for certified standards were satisfactory and consistent, e.g. 101 % for BDE 153, 91 % for p,p′-DDE and 92 % for PCB 138.
The lipid contents of eels were determined gravimetrically for both the historic 1986 (Soxhlet extraction, on one composite tissue sample per site) and the 2004–2008 (ASE, on each individual eel) datasets and were expressed as percentage wet muscle mass.
For a subset of the 2004–2008 sampling sites (Fig. 1, Table 1), the age of eels was determined by extracting and examining otoliths. Otoliths were prepared and analysed using the burning and cracking technique as described by the ICES Advisory Committee (ICES 2009).
Condition assessments
A condition index (K) was calculated following Le Cren’s relative condition factor as recommended by Froese (2006); for each individual, the observed mass in grams (W) is divided by an ideal mass (W′) that is determined from the simple length–weight regression (W = aL b) for the whole population. Here, we calculated the length–weight regression using data from both Macgregor et al. (2010; data from all individual eels) and Wells et al. (1987; length and mass average per site) as well as that from an additional site not previously included in published works (Cessnock Water, site 0 in Table 1). For the subset of the 2004–2008 sites that had eel ages determined (i.e. the aged eel subset), physical parameters and condition factors relative to age (i.e. log mass/age; length/age; lipid content/age) were also assessed to identify any differences among sites.
Tissue concentrations of the persistent organic pollutants were examined for any relationship to condition index and/or eel quality indicators. The aged eel data set represents eels most recently assessed (2006–2008) and consequently had the most comprehensive range of analyses performed (i.e. BDE and a wider array of PCB congeners). Some of the chemical impact evaluations conducted here are therefore limited to these eels.
Statistical evaluations
As appropriate for the varying comparisons intended (i.e. between time periods and amongst sites) and for the distributions of the datasets examined, a statistical approach combining multiple linear regression, t tests and analysis of variance (ANOVA) plus non-parametric tests (Kruskal–Wallis and Mann–Whitney, for non-normally distributed data) was employed. Pearson correlations were also employed. Following standard convention, significance levels for all tests used α = 0.05. Statistical evaluations were conducted using the software packages Analyse-it (v2.2) and Sigmaplot (v10).
Results and discussion
Eel condition trends 1986–2008
Treating all eels in our datasets (including values from Macgregor et al. (2010) and Wells et al. (1987)) as being part of a single population, the length–weight (mass) relationship was defined by log weight (g) = 3.159 log length (cm) − 3.008 (r 2 0.952; Fig. 2). The relationship was used to calculate Le Cren’s condition index (K) by rearranging the regression into the form W = aL b, which identified the parameters a and b as 0.000983 and 3.159, respectively. If the regression was performed considering only the eels collected during 2004–2008 (i.e. excluding the historic length–weight data from 1986), the parameter values were calculated as a = 0.000758 and b = 3.2268. On both counts, the parameter values are comparable to those determined for eels from the Camargue Nature Reserve in France (Oliveira Ribeiro et al. 2005).
Length, weight and lipid percentage, as well as calculated condition index (K) values, were compared between the 1986 and 2004–2008 eels. There was no difference in the length or mass of eels sampled in the two time periods (Mann–Whitney U tests, p > 0.05; Fig. 3), indicating that meaningful comparisons could be made with respect to other potential differences in other physical parameters between sample periods. The condition index K was statistically lower for the 2004–2008 eels compared to the 1986 eels (i.e. t test p < 0.001, with 1986 mean of 1.088 compared with a 2004–2008 mean of 0.995; Fig. 3), but whether this small numeric difference in K has any ecological relevance is difficult to determine. However, similar regression equations for log10 length versus log10 mass for the 1986 (y = 2.975x − 2.695; R 2 0.983) and 2004–2008 (y = 3.227x − 3.120; R 2 0.95) data (regressions not shown) may indicate that the ecological significance of the difference in K between time periods is limited.
