Abstract
Despite the fact that Anguilla rostrata (American eel) are frequently captured in salt marshes, their role in salt marsh food webs and the influence of human impacts, such as tidal restrictions, on this role remains unclear. To better understand salt marsh trophic support of A. rostrata, eels were collected from tidally restricted and unrestricted salt marsh creeks within three New England estuaries. Gut contents were examined, and eel muscle tissue was analyzed for carbon and nitrogen stable isotope values and entered into MixSir mixing models to understand if salt marsh food sources are important contributors to eel diet. Data suggest that eel prey rely heavily on salt marsh organic matter and eels utilize salt marsh secondary production as an energetic resource over time, and thus can be considered salt marsh residents. Gut contents indicate that A. rostrata function as top predators, feeding primarily on secondary consumers including other fish species, crustaceans, and polychaetes. Higher A. rostrata trophic position measured upstream of reference creeks suggests that severe tidal restrictions may result in altered food webs, but it is not clear how this impacts the overall fitness of A. rostrata populations in New England salt marshes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The American eel, Anguilla rostrata, ranges throughout the western North Atlantic and has a unique life history where juvenile eels remain inshore in estuaries and freshwater habitats in the “yellow” life stage before undergoing a spawning migration to the Sargasso Sea up to 20 years later (Jessop 1987; Tesch 2003). Historically, A. rostrata was abundant in the Gulf of Maine (Goode 2006) and served as an important source of income and sustenance throughout northern New England and Canada (Bolster 2002; SRSF 2002). While eels are not highly valued in the USA as a food source, increasing demand for American eels for overseas aquaculture operations has resulted in an increase in both fishing pressure and the economic value of the commercial fishery (Haro et al. 2000; Jessop 1997). However, A. rostrata is in decline over the entirety of its range (Haro et al. 2000). Potential causes include the introduction of a nonnative nematode parasite (Barse and Secor 1999), dioxin-like contaminants (Palstra et al. 2006), migration barriers, hydroturbine mortality, and overfishing and habitat loss (Haro et al. 2000). Due to the lack of knowledge on eel ecology in estuaries and potential severity of habitat loss impacts on A. rostrata, the Atlantic States Marine Fisheries Commission Interstate Fishery Management Plan for the American Eel lists use of inshore habitat and impacts of habitat loss as high priority research needs (ASMFC 2000).
The conventional understanding of Anguillids’ inshore habitat use has been obligated in catadromy; however, a high degree of residency (Jessop et al. 2002; Jessop et al. 2004; Tsukamoto and Arai 2001; Tsukamoto et al. 1998; Tsukamoto et al. 2002) and faster growth in estuaries at higher latitudes (Jessop et al. 2004; Morrison et al. 2003; Oliveira 1999) suggests that northern estuarine habitats may be favored more than freshwater habitats. For example, A. rostrata are frequently captured in New England salt marsh habitats (e.g., Dionne et al. 1999; Eberhardt et al. 2011; Nixon and Oviatt 1973) and in some studies were found to comprise the majority of fish biomass (Dionne et al. 1999). Despite the abundance of A. rostrata in northeast salt marshes, little is known about their use of these habitats. Evidence exists for a limited home range of approximately 1 km in salt marsh creeks (Bozeman et al. 1985; Ford and Mercer 1986; Helfman et al. 1983) suggesting that salt marshes provide sufficient trophic support for A. rostrata. However, with few exceptions (e.g., Wenner and Musick 1975), the majority of knowledge of yellow eel foraging ecology comes from freshwater habitats (Aoyama and Miller 2003; Tesch 2003). In light of the potential for the yellow life stage to remain resident in estuaries for many years (Jessop 1987; Tsukamoto and Arai 2001) and the habitat value that intact salt marshes provide, a need exists for greater understanding of A. rostrata use of salt marshes in terms of trophic support.
Coastal habitats such as salt marshes are particularly vulnerable to habitat impacts due to high rates of coastal development and their use as transportation corridors. Structures such as culverts are frequently installed to provide varying degrees of tidal connectivity where roadways intersect salt marsh creeks but often have negative impacts on intact marsh ecosystems and the many ecological values that they provide (Roman and Burdick 2012). Many culverts do not accommodate the full tidal regime, resulting in a tidally restricted system upstream where halophytic vegetation is replaced by invasive species such as Phragmites australis (common reed; Burdick et al. 1997; Chambers et al. 2012; Roman et al. 1984). Colonization by invasive species as well as changes to the infaunal communities (Fell et al. 1991) may shift the food base of tidally restricted salt marshes resulting in an altered food web. Furthermore, decreased flooding and accelerated water velocity through undersized culverts can limit fish movement and access to food resources (Eberhardt et al. 2011; Weisberg and Lotrich 1982). Such barriers may result in changes to A. rostrata or prey movement as well as habitat degradation upstream, and as a result, some marsh areas may contribute disproportionately to fish populations within larger estuaries (Gillanders 2005). In turn, this may limit the export of marsh production to open water habitats via fish migration (Kneib 1997). Examining the functional differences of tidally restricted and unrestricted salt marshes in the trophic support of A. rostrata will improve our understanding of how eels use tidal marshes and how human influence alters the habitat value salt marshes provide for eels.
Stable isotope and gut content analyses were used to evaluate the functional equivalency of both tidally restricted and unrestricted salt marshes in the support of A. rostrata in three New England (USA) estuaries. A. rostrata and their potential food resources were collected from three estuaries to test the hypotheses that (1) salt marsh primary and secondary production serve as important energetic resources for A. rostrata and its prey; (2) trophic position of A. rostrata is altered in tidally restricted salt marshes relative to unrestricted systems; and (3) A. rostrata nutritional sources continue to be represented by salt marsh sources over time suggesting that eels are resident in salt marshes.
Methods
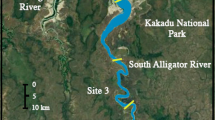
To evaluate the foraging ecology of A. rostrata in salt marsh habitats, three estuaries containing extensive marsh complexes were selected: the Webhannet Estuary, Maine (WEB); the Hampton-Seabrook Estuary, New Hampshire (HSE); and the Parker River Estuary, Massachusetts (PRE; Fig. 1; Table 1). Within each marsh, one tidally restricted and one reference creek were sampled for a total of six creeks (n = 3 for each hydrology treatment). Creeks were selected to represent similar characteristics such as size and availability of intertidal and subtidal habitats. Samples were collected from locations upstream and downstream of the culvert in tidally restricted creeks, and in comparable upstream and downstream sections of reference creeks to examine foraging patterns; only upstream data were analyzed to test for effects of tidal restriction. A. rostrata were collected from each creek using eel pots and were measured for length to the nearest millimeter (mm). Captured eels were anesthetized, sacrificed, and frozen according to a protocol approved by the Institutional Animal Care and Use Committee of the University of New Hampshire (IACUC permit 070702). In the laboratory, fish muscle tissue was dissected and dried for 48 h at 60 °C to achieve constant weight. Primary consumers representing potential prey species were also collected from the marsh to provide baseline data for the calculation of A. rostrata trophic position. Gastropods were removed from the shell, rinsed with distilled water, and analyzed whole. The adductor muscle was dissected out for analysis of stable isotope values of bivalve species.
To determine the relative contribution of salt marsh primary production to A. rostrata diet, organic matter was collected from restricted and reference creeks from within each estuary. Samples of the most abundant species were collected including the C3 plants P. australis and the cattail species Typha latifolia and Typha angustifolia (hereafter referred to as “Typha”), and the C4 plants Spartina alterniflora (cordgrass) and Spartina patens (salt hay). Leaves from plants of each species were rinsed with distilled water, scraped for removal of epiphytes, and dried for 48 h at 60 °C to achieve constant weight. Nekton and vegetation samples were ground using a coffee grinder and weighed into aluminum tins in preparation for stable isotope analysis.
Benthic microalgae were collected on a 210 μm mesh screen according to the protocol outlined by Levin and C. Currin (2012). At the start of the ebb tide, ashed silica was sprinkled on the sediment. The mesh screen was sprayed with filtered seawater, placed over the silica layer, and air bubbles were removed with a plastic spatula. An additional layer of silica was sprinkled on top of the mesh screen and then a layer of fiberglass screen elevated off the substrate with a foam ring was installed to provide shade and prevent desiccation. After several hours, the screen was removed, rinsed with distilled water, and stored on ice. In the laboratory, samples were decanted and filtered onto precombusted (450 °C for 4 h) 47-mm glass fiber filters (GF/F) using a low-pressure vacuum pump filtration system. Filters were examined under a microscope to remove debris and then dried at 60 °C until a constant weight was reached.
Particulate organic matter (POM) was collected from the restricted and reference creeks in each estuary by filtering 4 L of water through a 64-μm mesh. Samples were filtered through ashed GF/F at low pressure in the laboratory and dried at 60 °C until a constant weight was reached. Filters for both benthic microalgae and POM were stored in a desiccator prior to analysis. Material was removed from each filter using forceps and placed into tin capsules for analysis of stable isotopes. Stable isotope values for primary producers in adjacent habitats (i.e., terrestrial and marine) were taken from the literature to serve as end members in mixing models. Data were obtained from projects that occurred in the same estuaries (i.e., WEB and PRE) to represent marine (marine POM values; Deegan 2004) and terrestrial (Quercus rubra, Deegan 2004; Vincent, unpublished data) sources of primary production.
All primary producer, invertebrate, and nekton samples were analyzed for carbon (δ13C) and nitrogen (δ15N) stable isotopes at the University of New Hampshire Stable Isotope Laboratory with a Costech ECS4010 Elemental Analyzer coupled to a Delta Plus XP mass spectrometer (Thermo Finnigan). Stable isotope ratios are reported in delta notation per mil units (‰) as follows:
where X is the 13C or 15 N and R is the 13C/12C or 15 N/14 N, respectively. Stable isotope ratios were determined using Vienna Pee Dee Belemnite (VPDB) as the reference material for carbon and atmospheric N2 (air) for nitrogen. Delta 15 N values are reported on the VPDB scale using International Atomic Energy Agency-N1 (IAEA; 0.4 ‰) and IAEA-N2 (20.3 ‰). Repeated analyses of laboratory standards (tuna for eels and invertebrates, and apple leaves for plants) varied less than 0.15 ‰ for both δ15N and δ13C.