A more significant difference was identified for lipid content, with values from 2004–2008 being higher than those from 1986 (1986 mean = 21 %; 2004–2008 mean 37 %, Figs. 3 and 4). The significant difference was consistent when comparing both (i) 1986 site composite sample lipid percentages versus 2004–2008 individual eel lipid percentages (Mann–Whitney, p < 0.0001) and (ii) 1986 site composite sample lipid percentages versus 2004–2008 site average lipid percentages (t test, p < 0.0001). Moreover, the significant differences were consistent even when values from only the 19 sites surveyed in both time periods were compared. Thirty-seven percent is an unusually high fat content for yellow eels (Tesch 2003), and this apparent increase in mean lipid content since the 1980s is contrary to the trends observed in Belgium and the Netherlands over a similar period, where lipid levels have fallen (Belpaire et al. 2009; de Boer et al. 2010). This unexpected increase in lipid % with time thus demanded close examination of our methodology to rule out the possibility that any procedural artefacts within the study could have influenced the outcome, most notably the use of slightly differing lipid analysis techniques between 1986 and 2004–2008. In both periods, gravimetric methods were used, following ASE for 2004–2008 and Soxhlet extraction for 1986, on tissue from the tail region after skin removal. Comparisons of the two methods have previously shown them to be equivalent; ASE lipid determinations averaged 102 % of Soxhlet measurements, with all samples within 90–111 %, when compared for meat tissues with lipid contents of 32–67 % (DIONEX 2011). Similar reassurance can be gained from de Boer et al. (2010), who discuss the equivalence of related lipid determination methods. Furthermore, examination of individual site data for the 19 sites that were sampled in both time periods assessed here revealed that the observed increase in lipid content was not uniform but rather varied amongst sites, e.g. by a factor of 1.1 for River Tweed, 1.6 for River Ness, 2.0 for River Leven, 2.8 for River Don, 3.2 for River Dee and 4.0 for River Cree. This indicates that the increases were not systematic and thus cannot be ascribed to, e.g. minor differences in methodology. The size of eels examined was similar in both time periods, and so, no systematic difference in size can account for the observed result.
The reason for this significant increase in the lipid content of eels is not clear, but it does correspond to a notable decrease in the body burdens of organochlorine pesticides (DDE and γ-HCH) over the same time period, as previously identified (Macgregor et al. 2010). Stepwise multiple linear regression by backwards elimination (assessing the variables site, year, log mass, log length, log γ-HCH and log DDE) for all 1986–2008 data did nominally identify DDE and γ-HCH concentration as variables with statistical significance in relation to predicting lipid content (Table 2), but the percentage variance they accounted for in the model was very low (<3 %). Indeed, when regressed individually (i.e. log DDE vs log lipid content and log γ-HCH vs log lipid content), DDE was found to have no significant relationship to lipid level (p = 0.169), while there was only a slight negative relationship between γ-HCH concentration and lipid content (p = 0.026; R 2 = 0.03; slope −0.031). This latter relationship may in any case simply reflect the phenomenon reported elsewhere of pollutants becoming diluted with increasing fat levels (Ferrante et al. 2010; Larsson et al. 1991). Therefore, the influence of decreases in γ-HCH concentration on lipid contents of eels in Scottish freshwaters over time is likely to have been negligible.
Water temperature has been linked to lipid content in eels (Andersson et al. 1991), but while there is some evidence for minor increases in water temperature in Scotland between the two sampling periods (Anderson et al. 2010), the effect size potentially due to this increase is likely trivial (c.f. Dosoretz and Degani 1987). Reduction in eel density might influence body composition by providing improved feeding opportunities; however, despite a general decline in the European eel, there is no evidence for changes in eel population density between the two periods, at least not in Western Scotland where this has been assessed (Adams et al. 2013). Therefore, population density change is unlikely to be the explanation here. Changes in environmental concentrations of (unidentified) endocrine-disrupting substances were thus a possible explanation worth exploring (see “Examination of pollutant influence on eel status (2004–2008 age determined samples)”).
The large observed increase in lipid content and concomitant minor decrease in body condition index (Figs. 3 and 4) is a counter-intuitive finding and could indicate physiological changes such as relative decreases in protein levels or possibly decreases in skeletal or intestinal bulk. Perhaps the most likely explanation, however, is that the relative water content of the eels has decreased approximately in line with lipid increase, as typically observed in many fish species (Love 1970). However, Belpaire et al. (2009) observed that declines in fat content were accompanied by declines in condition factor in Belgian eels, so this aspect may warrant further study.
Condition status of eels sampled 2004–2008
Considering sites from 2004–2008 (Fig. 1, Table 1 and see also Macgregor et al. (2010)), 29 of 31 sites had median eel lipid contents in excess of the 20 % threshold that has been assumed as necessary to undergo normal migration and reproduction in silver eels (Belpaire et al. 2009; Boetius and Boetius 1980). This is in sharp contrast to the lipid levels observed in a recent comprehensive study of five catchments in Ireland (McHugh et al. 2010) where the majority of eels examined were below this threshold. Multiple linear regression of the 2004–2008 dataset revealed that log mass and log length were the only significant predictors for lipid content, but that the model generated from these parameters could only account for 15 % of the variance (Table 3). Notably, ‘site’ (location) was not a significant variable and this was further confirmed by a Kruskal–Wallis test of lipid contents of eels from different sites in the 2004–2008 dataset (p = 0.0832). None of the POPs for which data are available for the whole 2004–2008 dataset (i.e. DDE, γ-HCH and Σ7PCB; see Macgregor et al. (2010) for concentration values) were identified as a significant predictor of lipid levels.
There was a weak but significant relationship between condition index K and lipid content (regression R 2 0.1218, p < 0.001), suggesting a link between the two parameters. There were weak and positive relations between K and DDE (correlation r 0.025, p 0.01) and Σ7PCB (correlation r 0.23, p 0.015), again likely reflecting the point that larger eels can accumulate more POPs when present. Other than this, there were no apparent relationships between K and other parameters. Upon examining K values for individual sites, it was apparent that eels from the River Nith had relatively low values (Table 1 and Supplementary Information Table 1). Statistical evaluation (Kruskal–Wallis test, pairwise comparison to River Nith eels, p = 0.0066) confirmed that significant differences existed between K values for River Nith eels and those from seven other sites in the full 2004–2008 dataset (River Irvine, White Cart Water–Hammils, White Cart Water–Pollock, Annick Water, Whiteadder Water, River Ness and River Don; sites 3, 4, 5, 7, 20, 28 and 30, respectively, Supplementary Information Table 1). The lower condition index K for the River Nith eels could not simply be attributed to any unusually low length, mass or lipid values for the fish, as for both length and mass the River Nith eels ranked in the mid range of values observed (Table 1, Supplementary Information Table 2) and, as noted above, there were no statistically significant differences in lipid levels amongst fish from different sites. Our previous study (Macgregor et al. 2010) also indicated that eels from the River Nith are not particularly contaminated with POPs.
For the smaller dataset of eels for which age was determined (Table 1), no statistical differences were found between sites in terms of the age of eels examined (Kruskal–Wallis, p > 0.05). This allowed meaningful comparisons to be made among sites for parameters length, lipid and mass relative to age (i.e. potential growth rate indicators). However, statistical examinations found no differences between sites in terms of lipid content/age, length/age or mass/age (Kruskal–Wallis and, where appropriate, ANOVA on log-transformed data, using pairwise comparisons with Bonferroni corrections, p > 0.05). This lack of identified difference between sites for these parameters may have arisen on account of the considerable intra-site variability observed at some locations (Fig. 5).
Inter-quartile range of length in cm/age (upper panel) and log mass in g/age (lower panel) for eels that had ages determined (a subset of sites sampled 2004–2008). Medians (solid line) and means (dotted line) are indicated (see Table 1 for site names). Note, no significant differences were identified between sites
Pearson correlations between eel age and the parameters mass, length and lipid content were performed on the aged eel dataset (considering individual eels from all sites). The correlations indicated significant but modest relationships across the whole dataset for age versus mass (r = 0.43, p = 0.001, n = 54) and age versus length (r = 0.45, p = 0.001, n = 54), and a non-significant relationship for age versus lipid content (r = 0.07, p = 0.670, n = 41), which contrasts with the higher correlation coefficients observed for eels in a Swedish study (Larsson et al. 1991) where r values of 0.78 and 0.83 (p < 0.01, n = 52) were recorded for age versus mass and age versus length, respectively. The stronger correlations in the Swedish study may be due to the eels all coming from a single site and thus possibly having experienced less variation in terms of food resource availability, water temperature, predation and chemical exposure, but any differences between studies in terms of the age ranges examined could also be a factor (i.e. if the Swedish study included eels from a wider age range). The possibility that differences in chemical burdens might account for the variability in the current study is discussed in the next section.