Carbon and nitrogen stable isotope values of A. rostrata captured in upstream regions were evaluated for differences between hydrologic regimes with an analysis of covariance (ANCOVA) using JMP statistical software (JMP 11.0; SAS Institute, Cary, North Carolina, USA). The hydrology of the creek (restricted or restored) served as the main factor with eel length as the covariate to account for ontogenetic change in diet (Ogden 1970; Facey and Labar 1981). The estuary was included as a block to remove the variability in A. rostrata diet associated with latitudinal differences among estuaries (as in Tesch 2003). Residuals were examined for normality and homogeneity of variance; all data met the assumptions of the general linear model.
Mixing models were developed from stable isotope data with MixSir software (Moore and Semmens 2008) to examine the relative contributions of salt marsh primary producers to A. rostrata diets in each marsh treatment (i.e., upstream restricted and downstream reference). The input parameters for MixSir include δ15N and δ13C data for individual A. rostrata, means and standard deviations for potential primary producer sources specific to each estuary, and tissue-diet discrimination factors and associated standard deviations. All mixing models met the diagnostic requirements of MixSir (i.e., posterior draws, duplicate draws, and the ratio between the posterior at the best draw and the posterior density; Moore and Semmens 2008). However, it should be noted that low sample sizes for some treatment combinations (e.g., Webhannet/downstream/restricted and Webhannet/downstream/reference) decrease confidence in those results for inference to eel populations in general.
Estimates of contributions of prey items to consumer diets as well as consumer trophic position are subject to multiple sources of uncertainty (Moore and Semmens 2008; Vander Zanden and Rasmussen 2001), including changes in isotope ratios as prey are assimilated into consumer tissues (discrimination) and variation in the rate at which the diet is assimilated (turnover; Fry 2006). Many food web investigations using stable isotopes rely upon discrimination factors documented in the literature; however, evidence exists for species- and tissue-specific variability in both discrimination and turnover estimates (Hobson and Clark 1992; Logan et al. 2006; Pinnegar and Polunin 1999; Tieszen et al. 1983; Vander Zanden and Rasmussen 2001). To address these potential sources of uncertainty, A. rostrata discrimination factors and turnover rates were determined in a laboratory diet switch experiment. A. rostrata were fed a cultured earthworm diet of known carbon and nitrogen isotope values, and tissues were sampled over time to calculate discrimination and turnover. A. rostrata muscle turnover rate was estimated to be 191 days (Eberhardt, unpublished data). The discrimination factor was calculated as Δ15N = 1.18 (±0.14) and Δ13C = 1.99 (±0.38) for A. rostrata muscle (Eberhardt, unpublished data). Trophic position was calculated for A. rostrata (Vander Zanden and Rasmussen 1999) and estimated to be 4.0. Similarly, Persic et al. (2004) estimated yellow stage Anguilla anguilla to feed at a trophic level of 4.1. As such, discrimination estimates were adjusted to reflect eels feeding at the 4th trophic level in order to increase model robustness.
To evaluate impacts of restricted hydrology on A. rostrata diet, the trophic position of A. rostrata captured from upstream regions was calculated from δ15N data for eels measuring between 20 and 40 cm. Trophic position was calculated as TPeel = (δ15Neel - δ15N PC/Δ15Neel)/2 (Vander Zanden and Rasmussen 1999) where TPeel is the trophic position of the eel, δ15Neel is the eel nitrogen isotope value, δ15NPC is the nitrogen isotope value of primary consumers, and Δ15Neel is the A. rostrata nitrogen isotope discrimination value for one trophic level. The model uses primary consumers as the baseline trophic level to account for variation in δ15N values of basal resources. Stable isotope data for Geukensia demissus (ribbed mussel), Mytilus edulis (blue mussel), and Littorina littorea (common periwinkle) were collected and analyzed specific to each estuary and hydrology treatment for the calculation of trophic position. Data for trophic position were tested for effects of site, hydrology, and the interaction with a two-way ANOVA. Significant results for the interaction term were further evaluated with a Tukey-Kramer post hoc test.
Gut contents were analyzed from all captured eels to evaluate the importance of salt marsh secondary production to eel diet. Guts were removed from eels, and the relative fullness was estimated visually and assigned to one of three general categories: <50 % full, 50–75 % full, or 75–100 % full. Gut contents were examined with a dissecting microscope and identified to major taxon. To identify the contribution of each food type to the diet, the relative volume of each food type for each gut was calculated as the wet weight of each item as a proportion of the total gut contents (Hyslop 1980). Gut contents of A. rostrata with guts ≥50 % full were compared among tidally restricted and reference salt marsh creeks with a one-factor analysis of similarity (ANOSIM), a nonparametric equivalent to multivariate ANOVA, with PRIMER statistical software (Clarke and Green 1988).
Gut contents indicate intraday diet. Using two half-lives to define the period of integration for the isotope data, muscle data indicate A. rostrata diet integrated over approximately 1 year. While gut content data indicate what an animal has ingested hours before capture, stable isotope data provide information on what prey items an organism has assimilated over the past year. As such, proportions of prey items from gut contents were considered with isotope data to provide a time series of resource use by A. rostrata.
Results
The stable isotope values of A. rostrata muscle tissue ranged from 6.5 to 12.8 for δ15N and from −22.3 to −13.0 for δ13C (Fig. 2). Both δ15N and δ13C values of A. rostrata muscle tissue were significantly different among estuaries but did not differ between restricted and reference creeks, by eel length, or the interaction term between hydrology and eel length (ANCOVA; Table 2). Potential sources of primary production included red oak, benthic microalgae, estuarine POM, salt marsh grasses, brackish species and marine POM. Mean δ15N values for primary producers ranged from 1.2 to 7.0 ‰, and mean δ13C ranged from −28.4 to −13.7 ‰ (Table 3).
Biplots for carbon and nitrogen stable isotope values used in mixing models, including data for individual Anguilla rostrata and the means (±standard deviation) of potential prey items. Data are presented for each of the three estuaries sampled: a Webhannet Estuary, Maine (WEB), b Hampton-Seabrook Estuary, NH (HSE), and c Parker River Estuary, Massachusetts (PRE)
Mixing model outputs are more robust when the number of sources is limited; similarly, source items with similar isotope ratios can confound modeled diet distributions (Moore and Semmens 2008). As a result, data were pooled where isotope values were statistically similar; data for the salt marsh halophytes S. alterniflora and S. patens were not significantly different (one-way ANOVA) nor were the brackish/freshwater species P. australis and Typha; so, they were pooled into “marsh grasses” and “brackish species” categories, respectively. Primary producer values specific to each estuary were used in mixing models.
Mixing model outputs suggest that multiple sources of primary production support the diet of A. rostrata (Table 4). POM represented a common source of primary production to the prey of A. rostrata; evidence of trophic pathways based on POM from marine and/or estuarine sources was found at all sites. The contribution of salt marsh macrophytes to eel basal diet was modeled in downstream regions of all estuaries (9–23 % of diet) regardless of hydrology (with the exception of PRE/restricted/downstream), whereas salt marsh macrophtyes were not a primary contributor to eels captured in upstream locations. Model results suggest important differences in basal source contributions among estuaries. Marine POM was the dominant basal resource for A. rostrata captured from PRE (47–95 % of diet). The northern two estuaries (WEB and HSE) were less saline (Table 1), with dietary contributions from primarily estuarine POM and salt marsh grasses in addition to POM marine. In one case (HSE/restricted/upstream), the dominant basal source was modeled as brackish species (80 % of diet) where the tide was restricted and brackish species dominate.
Comparison of mixing model outputs for upstream data between tidally restricted and reference creeks provides insight into the impact of tidal restriction on the trophic ecology of eels. Evidence for an effect of tidal restriction varied by estuary, perhaps due to the magnitude of the restriction. A. rostrata basal resource use in PRE and WEB did not differ between reference and restricted creeks within each estuary. However, the organic matter sources supporting A. rostrata captured upstream were different between restricted and reference creeks in HSE, the site with the greatest degree of restriction (Table 1). A. rostrata from the reference creek of HSE were supported by primarily marine POM, while A. rostrata from the restricted creek were supported by brackish/invasive plant production as the primary carbon source.
Gut content data were analyzed for A. rostrata that had guts at least 50 % full (n = 31). The guts were composed primarily of species resident to salt marsh and other estuarine habitats. The common salt marsh resident fish F. heteroclitus and polychaete worms (Nereid species and Polynoidae) were the most abundant items in A. rostrata guts occupying 39 and 33 % of guts, respectively (Fig. 3). Other species present included shrimp (Crangon septemspinosa and Palaemonetes species), Gammarid amphipods, fish species not resident to salt marshes (classified as such due to the size of scales, opercula, and pharyngeal jaws), as well as plant material (Fig. 3). The gut of one small eel (15.9 cm) contained 57 mosquito larvae. Gut content composition of A. rostrata collected upstream of tidal restrictions did not differ from those captured upstream in reference creeks (one way ANOSIM, R = −0.06, p = 0.89).
Trophic position was calculated for A. rostrata ranging from 20 to 40 cm using eel muscle δ15N data (Table 5). The 20- to 40-cm size range was selected to analyze eels within the same feeding guild (Ogden 1970; Facey and Labar 1981; Tesch 2003) while maintaining an adequate sample size. The mean trophic position of A. rostrata varied between hydrology, sampling region, and their interaction (two-way ANOVA, df = 2, F = 4.0, p < 0.032). A posterior test (Tukey’s HSD) revealed that the trophic position of A. rostrata collected in upstream reference creeks was significantly greater than A. rostrata collected in upstream restricted creeks in two of the three sites sampled (WEB and HSE).