Examination of pollutant influence on eel status (2004–2008 age determined samples)
In addition to revealing a notable decrease in organochlorine pesticide concentrations in eel tissues at most sites since the 1980s, previous work on eels in Scotland revealed that DDT derivatives were still detectable at most locations examined and that there was widespread detection of PCBs and, to a lesser degree, BDEs (Macgregor et al. 2010).
Figure 6 presents PCB congener profiles of eels in the age determined dataset (i.e. the eels for which BDE and the full range of PCB congener data is available) and shows that, while varying congener mixtures were apparent, the profiles at numerous sites reflect the commercial mixtures of Aroclor 1254 and 1260. The variations observed reveal some degree of difference in terms of input sources amongst sites, probably resulting from varying mixtures of local and distant sources. Notably, the dioxin-like congener PCB-81 formed a substantial component of the PCB burden of eels at sites 11, 12, 21, 22 and 23, while another dioxin-like congener, PCB-126, was also notable at sites 22 and 25. However, linear regressions of PCB-81 and PCB-126 concentrations against K and against the log-transformed parameters length/age, lipid/age, lipid content and mass/age, considering all individual eels in the aged fish dataset, revealed no negative associations (i.e. p > 0.05; except for log lipid vs PCB-81, where p = 0.048, but the association was positive and thus did not indicate any potential negative effect).
Mean PCB congener profiles (percentage contribution from each congener) for eels at each site in the age-determined dataset. Values above columns indicate the total PCB burden in μg/kg (sum of detectable PCB congeners considering all those measured for 13, 28, 52, 49, 47, 44, 66, 101, 81, 110, 77, 151, 123, 118, 114, 153, 105, 141, 138, 126, 187, 183, 128, 167, 156, 157, 180, 169, 170, 189, and 194; data compiled from this study and Macgregor et al. 2010) (see Table 1 for site names). Congener profiles for Aroclor mixtures 1254a, 1254 g and 1260 are also shown (excluding minor constituents representing <3 %, for ease of viewing)
The potential influence of all the dioxin-like PCBs detected in eels were assessed by calculating toxic equivalent values (TEQs, relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)) using the WHO toxic equivalent factors (TEFs) for fish (Van Den Berg et al. 1998). Linear regressions considering all individual fish in the aged eel dataset failed to identify any associations between TEQs and the parameters K, length/age, lipid/age, mass/age and lipid content (p > 0.05; parameters log-transformed where appropriate). This lack of association for TEQs was not surprising considering the low concentrations observed; the maximum TEQ observed in a single eel was 0.06 μg/kg, for one fish from site 22 (River Esk), while mean TEQ values for each site, calculated from detected and quantified PCB concentrations only, were below 0.04 μg/kg. Therefore, together with the findings discussed in “Condition status of eels sampled 2004–2008” (i.e. regarding no negative associations of Σ7PCBs with either lipid content or K when considering all eels captured 2004–2008), no negative influences from any PCBs (dioxin-like or otherwise) were discernable in the parameters examined in this study for eels in Scotland in the continental life stage.
When BDE and K data were examined across the aged eel dataset using multiple linear regression, neither site nor BDE concentration significantly accounted for the variance observed for K (p > > 0.05; Supplementary Information Table 3). This was supported by Pearson correlations on an individual site basis (BDE vs K for individual fish at each site, with Bonferroni corrections for multiple tests) that found no significant correlations (Supplementary Information Table 4). Linear regression of all eels in the aged eel dataset revealed a weak positive association between log BDE concentration and the growth parameter log mass/age (p = 0.016, R 2 = 0.11, n = 54; Supplementary Information Table 3), possibly again simply indicating that larger eels tend to have accumulated more BDEs when present. No relationships were found between log BDE concentration and log lipid content/age or length/age (e.g. Supplementary Information Table 3).
Consistent with the finding that DDE concentration had no significant negative association with K when all eels in the entire database were examined (sections “Eel condition trends 1986–2008” and “Condition status of eels sampled 2004–2008”), when individual sites in the aged eel dataset were assessed no site returned a significant correlation between K and DDE once correction for multiple tests were made (Supplementary Information Table 4). Correction for multiple tests can conceivably increase the likelihood of false negatives (or type II errors) (Perneger 1998), but even without multiple test correction, there were only three sites with a non-corrected p value <0.05 (Supplementary Information Table 4). Linear regression of DDE concentration against other physical and quality parameters (lipid content, mass/age, length/age, lipid/age and age, log transformed where appropriate), considering all individual eels with available data, found no significant relationships (p > 0.05).
Conclusions
Within the limitations of our dataset, which comprises eels from two sample periods only (1986 and 2004–2008), a detectable change in condition index (K) was identified between 1986 and 2004–2008. A more significant shift in lipid contents was observed between time periods, with eels from the 2004–2008 sampling period having greater lipid levels. This is in direct contrast to the trends recorded in Belgium and the Netherlands though the mechanisms involved and causative factors remain unclear. For the most recently sampled eels (2004–2008 period), no links between physical/quality parameters and persistent organic pollutants were identified, indicating that POPs do not appear to have discernable negative impacts on eel quality in Scotland during the continental life stage.
References
Adams CE, Godfrey JD, Dodd JA, Maitland PS (2013) Is proximity to the North Atlantic Drift and the Continental Shelf Current sustaining freshwater European eel populations in west Scotland? Freshw Biol 58:1–9
Anderson H, Futter M, Oliver IW, Redshaw CJ (2010) Trends in Scottish river water quality. Macaulay Land Use Research Institute and Scottish Environment Protection Agency, Edinburgh
Andersson J, Sandström O, Hansen HJM (1991) Elver (Anguilla anguilla L.) stockings in a Swedish thermal effluent-recaptures, growth and body condition. J Appl Ichthyol 7:78–89
Belpaire C, Geeraerts C, Evans D, Ciccotti E, Poole R (2011) The European eel quality database: towards a pan-European monitoring of eel quality. Environ Monit Assess 183:273–284
Belpaire CGJ, Goemans G, Geeraerts C, Quataert P, Parmentier K, Hagel P, De Boer J (2009) Decreasing eel stocks: survival of the fattest? Ecol Freshw Fish 18:197–214
Boetius I, Boetius J (1980) Experimental maturation of female silver eels, Anguilla anguilla. Estimates of fecundity and energy reserves for migration and spawning. Dana 1:1–28
de Boer J, Dao QT, van Leeuwen SPJ, Kotterman MJJ, Schobben JHM (2010) Thirty year monitoring of PCBs, organochlorine pesticides and tetrabromodiphenylether in eel from The Netherlands. Environ Pollut 158:1228–1236
de Boer J, Hagel P (1994) Spatial differences and temporal trends of chlorobiphenyls in yellow eel (Anguilla anguilla) from inland waters of the Netherlands. Sci Total Environ 141:155–174
Dekker W, Pawson M, Wickstrom H (2007) Is there more to eels than slime? An introduction to papers presented at the ICES Theme Session in September 2006. ICES J Mar Sci: J Conseil 64:1366–1367
DIONEX (2011) Rapid determination of fat in meat using Accelerated Solvent Extraction (ASE)—Application Note 334
Dosoretz C, Degani G (1987) Effect of fat rich diet and temperature on growth and body composition of European eels (Anguilla anguilla). Comp Biochem Physiol A Physiol 87:733–736
EC (European Commission) (2000) Directive 2000/60/EC (the Water Framework Directive) of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Communities L327(2000):1–72
EC (European Commission) (2007) Council Regulation (EC) No 1100/2007 of 18 September 2007 establishing measures for the recovery of the stock of European eel. Offic J European Union 22.9.2007 L 248:17–23
Ferrante MC, Clausi MT, Meli R, Fusco G, Naccari C, Lucisano A (2010) Polychlorinated biphenyls and organochlorine pesticides in European eel (Anguilla anguilla) from the Garigliano River (Campania region, Italy). Chemosphere 78:709–716