Discussion
Mixing models indicate that A. rostrata foods webs are supported by a diversity of organic matter sources that vary across estuaries. While it is clear from the data that A. rostrata basal diet stems from many available sources (e.g., estuarine, marine, brackish/invasive species), strong evidence exists for A. rostrata support from salt marsh primary production in the form of salt marsh grasses and/or POM. The relative contribution of salt marsh macrophyte detritus to POM can be quite variable depending on the coastal geomorphology of the system, recent meteorological events and the size and proximity of vegetated marsh habitat (Odum et al. 1979; Roman and Daiber 1989; Sullivan and Moncreiff 1990; McClelland and Valiela 1998). Due to considerable evidence for S. alterniflora detritus across salt marsh dominated estuaries of the eastern USA (Teal 1962; Odum and de la Cruz 1967; Roman and Daiber 1989), it is reasonable to assume that assimilation of POM by A. rostrata and its prey represents, in part, use of salt marsh production. It should be noted that A. rostrata are likely not grazing directly on salt marsh macrophytes since direct herbivory is considered uncommon (Tesch 2003). Rather, A. rostrata may directly ingest small amounts of salt marsh macrophytes as detritus while foraging for prey. More likely, the majority of the carbon and nitrogen fixed by vegetation is assimilated into A. rostrata diets indirectly as their prey digest vegetation tissue or bacteria and fungus growing on the vegetation (i.e., detrital food web).
A. rostrata are considered opportunistic omnivores (Tesch 2003), and the abundance of prey items identified in A. rostrata guts supports this. Secondary consumers were common in guts of A. rostrata, and it is clear that A. rostrata are functioning as a top predator in salt marsh food webs. The abundance of salt marsh resident (F. heteroclitus) and dependent (e.g., C. maenus, Palaemonetes spp., C. septemspinosa) species in gut contents confirms that A. rostrata diet relies heavily on salt marsh secondary production. POM has been found to be an important energetic resource to salt marsh infauna, shrimp, and F. heteroclitus in New England salt marshes (Dibble and Meyerson 2013). Therefore, it is likely that the dominance of POM as a basal resource at most sites is due to eel foraging on suspension feeding primary consumers such as polychaetes resulting in trophic transfer of POM to A. rostrata.
Estuaries appeared to function differently in terms of the basal support of eels. Furthermore, evidence exists for impacts of tidal restriction in some systems, but not others. In the upstream area of the HSE restricted marsh, mixing model results indicate that trophic pathways were driven by Typha and P. australis, species common to tidally restricted systems (Chambers et al. 2012; Roman et al. 1984) whereas the reference creek in HSE was driven by marine POM. Additionally, eels from the restricted area of HSE fed at one trophic level lower than those captured in the reference creek of the same estuary. Of all estuaries sampled, the tidal restriction at HSE results in the greatest reduction in tidal amplitude (Table 1), and as a result, the upstream area is colonized by primarily invasive and brackish vegetation. Eels captured from WEB, the estuary with the intermediate degree of restriction of the estuaries sampled, were supported by primarily estuarine basal sources in the upstream regions of both the restricted and reference creeks; however, the reduced trophic position in eels captured from the restricted system (Table 5) suggest that, like in HSE, the structure of the restricted creek food web is altered relative to the reference creek. Finally, eels captured from PRE, the site with the most tidal flushing and smallest restriction, had a basal diet driven by marine organic matter sources in both reference and restricted creeks. Although the experiment was not designed to explicitly test for the degree of restriction, it appears that in creeks with severe tidal restrictions, more food is eaten locally and at a lower trophic level.
PRE is a well-mixed estuary and the undersized culvert in the restricted creek reduced the tidal regime only slightly (Table 1). As such, it is possible that A. rostrata captured in PRE had isotope signatures reflecting basal marine sources because either prey were relying on marine carbon sources or eels had recently moved from marine to estuarine habitats.
Evidence for A. rostrata feeding at a lower trophic level in the restricted creeks of two of the three estuaries sampled suggests that the trophic structure of highly restricted systems is altered. Although the food web structure of middle trophic levels in estuarine systems is highly complex and poorly understood (Douglass et al. 2011), changes to the infaunal community composition have been documented as a result of tidal restriction (Fell et al. 1991). Decreased prey diversity, and subsequently the loss of trophic levels have been documented in tidally restricted mangrove systems (Layman et al. 2007). Furthermore, decreased movement of important prey species (e.g., F. heteroclitus, C. septemspinosa) between upstream and downstream regions of tidally restricted marshes (Eberhardt et al. 2011) may result in a loss of prey resources for A. rostrata in some marsh areas. As a result, the observed lower trophic position of eels in highly restricted systems may indicate an altered trophic structure in marshes subject to reduced tidal flushing.
Little data exist regarding A. rostrata use of salt marsh habitats as a food source (e.g., Wenner and Musick 1975); so, our results provide important new information regarding the trophic support of A. rostrata in New England salt marshes. Data indicate that A. rostrata rely on salt marsh primary production (isotope data) as a basal resource and secondary production (gut contents) to meet energetic demands. The spatial variation observed in basal food resources among estuaries supports previous research in New England marshes suggesting that upper trophic levels derive organic matter from local sources (Deegan and Garritt 1997) and also suggests that A. rostrata are resident in these systems. Microchemical analysis of A. rostrata otoliths has documented use of estuarine habitats by eels (e.g., Tsukamoto and Arai 2001; Jessop et al. 2002), and our results provide compelling evidence that salt marshes serve as critical habitats in the life history of A. rostrata as a sustained foraging area over time. Prior to this study, little evidence existed for sustained use of New England salt marshes over time by A. rostrata. Most assessments of salt marsh food webs go no higher on the trophic spectrum than F. heteroclitus, likely because higher trophic levels in northern New England salt marshes are thought to be primarily transient (Ayvazian et al. 1992). Our data indicate that A. rostrata can function as resident top predators in New England salt marshes.
Human impacts including habitat loss have resulted in a decline in A. rostrata populations (Haro et al. 2000). Given the clear importance of salt marsh primary and secondary production to A. rostrata diet (observed through mixing models and gut contents) as well as the altered trophic support (as measured by trophic position) upstream of tidal restrictions, habitat functional impacts through tidal restrictions and other mechanisms have resulted in an alteration of trophic resources for eels. As 37 % of New England salt marshes have been lost over the last 200 years (Bromberg and Bertness 2005), the cumulative impact of marsh loss is likely a contributing factor in the decline of A. rostrata populations.
Fish can play an important role in exporting energy and nutrients out of estuarine habitats to downstream open water (Kneib 1997; Nelson et al. 2013). Considering that A. rostrata are residents that exploit a diversity of food resources in the salt marsh, and that A. rostrata undergo a spawning migration of thousands of miles, the potential exists for salt marsh-derived production to support marine food webs as eels are preyed upon along their migratory paths (Béguer-Pon et al. 2012). Furthermore, as semelparous organisms, eels that complete the spawning migration will enter the detrital food web of the Sargasso Sea. This potential mechanism represents a large-scale movement of nutrients and energy from the highly productive salt marshes of New England to distant open ocean habitats. Given the decline in A. rostrata populations and the important role that salt marshes play as a foraging resource, as well as the potential role of A. rostrata as a vector of trophic transfer of marsh production to open ocean habitats, it is critical to maintain the ecological integrity of salt marshes (e.g., through tidal restoration) in support of A. rostrata populations.
References
Adamowicz, S. and K. O’Brien. (2012). Drakes Island Tidal Restoration. In Tidal Marsh Restoration: A Synthesis of Science and Management, ed. C. Roman and D. Burdick, 315-332: Island Press/Center for Resource Economics.
Aoyama, J., and M.J. Miller. 2003. The silver eel. In Eel Biology, ed. K. Aida, K. Tsukamoto, and K. Yamauchi, 107–117. Tokyo: Springer-Verlag.
Atlantic States Marine Fisheries Commission. (2000). Interstate fishery management plan for American eel (Anguilla rostrata). Atlantic States Marine Fisheries Commission, Washington, D.C. Fishery Management Report No. 36. 79 pp.
Ayvazian, S.G., L.A. Deegan, and J.T. Finn. 1992. Comparison of habitat use by estuarine fish assemblages in the Acadian and Virginian zoogeographic provinces. Estuaries 15: 368–383.
Barse, A. M., and D. H. Secor. 1999. An exotic nematode parasite of the American eel. Fisheries 24:6–10.
Béguer-Pon, M., J. Benchetrit, M. Castonguay, K. Aarestrup, and S.E. Campana. 2012. Shark Predation on Migrating Adult American Eels (Anguilla rostrata) in the Gulf of St. Lawrence. PLoS ONE 7(10): e46830.
Bolster, W.J. 2002. Cross-grained and wily waters. A guide to the Piscataqua maritime region. Portsmouth, N.H.: Peter E. Randall
Bozeman, E.L., G.S. Helfman, and T. Richardson. 1985. Population size and home range of American eels in a Georgia tidal creek. Transactions of the American Fisheries Society 114: 821–825.
Bromberg, K., and M. Bertness. 2005. Reconstructing New England salt marsh losses using historical maps. Estuaries 28: 823–832.
Burdick, D.M., M. Dionne, R.M. Boumans, and F.T. Short. 1997. Ecological responses to tidal restorations of two northern New England salt marshes. Wetlands Ecology and Management 4: 129–144.
Burdick, D.M., R.M. Boumans, M. Dionne, and F.T. Short. 1999. Impacts to salt marshes from tidal restrictions and ecological responses to Tidal Restoration. Silver Spring: Final Report. NOAA Reserves and Sanctuaries Division.
Burdick, D.B., C.R. Peter, G.E. Moore, and G. Wilson. 2010. Comparison of restoration techniques to reduce dominance of Phragmites australis at Meadow Pond, Hampton. Portsmouth: New Hampshire. Report to the New Hampshire Coastal Program.
Chambers, R., L. Meyerson, and K. Dibble. (2012). Ecology of Phragmites australis and responses to tidal restoration. In Tidal marsh restoration: A synthesis of science and management, ed. C. Roman and D. Burdick, 81-96: Island Press/Center for Resource Economics.
Clarke, K.R., and R.H. Green. (1988). Statistical design and analysis for a “biological effects” study.
Deegan, L. 2004. Stable isotope (carbon and nitrogen) data for functional groups in the Plum Island Sound Estuary. Long Term Ecological Research Network. doi:10.6073/pasta/d2af1a946689c48ece10b072b6ef1172.
Deegan, L.A., and R.H. Garritt. 1997. Evidence for spatial variability in estuarine food webs. Marine Ecology Progress Series 147: 31–47.
Dibble, K., and L. Meyerson. 2013. The effects of plant invasion and ecosystem restoration on energy flow through salt marsh food webs. Estuaries and Coasts 1–15.