Freyhof J, Kottelat M, (2008) Anguilla anguilla, International Union for Conservation of Nature (IUCN) Red List of Threatened Species 2010
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Geeraerts C, Focant JF, Eppe G, De Pauw E, Belpaire C (2011) Reproduction of European eel jeopardised by high levels of dioxins and dioxin-like PCBs? Sci Total Environ 409:4039–4047
ICES (2006) Report of the 2006 session of the Joint EIFAC/ICES Working Group on Eels. Rome, 23–27 January 2006. EIFAC occasional paper 38. ICES CM 2006/ACFM:16
ICES (2009) Workshop on age reading of European and American eel (WKAREA), 20–24 April 2009, Bordeaux, France. ICES CM 2009\ACOM: 48. pg 66 International Council for the Exploration of the Sea (ICES)
ICES (2011) Report of the 2011 Session of the Joint EIFAAC/ICES Working Group on Eels, Lisbon, Portugal, 5–9 September 2011. EIFAAC occasional paper 48. ICES CM 2011/ACOM:18
Knights B (2003) A review of the possible impacts of long-term oceanic and climate changes and fishing mortality on recruitment of anguillid eels of the Northern Hemisphere. Sci Total Environ 310:237–244
Larsson P, Hamrin S, Okla L (1991) Factors determining the uptake of persistent pollutants in an eel population (Anguila anguilla L.). Environ Pollut 69:39–50
Love RM (1970) The chemical biology of fishes. Academic, London, p 547
Macgregor K, Oliver IW, Harris L, Ridgway IM (2010) Persistent organic pollutants (PCB, DDT, HCH, HCB & BDE) in eels (Anguilla anguilla) in Scotland: current levels and temporal trends. Environ Pollut 158:2402–2411
McHugh B, Poole R, Corcoran J, Anninou P, Boyle B, Joyce E, Barry Foley M, McGovern E (2010) The occurrence of persistent chlorinated and brominated organic contaminants in the European eel (Anguilla anguilla) in Irish waters. Chemosphere 79:305–313
Oliveira Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat Toxicol 74:53–69
Perneger TV (1998) What's wrong with Bonferroni adjustments. Br Med J 316:1236–1238
Roose P, Van Thuyne G, Belpaire C, Raemaekers M, Brinkman UAT (2003) Determination of VOCs in yellow eel from various inland water bodies in Flanders (Belgium). J Environ Monit 5:876–884
Szlinder-Richert J, Usydus Z, Pelczarski W (2010) Organochlorine pollutants in European eel (Anguilla anguilla L.) from Poland. Chemosphere 80:93–99
Tesch FW (2003) In: Thorpe JE (ed) The eel. Blackwell Science, Oxford, 408 pp
Van Den Berg M, Birnbaum L, Bosveld ATC, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, Van Leeuwen FXR, Liem AKD, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T (1998) Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106:775–792
van Ginneken V, Palstra A, Leonards P, Nieveen M, van den Berg H, Flik G, Spanings T, Niemantsverdriet P, van den Thillart G, Murk A (2009) PCBs and the energy cost of migration in the European eel (Anguilla anguilla L.). Aquat Toxicol 92:213–220
Wells DE, Cowan AA, Christie AEG (1987) A survey of organochlorine residues and related compounds in the European eel, Anguilla anguilla in Scottish fresh waters between September 1986 and February 1987. Report to the working party on pesticide residues (sub-group on wildlife and the environment)
Acknowledgments
The authors acknowledge the contribution of SEPA field staff involved in eel collection and the support of technical staff in the Trace Organics laboratory. In addition, the work of Iain McLaren (Marine Science Scotland) in relation to eel age determination is gratefully acknowledged.
Conflict of interest
Any opinions expressed in this article are those of the authors and are not necessarily those of their host organisations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information Table 1
(DOC 76 kb)
Supplementary Information Table 2
(DOC 75 kb)
Supplementary Information Table 3
(DOC 49 kb)
Supplementary Information Table 4
(DOC 64 kb)
Rights and permissions
About this article
Cite this article
Oliver, I.W., Macgregor, K., Godfrey, J.D. et al. Lipid increases in European eel (Anguilla anguilla) in Scotland 1986–2008: an assessment of physical parameters and the influence of organic pollutants. Environ Sci Pollut Res 22, 7519–7528 (2015). https://doi.org/10.1007/s11356-015-4116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4116-4