Dionne, M., F. Short, and D. Burdick. 1999. Fish utilization of restored, created and reference salt-marsh habitat in the Gulf of Maine. American Fisheries Society Symposium 22: 384–404.
Douglass, J.G., J.E. Duffy, and E.A. Canuel. 2011. Food web structure in a Chesapeake Bay eelgrass bed as determined through gut contents and 13C and 15N isotope analysis. Estuaries and Coasts 34: 701–711.
Eberhardt, A.L., D.M. Burdick, and M. Dionne. 2011. The Effects of Road Culverts on Nekton in New England Salt Marshes: Implications for Tidal Restoration. Restoration Ecology 19: 776–785.
Facey, D.E., and G.W. LaBar. 1981. Biology of American eels in Lake Champlain, Vermont. Transactions of the American Fisheries Society 110: 396–402.
Fell, P.E., K.A. Murphy, M.A. Peck, and M.L. Recchia. 1991. Re-establishment of Mylampus bidentatus (Say) and other macroinvertebrates on a restored impounded salt marsh: comparison of populations above and below the impoundment dyke. Journal of Experimental Marine Biology and Ecology 152: 33–48.
Ford, T.E., and E. Mercer. 1986. Density, size distribution, and home range of American eels, Anguilla rostrata, in a Massachusetts salt marsh. Environmental Biology of Fishes 17: 309–314.
Fry, B. 2006. Stable isotope ecology. New York: Springer.
Gillanders, B.M. 2005. Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuarine, Coastal and Shelf Science 64: 47–57.
Goode, A. 2006. The plight and outlook for migratory fish in the Gulf of Maine. Journal of Contemporary Water Research and Education 134: 23–28.
Haro, A., W. Richkus, K. Whalen, A. Hoar, W.D. Busch, S. Lary, T. Brush, and D. Dixon. 2000. Population decline of the American eel: Implications for research and management. Fisheries 25: 7–16.
Helfman, G.S., D.L. Stoneburner, E.L. Bozeman, P.A. Christian, and R. Whalen. 1983. Ultrasonic telemetry of American eel movements in a tidal creek. Transactions of the American Fisheries Society 112: 105–110.
Hobson, K.A., and R.G. Clark. 1992. Assessing avian diets using stable isotopes 2. Factors influencing diet-tissue fractionation. Condor 94: 189–197.
Hyslop, E.J. 1980. Stomach contents analysis - A review of methods and their application. Journal of Fish Biology 17: 411–429.
Jessop, B.M. 1987. Migrating American eels in Nova Scotia. Transactions of the American Fisheries Society 116: 161–170.
Jessop, B.M. (1997). An overview of European and American eel stocks, fisheries and management issues. pp. 6-20. In R. H. Peterson (ed.). The American eel in eastern Canada: stock status and management strategies. Proceedings of Eel Workshop, January 13-14, 1997, Quebec City, Quebec. Biological Station. St. Andrews, NB. Canadian Technical Report of Fisheries and Aquatic Sciences. No. 2196. 174 pp.
Jessop, B.M., J.C. Shiao, Y. Iizuka, and W.N. Tzeng. 2002. Migratory behavior and habitat use by American eels Anguilla rostrata as revealed by otolith microchemistry. Marine Ecology-Progress Series 233: 217–229.
Jessop, B.M., J.C. Shiao, Y. Iizuka, and W.N. Tzeng. 2004. Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Marine Ecology-Progress Series 272: 231–244.
Kneib, R.T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: An Annual Review 35: 163–220.
Layman, C.A., J.P. Quattrochi, C.M. Peyer, and J.E. Allgeier. 2007. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecology Letters 10: 937–944.
Levin, L.A., and C. Currin. (2012). Stable Isotope Protocols: Sampling and Sample Processing.
Logan, J., H. Haas, L. Deegan, and E. Gaines. 2006. Turnover rates of nitrogen stable isotopes in the salt marsh mummichog, Fundulus heteroclitus, following a laboratory diet switch. Oecologia 147: 391–395.
McClelland, J.W., and I. Valiela. 1998. Changes in food web structure under the influence of increased anthropogenic nitrogen inputs to estuaries. Marine Ecology Progress Series 168: 259-271.
Moore, J.W., and B.X. Semmens. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11: 470–480.
Morrison, W.E., D.H. Secor, and P.M. Piccoli. 2003. Estuarine habitat use by Hudson River American eels. American Fisheries Society Symposium 33: 87–99.
Nelson, J.A., C.D. Stalling, W.M. Landing, and J. Chanton. 2013. Biomass transfer subsidizes nitrogen to offshore food webs. Ecosystems 16(6): 1130–1138.
Nixon, S.W., and C.A. Oviatt. 1973. Ecology of a New England salt marsh. Ecological Monographs 43: 463–498.
Odum, E.P. and A.A. de la Cruz. 1967. Particulate detritus in a Georgia salt marsh estuarine ecosystem. In Estuaries, ed. G.H. Lauff, 381-388. American Association for the Advancement of Science Publication 83. Washington, D.C.
Odum, W.E., J.S. Fisher, and J. Pickral. 1979. Factors controlling the flux of particulate organic carbon from estuarine wetlands. In Ecological processes in coastal and marine systems. Ecological Study Series, No. 10, ed. R.J. Livingston, 69–80. New York: Plenum.
Ogden, J.C. 1970. Relative abundance, food habits, and age of the American eel, Anguilla rostrata (LeSueur), in certain New Jersey streams. Transactions of the American Fisheries Society 99: 54–59.
Oliveira, K. 1999. Life history characteristic and strategies of the American eel, Anguilla rostrata. Canadian Journal of Fisheries and Aquatic Sciences 56: 795–802.
Palstra, A. P., V. J. T. van Ginneken, A. J. Murk, and G. van den Thillart. 2006. Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 93:145–148.
Persic, A., H.Roche, and F. Ramade. 2004. Stable carbon and nitrogen isotope quantitative structural assessment of dominant species from the Vaccarès Lagoon trophic web (Camargue Biosphere Reserve, France). Estuarine, Coastal and Shelf Science 60(2): 261–272.
Pinnegar, J.K., and N.V.C. Polunin. 1999. Differential fractionation of delta C-13 and delta N-15 among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225–231.
Roman, C., and D. Burdick. (2012). A Synthesis of Research and Practice on Restoring Tides to Salt Marshes. In Tidal Marsh Restoration: A Synthesis of Science and Management, ed. C. Roman and D. Burdick, 3-10: Island Press/Center for Resource Economics.
Roman, C.T. and F.C. Daiber. 1989. Organic carbon flux through a Delaware Bay salt marsh: tidal exchange, particle size distribution, and storms. Marine Ecology Progress Series 54: 149–156.
Roman, C. T., W. A. Niering, and R. S. Warren. 1984. Salt marsh vegetation change in response to tidal restriction. Environmental Management 8:141-150.
Social Research for Sustainable Fisheries (SRSF). 2002. The Paq’tnkek Mi’kmaq and Kat American Eel - Anguilla rostrata) - A Preliminary Report of Research Results, Phase 1
Sullivan, M.J., and C.A. Moncreiff. 1990. Edaphic algae are animportant component of salt marsh food webs: evidencefrom multiple stable isotope analyses. Marine Ecology Progress Series 62:149–159.
Teal, J.M. 1962. Energy flow in the salt marsh ecosystem of Georgia. Ecology 43(4): 614–624.
Tesch, F.W. 2003. The Eel. Oxford: Blackwell Science.
Tieszen, L.L., T.W. Boutton, K.G. Tesdahl, and N.A. Slade. 1983. Fractionation and turnover of stable carbon isotopes in animal tissues - implications for delta C13 analysis of diet. Oecologia 57: 32–37.
Tsukamoto, K., and T. Arai. 2001. Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Marine Ecology-Progress Series 220: 265–276.
Tsukamoto, K., I. Nakai, and W.V. Tesch. 1998. Do all freshwater eels migrate? Nature 396: 635–636.
Tsukamoto, K., J. Aoyama, and M.J. Miller. 2002. Migration, speciation, and the evolution of diadromy in anguillid eels. Canadian Journal of Fisheries and Aquatic Sciences 59: 1989–1998.
Vander Zanden, M.J., and J.B. Rasmussen. 1999. Primary consumer d13C and d15N and the trophic position of aquatic consumers. Ecology 80: 1395–1404.
Vander Zanden, M.J., and J.B. Rasmussen. 2001. Variation in delta N-15 and delta C-13 trophic fractionation: Implications for aquatic food web studies. Limnology and Oceanography 46: 2061–2066.
Weisberg, S.B., and V.A. Lotrich. 1982. The importance of an infrequently flooded intertidal salt marsh surface as an energy source for the mummichog, Fundulus heteroclitus - an experimental approach. Marine Biology 66: 307–310.
Wenner, C., and J. Musick. 1975. Food habits and seasonal abundance of the American eel, Anguilla rostrata, from the lower Chesapeake Bay. Chesapeake Science 16: 62–66.
Acknowledgments
The authors are grateful to Chris Peter, Chris Cavalieri, Carol Eberhardt, and Sandra Pimentel for field assistance, stable isotope sample preparation, and gut content analysis, and to Raymond Grizzle for assistance in identifying invertebrates in gut content samples. The authors also extend thanks to Andy Ouimette at the University of New Hampshire (UNH) Stable Isotope Laboratory for assistance in stable isotope sample analysis as well as Linda Deegan, Charles Hopkinson, and Hap Garritt for permission to use Quercus rubra and marine particulate organic matter stable isotope values. New Hampshire Sea Grant Development Funds, the UNH Marine Program William R. Spaulding Endowment, and the Natural Resources and Earth Systems Science doctoral program at UNH provided funding for this research. The Rachel Carson National Wildlife Refuge granted permission to sample in Wells, Maine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wayne S. Gardner
Rights and permissions
About this article
Cite this article
Eberhardt, A.L., Burdick, D.M., Dionne, M. et al. Rethinking the Freshwater Eel: Salt Marsh Trophic Support of the American Eel, Anguilla rostrata . Estuaries and Coasts 38, 1251–1261 (2015). https://doi.org/10.1007/s12237-015-9960-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-9960-4